Abstract
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a novel family of glucose-lowering agents. Accumulating evidence suggests that DPP-4 inhibitors preserve pancreatic beta-cell function, but results in previous studies have been inconsistent. We assessed the effects of DPP-4 inhibitors on the homoeostasis model assessment beta-cell function (HOMA-B) or insulin resistance (HOMA-IR) index in patients with type 2 diabetes through a systematic review and meta-analysis of randomized controlled trials (RCTs). Relevant articles were identified from PubMed, Embase, and Cochrane Library databases up to December 27, 2016. We calculated weighted mean differences (WMDs) and 95% confidence intervals (CIs) in each included trial and pooled the data using a random-effects model. Fifty-two trials were included in the present analysis. Compared with placebo control, DPP-4 inhibitors as monotherapy significantly improved HOMA-B (WMD 9.15; 95% CI 7.48, 10.81). Similarly, DPP-4 inhibitors as add-on therapy in combination with other drugs showed significant improvement in HOMA-B (WMD 9.04; 95% CI 5.72, 12.37). However, we found no significant improvement in HOMA-IR following treatment with DPP-4 inhibitors as mono-therapy or as add-on therapy. In conclusion, DPP-4 inhibitors as monotherapy or as add-on therapy significantly improved beta-cell function but had no significant effect on insulin resistance in type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by a progressive decline in beta-cell function with insulin resistance1,2,3. Beta-cell dysfunction and insulin resistance are the central mechanisms in the pathogenesis of T2DM. As a surrogate marker for measuring beta-cell function and insulin sensitivity, the homoeostasis model assessment (HOMA) indexes, which are based on fasting glucose and insulin levels, have been widely used for decades in clinical and epidemiological research4,5. The validity of HOMA indexes have been confirmed against the hyperglycemic and euglycemic clamps and the intravenous glucose tolerance test4. Recently, the Whitehall II study has shown that beta-cell function and insulin sensitivity as measured by HOMA beta-cell function (HOMA-B) and HOMA insulin resistance (HOMA-IR) may undergo significant reduction several years before the diagnosis of T2DM6. The UK Prospective Diabetes Study (UKPDS) has demonstrated that HOMA-B continues to deteriorate in association with progressively increasing hyperglycemia despite treatment7. These data highlight the importance of preserving beta-cell function and insulin sensitivity in the prevention and management of T2DM.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a novel family of glucose-lowering agents that are increasingly used in clinical practice in treating T2DM patients. DPP-4 is responsible for the degradation of incretin hormones such as glucagon-like peptide 1 (GLP-1)8. Inhibition of DPP-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in T2DM patients9. DPP-4 inhibitors have many favorable features including a low risk of hypoglycemia and a neutral effect on body weight8. In addition, they are efficacious and well tolerated as mono-therapy and also as add-on therapy in combination with commonly prescribed anti-diabetic agents and are suitable for once-daily oral dosing8,10. As a result, the American Diabetes Association and the European Association for the Study of Diabetes recommend DPP-4 inhibitors as part of a combination therapy with metformin and/or other agents or as a preferable monotherapy choice for patients in whom metformin is contraindicated or not tolerated11,12.
The underlying mechanisms of DPP-4 inhibitors in the management of T2DM remain to be understood, and the roles of DPP-4 inhibitors on beta-cell function and insulin sensitivity are of particular interest. Experimental data show that DPP-4 inhibitors may help preserve pancreatic beta-cell function13,14. However, results from previous studies in humans have been inconsistent, partly because of the limited sample size and insufficient statistical power in some individual studies. In this study, we aimed to systematically review the available evidence and quantitatively summarize the findings by performing a meta-analysis of randomized controlled trials (RCTs).
Material and Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement15.
Study selection
Articles were eligible for inclusion if they fulfilled all the following criteria: (i) they were RCTs; (ii) the participants were patients with T2DM; (iii) they compared a DPP-4 inhibitor as monotherapy or add-on therapy with an appropriate control; (iv) they provided information on HOMA-estimated beta-cell function or insulin resistance; (v) the study duration was no less than 12 weeks; and (vi) the results were published in peer-reviewed journal as a full paper. We excluded the following types of articles: review articles or editorials, non-human studies (i.e., cell culture or animal studies), studies that did not include DPP-4 inhibitor treatment in the intervention group, and studies that did not evaluate beta-cell function or insulin resistance. Disagreement about eligibility was resolved by consensus between all authors.
Literature search
We performed a comprehensive literature search in the PubMed, EMBASE, and Cochrane Library databases. The last search update was conducted on up to December 27, 2016. In terms of the database search strategy, we used a combination of free text (e.g., type 2 diabetes) and subheadings from MeSH (e.g., “Diabetes Mellitus, Type 2” [Mesh]) or EMTREE terms (e.g., ‘non insulin dependent diabetes mellitus’/exp). In addition to using the generic term for DPP-4 inhibitors, we also specifically named each major DPP-4 inhibitor (e.g., Sitagliptin, Vildagliptin, Saxagliptin, Linagliptin, Anagliptin, Teneligliptin, Alogliptin, Gemigliptin, and Dutogliptin) when conducting the literature search. More detailed search terms are listed in the Supplementary Materials. The reference lists of relevant studies and review articles were also checked to identify additional relevant studies.
Data extraction
The following data were extracted from each eligible article: the first author’s name, year of publication, sample size, participants’ age, T2DM duration, types of DPP-4 inhibitors tested, study duration, mean baseline HbA1c, and mean and standard deviation of HOMA indexes in the comparison groups. When necessary, we contacted the corresponding authors of the original articles by email to request relevant data or information. If multiple articles were published using data from the same study, we extracted the report with the information most relevant to the analysis. If the same trial reported data at different follow-up durations16,17,18, we extracted the data corresponding to the longest follow-up period18. If a study reported results on the effects of DPP-4 inhibitors at different doses, we extracted data corresponding to the standard dosages for each DPP-4 inhibitor (e.g., sitagliptin 100 mg/day; saxagliptin 5 mg/day; vildagliptin 100 mg/day; linagliptin 5 mg/day; teneligliptin 20 mg/day; and alogliptin 25 mg/day) unless otherwise specified.
Quality assessment of the included RCTs
Assessment of risk of bias in the included RCTs was performed according to the Cochrane Collaboration’s tool19, which includes the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias in each domain was assessed as low, high, or unclear.
Data synthesis and statistical analysis
We calculated weighted mean differences (WMDs) and 95% confidence intervals (CIs) for the change in HOMA indexes from baseline to the end of the trial for DPP-4 inhibitors versus controls reported in each of the included trials and pooled the data in the meta-analysis using a random-effects model20. If both adjusted (typically derived from analysis of covariance) and unadjusted changes from baseline were reported in a trial, we selected the adjusted estimates as they accounted for possible baseline imbalance and the correlation between baseline and follow-up measures. When the adjusted estimates were not available, we limited meta-analyses to those trials with baseline balance in HOMA indexes21. When standard deviations of the change from baseline were not reported in the article, we converted standard errors or 95% CIs to standard deviations.
We used forest plots to visualize the effect sizes in each individual study and the pooled overall effect size. We assessed between-study heterogeneity using the χ2-based Cochrane’s Q statistic and the I2 metric (I2 values of 25%, 50%, and 75% were considered to indicate low, medium, and high heterogeneity, respectively)22. Potential of publication bias is assessed by funnel plots, in which asymmetrical plot indicates presence of reporting bias. To explore the possible differences in the effectiveness of individual DPP-4 inhibitors on beta-cell function and insulin sensitivity, we performed stratification analysis according to the type of DPP-4 inhibitor tested. Sensitivity analyses were performed by omitting one study at a time and computing the pooled effect size of the remaining studies to evaluate whether the results were affected markedly by a single study. All statistical analyses were performed using Stata software version 11.0 (Stata Corp, College Station, TX, USA).
Results
Characteristics of the included studies
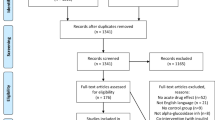
We identified 589 potentially relevant articles from database search and other resources. After screening, 278 articles were evaluated in detail and 52 articles presenting RCTs16,18,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72 were finally included in the meta-analysis (Fig. 1). Of them, 23 articles reported data from trials using DPP-4 inhibitors as monotherapy, 28 articles from trials using DPP-4 inhibitors as adds-on therapy, and the other one article involving mixed study design using DPP-4 inhibitors as both monotherapy and adds-on therapy.
The baseline characteristics of the included RCTs in the meta-analysis are shown in Table 1. The majority of studies were conducted in Caucasian or Asian populations. Sitagliptin was the most investigated drug among DPP-4 inhibitors as monotherapy and add-on therapy in the included studies. Most of the included RCTs had a relatively short study duration (typically 12 or 24 weeks). The risk of bias varied across the individual trials. All the included trials, except one56, involved appropriate double blind procedures, and none were associated with concerns about selective outcome reporting. However, in some of the included trials, HOMA-IR or HOMA-B data were missing for more than 20% of the participants, because these indicators were not the primary outcome and therefore were not assessed all participants. In addition, information regarding random generation sequence, allocation concealment, and blinding of outcome assessment was not clearly described in most trials (Supplementary Table 1).
Effects of DPP-4 inhibitors as monotherapy on beta-cell function and insulin resistance
Our meta-analysis of 23 RCTs showed that DPP-4 inhibitors as monotherapy significantly improved beta-cell function (Fig. 2). The pooled WMD (95% CI) for the HOMA-B was 9.15 (7.48, 10.81). We observed no evidence for significant heterogeneity (I2 = 4%, P = 0.41). For insulin resistance, we found no significant improvement after treatment with DPP-4 inhibitors as monotherapy (Fig. 3). Sensitivity analyses conducted by omitting one study at a time did not show significant alteration of the results.
Effects of DPP-4 inhibitors as add-on therapy on beta-cell function and insulin resistance
In a meta-analysis of 28 RCTs, we found that treatment with DPP-4 inhibitors as add-on therapy in combination with other drugs significantly improved beta-cell function (Fig. 4). The pooled WMD (95% CI) for the HOMA-B was 9.04 (5.72, 12.37). There was evidence for significant heterogeneity (I2 = 89%, P < 0.001). Similar to the findings for DPP-4 inhibitor monotherapy, we found no significant improvement in insulin resistance after treatment with DPP-4 inhibitors as monotherapy (Fig. 5). Sensitivity analyses conducted by omitting one study at a time did not show significant alteration of the results.
Publication bias
In the funnel plots, we observed evidence of publication bias (i.e., asymmetrical plots) in the meta-analysis of DPP-4 inhibitors as either monotherapy (Supplementary Figures 1 and 2) or add-on therapy (Supplementary Figures 3 and 4) on beta-cell function and insulin resistance. However, these results should be interpreted with caution, because asymmetrical plots may also be a result of other reasons, such as small study effects, rather than publication bias.
Comparative effects of individual DPP-4 inhibitors as monotherapy
In stratification analyses according to the types of DPP-4 inhibitors, we pooled data for each DPP-4 inhibitor. We found that treatment with all types of DDP-4 inhibitors except for linagliptin resulted in an increase in HOMA-B (Supplementary Figure 5). In the meta-analysis of the effects of individual DPP-4 inhibitors on insulin resistance (Supplementary Figure 6), we observed a significant improvement in insulin resistance with sitagliptin treatment without evidence of significant heterogeneity (WMD −0.38; 95% CI −0.69, −0.08; I2 = 0%, P for heterogeneity = 0.58). Linagliptin seemed to have a trend to increase insulin resistance, but the results should be interpreted cautiously due to the limited number of available studies. We found no significant effects of other DPP-4 inhibitors on insulin resistance.
Discussion
Our meta-analysis of data from 52 RCTs showed that DPP-4 inhibitors as monotherapy or as add-on therapy significantly improved beta-cell function as measured by the HOMA-B. However, there was no significant improvement in insulin resistance with the use of DPP-4 inhibitors as monotherapy or as add-on therapy in combination with other drugs. Consistent with the results of previous meta-analyses73,74, these findings provide important insight into the pathophysiological mechanisms of DPP-4 inhibitor action in the treatment of T2DM.
Our results regarding the effects of DPP-4 inhibitor treatment on beta-cell function were largely consistent with results from the largest trials using DPP-4 inhibitors as monotherapy75, adds-on therapy66, or mixed trial design involving multiple trials62. In the trial by Pratley et al.75, vildagliptin monotherapy yielded consistently robust improvement in beta-cell function, measured not only by HOMA-B based on fasting glucose and insulin but also by meal test-derived measures, across a broad spectrum of drug-naive patients with T2DM. In addition, our findings were consistent with those of other studies that measured dynamic beta-cell function after meal ingestion. For instance, in a meal tolerance test performed with a double tracer technique (3-(3)H-glucose iv and 1-(14)C-glucose orally), the DPP-4 inhibitor vildagliptin significantly increased the insulin secretion rate divided by plasma glucose by 29% in T2DM patients76. When added to metformin therapy in patients with T2DM, vildagliptin treatment resulted in a significant increase in insulin secretion (postmeal suprabasal area under the 0- to 30-min C-peptide curve divided by the 30-min increase in glucose)77.
The favorable effects of DPP-4 inhibitors for improving beta-cell function among patients with T2DM are biologically plausible78. DPP-4 inhibitor treatment in diabetic animal models stimulated beta-cell survival, facilitated islet neogenesis, enhanced insulin biosynthesis79, and preserved beta-cell mass and function13. Moreover, DPP-4 gene knockout mice showed better insulin sensitivity and less pancreatic islet hypertrophy and were resistant to streptozotocin-induced loss of beta-cell mass and hyperglycemia80. In humans with T2DM, DPP-4 inhibitors have demonstrated improvement of beta-cell function both in the fasting and postprandial statuses, and these beneficial effects were sustained in studies with a duration up to 2 years78. DPP-4 inhibitors may restore beta-cell function and survival in isolated human islets through glucagon-like peptide (GLP)-1 stabilization81. In addition, DPP-4 inhibitors also exert an anti-inflammatory effect82,83, which may alleviate the loss of beta-cell function84. Recently, it is reported that DPP-4 inhibitors may protect pancreatic beta-cells by elongating the telomere length85. However, there is a lack of evidence in humans to demonstrate the durable effects of DPP-4 inhibitors on beta-cell function after cessation of therapy in patients with T2DM, which warrants further investigation in long-term, well-designed trials with a sufficiently long washout period after discontinuation of the study drug78.
In general, DPP-4 inhibitors showed no effects on insulin resistance. While the underlying mechanisms remain to be elucidated, this finding may be at least partly due to the neutral effects of DPP-4 inhibitors on body weight8. Interestingly, our meta-analysis showed that sitagliptin, but not the other tested DPP-4 inhibitors, as monotherapy resulted in significant improvement in insulin resistance. This finding needs to be confirmed in future studies.
The study has strengths in that it was conducted using a systematic approach and the results are based on a large number of published RCTs. However, there are several limitations. First, most of the included studies had a follow-up duration of less than 6 months. The longer-term effects of DPP-4 inhibitor treatment on beta-cell function and insulin resistance in patients with T2DM warrant further investigation. Second, our study had a lack of power to compare the effectiveness of DPP-4 inhibitors in this study, because the number of available trials for some individual DPP-4 inhibitors was still limited. DPP-4 inhibitors have similar but still varying pharmacological features86,87, and thus, their effects on beta-cell function and insulin resistance may vary. Third, we used the HOMA-B as the indicator of beta-cell function. Because this parameter is originally estimated based on fasting glucose and insulin levels, it may not sufficiently represent postprandial beta-cell function. Fourth, publication bias may exist regarding the effects of DPP-4 inhibitors on beta-cell function.
In conclusion, among patients with T2DM, DPP-4 inhibitors as monotherapy or as add-on therapy in combination with other drugs significantly improved beta-cell function but had no significant effect on insulin resistance.
Additional Information
How to cite this article: Lyu, X. et al. Effects of dipeptidyl peptidase-4 inhibitors on beta-cell function and insulin resistance in type 2 diabetes: meta-analysis of randomized controlled trials. Sci. Rep. 7, 44865; doi: 10.1038/srep44865 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kahn, S. E. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86, 4047–58 (2001).
Fonseca, V. A. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 32 Suppl 2, S151–6 (2009).
Halban, P. A. et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37, 1751–8 (2014).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–9 (1985).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–95 (2004).
Tabak, A. G. et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 373, 2215–21 (2009).
Cnop, M. et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 30, 677–82 (2007).
Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract 60, 1454–70 (2006).
Ahren, B. et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89, 2078–84 (2004).
Dicker, D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 34 Suppl 2, S276–8 (2011).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–9 (2015).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–79 (2012).
Mu, J. et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55, 1695–704 (2006).
Mu, J. et al. Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol 623, 148–54 (2009).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Goldstein, B. J. et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 30, 1979–87 (2007).
Williams-Herman, D. et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 25, 569–83 (2009).
Williams-Herman, D. et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab 12, 442–51 (2010).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986).
Riley, R. D. et al. Meta-analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Stat Med 32, 2747–66 (2013).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003).
Ristic, S., Byiers, S., Foley, J. & Holmes, D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab 7, 692–8 (2005).
Aschner, P. et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 29, 2632–7 (2006).
Charbonnel, B., Karasik, A., Liu, J., Wu, M. & Meininger, G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 29, 2638–43 (2006).
Pratley, R. E., Jauffret-Kamel, S., Galbreath, E. & Holmes, D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res 38, 423–8 (2006).
Raz, I. et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 49, 2564–71 (2006).
Rosenstock, J., Brazg, R., Andryuk, P. J., Lu, K. & Stein, P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 28, 1556–68 (2006).
Hanefeld, M. et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 23, 1329–39 (2007).
Hermansen, K. et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 9, 733–45 (2007).
Scott, R., Wu, M., Sanchez, M. & Stein, P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 61, 171–80 (2007).
DeFronzo, R. A., Fleck, P. R., Wilson, C. A., Mekki, Q. & Alogliptin Study, G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 31, 2315–7 (2008).
Nonaka, K. et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 79, 291–8 (2008).
Pratley, R. E. et al. Robust improvements in fasting and prandial measures of beta-cell function with vildagliptin in drug-naive patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab 10, 931–8 (2008).
Raz, I. et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 24, 537–50 (2008).
Rosenstock, J., Sankoh, S. & List, J. F. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 10, 376–86 (2008).
Scott, R., Loeys, T., Davies, M. J., Engel, S. S. & Sitagliptin Study, G. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 10, 959–69 (2008).
Chacra, A. R. et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 63, 1395–406 (2009).
DeFronzo, R. A. et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 32, 1649–55 (2009).
Hollander, P., Li, J., Allen, E. & Chen, R. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 94, 4810–9 (2009).
Mohan, V. et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract 83, 106–16 (2009).
Rosenstock, J. et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin 25, 2401–11 (2009).
Forst, T. et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med 27, 1409–19 (2010).
Rhee, E. J. et al. A multicenter, randomized, placebo-controlled, double-blind phase II trial evaluating the optimal dose, efficacy and safety of LC 15-0444 in patients with type 2 diabetes. Diabetes Obes Metab 12, 1113–9 (2010).
Bosi, E., Ellis, G. C., Wilson, C. A. & Fleck, P. R. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab 13, 1088–96 (2011).
Del Prato, S. et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 13, 258–67 (2011).
Gomis, R., Espadero, R. M., Jones, R., Woerle, H. J. & Dugi, K. A. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 13, 653–61 (2011).
Kaku, K., Itayasu, T., Hiroi, S., Hirayama, M. & Seino, Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab 13, 1028–35 (2011).
Owens, D. R., Swallow, R., Dugi, K. A. & Woerle, H. J. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med 28, 1352–61 (2011).
Reasner, C. et al. The effect of initial therapy with the fixed-dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes Metab 13, 644–52 (2011).
Seino, Y., Fujita, T., Hiroi, S., Hirayama, M. & Kaku, K. Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin 27 Suppl 3, 21–9 (2011).
Seino, Y., Fujita, T., Hiroi, S., Hirayama, M. & Kaku, K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr Med Res Opin 27, 1781–92 (2011).
Taskinen, M. R. et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 13, 65–74 (2011).
Yoon, K. H. et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone on glycemic control and measures of beta-cell function in patients with type 2 diabetes. Int J Clin Pract 65, 154–64 (2011).
Kawamori, R. et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab 14, 348–57 (2012).
Kutoh, E. & Ukai, Y. Alogliptin as an initial therapy in patients with newly diagnosed, drug naive type 2 diabetes: a randomized, control trial. Endocrine 41, 435–41 (2012).
Pan, C. Y., Yang, W., Tou, C., Gause-Nilsson, I. & Zhao, J. Efficacy and safety of saxagliptin in drug-naive Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev 28, 268–75 (2012).
Seino, Y., Miyata, Y., Hiroi, S., Hirayama, M. & Kaku, K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab 14, 927–36 (2012).
Dobs, A. S. et al. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. J Diabetes 5, 68–79 (2013).
Kadowaki, T. & Kondo, K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 15, 810–8 (2013).
Zeng, Z. et al. Efficacy and safety of linagliptin added to metformin and sulphonylurea in Chinese patients with type 2 diabetes: a sub-analysis of data from a randomised clinical trial. Curr Med Res Opin 29, 921–9 (2013).
Heise, T. et al. The dipeptidyl peptidase-4 inhibitor linagliptin lowers postprandial glucose and improves measures of beta-cell function in type 2 diabetes. Diabetes Obes Metab 16, 1036–9 (2014).
Yokoyama, H. et al. Liraglutide Versus Sitagliptin in a 24-week, Multicenter, Open-label, Randomized, Parallel-group Study in Japanese Type 2 Diabetes Mellitus Patients Responding Inadequately to a Sulfonylurea and/or One or Two Other Oral Antidiabetic Drugs (JDDM 33). Jpn Clin Med 5, 33–41 (2014).
Fukui, K. et al. Dipeptidyl peptidase-4 inhibitor sitagliptin improves pancreatic beta-cell function in hypertensive diabetic patients treated with angiotensin receptor blockers. J Renin Angiotensin Aldosterone Syst 16, 1001–9 (2015).
Jung, C. H. et al. A randomized, double-blind, placebo-controlled, phase II clinical trial to investigate the efficacy and safety of oral DA-1229 in patients with type 2 diabetes mellitus who have inadequate glycaemic control with diet and exercise. Diabetes Metab Res Rev 31, 295–306 (2015).
Leibowitz, G. et al. Impact of treatment with saxagliptin on glycaemic stability and beta-cell function in the SAVOR-TIMI 53 study. Diabetes Obes Metab 17, 487–94 (2015).
Strozik, A., Steposz, A., Basiak, M., Drozdz, M. & Okopien, B. Multifactorial effects of vildagliptin added to ongoing metformin therapy in patients with type 2 diabetes mellitus. Pharmacol Rep 67, 24–31 (2015).
Yokoh, H. et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1): A multicenter, randomized, open-label, non-inferiority trial. J Diabetes Investig 6, 182–91 (2015).
Zografou, I. et al. Effect of vildagliptin on hsCRP and arterial stiffness in patients with type 2 diabetes mellitus. Hormones (Athens) 14, 118–25 (2015).
Ba, J. et al. Randomized trial assessing the safety and efficacy of sitagliptin in Chinese patients with type 2 diabetes mellitus inadequately controlled on sulfonylurea alone or combined with metformin. J Diabetes(Aug 8, 2016), doi: 10.1111/1753-0407.12456.
Ekholm, E. et al. Combined treatment with saxagliptin plus dapagliflozin reduces insulin levels by increased insulin clearance and improves beta-cell function. Endocr Pract(Nov 16, 2016).
Oyama, J. et al. The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial. PLoS Med 13, e1002051 (2016).
Riche, D. M., East, H. E. & Riche, K. D. Impact of sitagliptin on markers of beta-cell function: a meta-analysis. Am J Med Sci 337, 321–8 (2009).
Lu, J., Zang, J. & Li, H. Impact of three oral antidiabetic drugs on markers of beta-cell function in patients with type 2 diabetes: a meta-analysis. PLoS One 8, e76713 (2013).
Pratley, R. E. et al. Robust improvements in fasting and prandial measures of (beta)-cell function with vildagliptin in drug-naive patients: Analysis of pooled vildagliptin monotherapy database. Diabetes, Obesity and Metabolism 10, 931–8 (2008).
Balas, B. et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 92, 1249–55 (2007).
Ahren, B., Pacini, G., Foley, J. E. & Schweizer, A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care 28, 1936–40 (2005).
van Genugten, R. E., van Raalte, D. H. & Diamant, M. Dipeptidyl peptidase-4 inhibitors and preservation of pancreatic islet-cell function: a critical appraisal of the evidence. Diabetes Obes Metab 14, 101–11 (2012).
Pospisilik, J. A. et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 52, 741–50 (2003).
Conarello, S. L. et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100, 6825–30 (2003).
Shah, P. et al. The DPP-4 inhibitor linagliptin restores beta-cell function and survival in human isolated islets through GLP-1 stabilization. J Clin Endocrinol Metab 98, E1163–72 (2013).
Makdissi, A. et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab 97, 3333–41 (2012).
Omar, B. A. et al. Enhanced beta cell function and anti-inflammatory effect after chronic treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin in an advanced-aged diet-induced obesity mouse model. Diabetologia 56, 1752–60 (2013).
Donath, M. Y. et al. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31 Suppl 2, S161–4 (2008).
Ma, D., Yu, Y., Yu, X., Zhang, M. & Yang, Y. The changes of leukocyte telomere length and telomerase activity after sitagliptin intervention in newly diagnosed type 2 diabetes. Diabetes Metab Res Rev 31, 256–61 (2015).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab 13, 7–18 (2011).
Baetta, R. & Corsini, A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs 71, 1441–67 (2011).
Acknowledgements
The study was supported by Merck & Co., Inc.
Author information
Authors and Affiliations
Contributions
L.X.F. and R.X.W. designed the study, conducted all searches, appraised all potential studies and wrote and revised the draft manuscript and subsequent manuscripts. Z.X.L. and Z.B. revised the draft manuscript and subsequent manuscripts. D.L. and C.D.W. assisted with the presentation of findings and assisted with drafting and revising the manuscript. W.C. and L.G.J. conceived and designed the study, assisted with searches, appraised relevant studies and assisted with drafting and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lyu, X., Zhu, X., Zhao, B. et al. Effects of dipeptidyl peptidase-4 inhibitors on beta-cell function and insulin resistance in type 2 diabetes: meta-analysis of randomized controlled trials. Sci Rep 7, 44865 (2017). https://doi.org/10.1038/srep44865
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44865
This article is cited by
-
Systematic review and meta-analysis of teneligliptin for treatment of type 2 diabetes
Journal of Endocrinological Investigation (2023)
-
Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity
Pulmonary Therapy (2023)
-
Reporting and methodological quality of systematic reviews of DPP-4 inhibitors for patients with type 2 diabetes mellitus: an evidence-based mapping
Acta Diabetologica (2022)
-
Differential Effects of Thiazolidinediones and Dipeptidyl Peptidase-4 Inhibitors on Insulin Resistance and β-Cell Function in Type 2 Diabetes Mellitus: A Propensity Score-Matched Analysis
Diabetes Therapy (2019)
-
Effect of Linagliptin and Voglibose on metabolic profile in patients with Type 2 Diabetes: a randomized, double-blind, placebo-controlled trial
BMC Pharmacology and Toxicology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.