Abstract
Sprint interval training has been reported to induce similar or greater mitochondrial adaptations to continuous training. However, there is limited knowledge about the effects of different exercise types on the early molecular events regulating mitochondrial biogenesis. Therefore, we compared the effects of continuous and sprint interval exercise on key regulatory proteins linked to mitochondrial biogenesis in subcellular fractions of human skeletal muscle. Nineteen men, performed either 24 min of moderate-intensity continuous cycling at 63% of WPeak (CE), or 4 × 30-s “all-out” cycling sprints (SIE). Muscle samples (vastus lateralis) were collected pre-, immediately (+0 h) and 3 (+3 h) hours post-exercise. Nuclear p53 and PHF20 protein content increased at +0 h, with no difference between groups. Nuclear p53 phosphorylation and PGC-1α protein content increased at +0 h after SIE, but not CE. We demonstrate an exercise-induced increase in nuclear p53 protein content, an event that may relate to greater p53 stability - as also suggested by increased PHF20 protein content. Increased nuclear p53 phosphorylation and PGC-1α protein content immediately following SIE but not CE suggests these may represent important early molecular events in the exercise-induced response to exercise, and that SIE is a time-efficient and possibly superior option than CE to promote these adaptations.
Similar content being viewed by others
Introduction
Exercise training induces mitochondrial biogenesis1 and leads to an improved capacity for substrate oxidation, greater mitochondrial content, and/or increased mitochondrial respiration2,3,4,5,6. Exercise is therefore a valuable tool to help prevent and treat a host of chronic diseases linked with reduced and/or compromised mitochondrial function7. Current recommendations are that adults engage in moderate-intensity continuous exercise (CE) for ~30 min/d on 5 d/wk, or in vigorous-intensity exercise for ~20 min/d on 3 d/wk, or a combination of both8. Considering that lack of time is often cited as the primary reason for physical inactivity9, time efficient forms of exercise could improve exercise adherence and help prevent chronic diseases linked with a sedentary lifestyle3,10. One such type of exercise is low-volume sprint interval exercise (SIE), which consists of short “all-out” efforts interspersed with passive recovery3. Although sprint interval training has been shown to induce similar and sometimes greater mitochondrial and metabolic adaptations compared to continuous training11,12, there is limited information about the effects of SIE on the early molecular events regulating mitochondrial biogenesis, especially in comparison to longer duration CE.
A key mediator of exercise-induced mitochondrial biogenesis is peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which modulates gene transcription in the cellular nucleus13. In rat skeletal muscle, the increase in whole-muscle PGC-1α protein content following 3 hours of recovery is preceded by an increase in nuclear PGC-1α protein content immediately after the termination of exercise14. The authors suggested this increase in nuclear PGC-1α protein content may constitute the initial phase of the exercise-induced adaptive response. There is controversy however, regarding the timing and magnitude of the increase in nuclear PGC-1α protein content in human skeletal muscle following different types of exercise. While a single bout of SIE has been shown to induce an increase in nuclear PGC-1α protein content following 3 hours of recovery15, the effects of CE are controversial. One study has reported an increase in nuclear PGC-1α protein content immediately post-exercise16, whereas other studies observed no change either immediately post-exercise17 or after 3 hours of recovery18. These discrepancies may relate to the small sample size of the three studies employing CE (n = 4 to 6)16,17,18, and the fact that participants’ fitness levels and previous physical activity history were also different between studies. Moreover, all three of these studies investigated only a single time point, hence providing no indication of the timing of this change. Therefore, to better understand the influence of CE and SIE on the timing and magnitude of the change in nuclear PGC-1α protein content it is important to directly compare participants of similar fitness levels within the same study.
p53 is also a key transcription factor mediating exercise-induced mitochondrial biogenesis19, as demonstrated by its transcriptional control of mitochondrial respiration20 and mitochondrial remodelling19,21,22, as well as by the reduced mitochondrial content and exercise capacity in p53 knockout mice20,23. However, little is known about the effects of exercise on changes in p53 protein content in the nucleus, where p53 exerts its transcriptional and biochemical activity24. An increase in nuclear p53 protein content has been observed 3 hours after a 60-min bout of CE in human skeletal muscle18, whereas both an increase25 and a decrease26 have been reported following CE in rodent skeletal muscle; no study has investigated the effects of SIE. In addition to changes in content, phosphorylation of p53 at serine 15 (p-p53Ser15) is an important post-translational modification that enhances p53 activity and stability27. A single bout of CE increased p-p53Ser15 in human skeletal whole-muscle lysates28, whereas the effects of SIE remain unknown. Given that induction of the p53 response to physiological stressors, such as exercise, occurs largely through p53 protein stabilisation and nuclear accumulation19,24, it is important to investigate the exercise-induced changes in nuclear p-p53Ser15 in human skeletal muscle, and to determine whether these changes (and those in nuclear p53 protein content) may be differentially regulated by different types of exercise.
An important regulator of p53 is plant homeodomain finger-containing protein 20 (PHF20), which induces both p53 protein stability29 and p53 transcription30 in the nucleus. Research in human skeletal muscle has observed increased protein content of PHF20 (as well as p53 and PGC-1α) in whole muscle lysates following four weeks of “all-out” sprint training, but not moderate-intensity continuous training, and suggested exercise intensity may be associated with greater upregulation of both PGC-1α- and p53-mediated mitochondrial biogenesis12. Despite these findings, no research has investigated the impact of a single bout of exercise on nuclear PHF20 protein content in human skeletal muscle, and determined if the potential effects are differentially modulated by different types of exercise.
The purpose of this study was to compare the effects of a single bout of CE and SIE on key regulatory proteins associated with mitochondrial biogenesis in nuclear- and cytosolic-enriched fractions of human skeletal muscle. It was hypothesised that, similar to PGC-1α, exercise would increase the nuclear protein content of p53 and PHF20, and Ser-15 phosphorylation of p53 in the nucleus. It was also hypothesised that these increases would be greater following SIE, despite a shorter time commitment and a much lower total work.
Results
Total work, performance parameters and blood lactate concentration ([La−]) during the biopsy trial
Total work during the biopsy trial was higher for CE compared with SIE (3.6-fold, P < 0.001; Table 1). In contrast, 1-s maximum and mean exercise intensity (expressed in Watts) were higher for SIE compared with CE (4.7- and 3.3-fold, respectively, all P < 0.001; Table 1). The exercise-induced increase in blood [La−] for the two types of exercise (both P < 0.001) was also greater in SIE compared with CE (2.9-fold, P < 0.001; Table 1).
Muscle analyses
Representative immunoblots, cellular fractions enrichment, and antibody specificity
Representative blots for the study are presented in Fig. 1a. The specificity of the p53 antibody was assessed by blotting samples beside a commercially-available, untagged, full-length p53 recombinant human protein (Aviva Systems Biology, AEP00002) (Fig. 1b). The same full-length p53 recombinant human protein, which was expressed in E. coli and was not phosphorylated, was also used as a negative control against the chosen p-p53Ser15 antibody. Results from Fig. 1b show that the p-p53Ser15 antibody did not recognise this protein, suggesting it is phospho-specific. The enrichment and purity of both nuclear and cytosolic fractions was confirmed by blotting the separated fractions against the nuclear protein histone H3, and the cytosolic protein lactate dehydrogenase A (LDHA). Histone H3 was only detected in nuclear fractions, whereas LDHA was only detected in cytosolic fraction (Fig. 1c), confirming the cellular fractionation protocol was successful. Images showing whole-lane Coomassie staining for both nuclear and cytosolic fractions, and histone H3 (nuclear) and GAPDH (cytosolic) immunoblots, were used to verify equal loading between lanes, and representative images are presented in Fig. 1d and e respectively.
(a) Representative immunoblots corresponding to total and phosphorylated protein content measured in the nuclear and cytosolic fractions, before (Pre), immediately (+0 h) and 3 h (+3 h) after the CE and SIE trials. p-p38 MAPKThr180/Tyr182: bottom band at ~38 kDa; PHF20: top band at ~105 kDa. No band was detected in the nuclear fractions for p-ACCSer79. (b) Confirmation of the p53 antibody specificity; samples were run beside an untagged full length p53 recombinant human protein and blotted against both total-p53 (positive control) and p-p53Ser15 (negative control) antibodies. S: sample; RP: p53 recombinant protein. (c) Histone H3 and LDHA were used as indicators of cytosolic and nuclear enrichment, respectively. N: nuclear fractions; C: cytosolic fractions. (d) Whole-lane Coomassie blue staining for both nuclear and cytosolic fractions, and (e) histone H3 (nuclear) and GAPDH (cytosolic), were used to verify equal loading between lanes. The immunoblot and whole-lane Coomassie images in this figure were cropped to improve the conciseness and clarity of the presentation.
p53 protein content
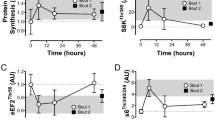
There were main effects of time (nuclear: P = 0.002; cytosolic: P = 0.012), with the content of p53 protein significantly increased in both the nuclear (2.0-fold; P = 0.001) and cytosolic (1.8-fold; P = 0.014) fraction at +0 h only. There was no interaction effect (nuclear: P = 0.283; cytosolic: P = 0.393), indicating no significant difference in p53 protein content between the two types of exercise in either the nuclear (1.5-fold; ES: 1.61; −0.76, 3.97) or cytosolic (1.4-fold; ES: 0.57; −0.35, 1.48) fraction at +0 h. There were no significant differences versus baseline or between the two types of exercise at +3 h in either fraction. (Fig. 2a and b).
Protein content of nuclear (a) and cytosolic (b) p53, of nuclear (c) and cytosolic (d) PHF20, and of nuclear (e) and cytosolic (f) p-p53Ser15 before (Pre), immediately (+0 h) and 3 h (+3 h) after the CE and SIE trials. Open circles (CE) and closed triangles (SIE) represent individual values. Primary effects were analysed using two-way ANOVA with repeated measures for time followed by Tukey’s honestly significant difference post-hoc test for pairwise comparisons. *P < 0.05 vs. Pre; ‡P < 0.05 vs. CE at the same time point. Individual data points and mean bars are plotted. n = 9 for each type of exercise.
PHF20 protein content
There were main effects of time (nuclear: P = 0.006; cytosolic: P < 0.001), with the content of PHF20 protein significantly increased in both the nuclear (1.9-fold; P = 0.004) and cytosolic (1.7-fold; P < 0.001) fraction at +0 h only. There was no interaction effect (nuclear: P = 0.864; cytosolic: P = 0.399), indicating no significant difference in PHF20 protein content between the two types of exercise in either the nuclear (1.3-fold; ES: 0.22; −0.83, 1.26) or cytosolic (1.1-fold; ES: 0.49; −0.37, 1.34) fraction at +0 h. There were no significant differences versus baseline or between the two types of exercise at +3 h in either fraction. (Fig. 2c and d).
p-p53Ser15 protein content
There was an interaction effect (P = 0.003), with nuclear p-p53Ser15 content increased both at +0 h (3.1-fold; P < 0.001), and at +3 h (2.1-fold; P = 0.007), following SIE but not CE (1.5-fold, P = 0.785 at +0 h, and 1.2-fold, P = 0.873 at +3 h). p-p53Ser15 content was significantly greater in SIE compared with CE at both time points (2.1-fold; P < 0.001; ES: 2.40; 0.88, 3.43 at +0 h, and 1.8-fold; P = 0.044; ES: 1.25; −0.06, 2.55 at +3 h). There was a main effect of time in the cytosolic fraction (P < 0.001), with p-p53Ser15 content significantly increased at both time points (1.8-fold; P < 0.001 at +0 h, and 1.6-fold; P = 0.008 at +3 h). There was no interaction effect (P = 0.068), indicating no significant difference in p-p53Ser15 content between the two types of exercise at both time points (1.5-fold; ES: 1.38; −0.05, 2.81 at +0 h, and 1.7-fold; ES: 1.56; −0.25, 3.37 at +3 h).(Fig. 2e and f).
Phosphorylation of Acetyl-CoA Carboxylase (ACC) at serine 79 (p-ACCSer79) protein content
p-ACCSer79 was not detected in nuclear fractions (Fig. 1a). There was a main effect of time in the cytosolic fraction (P < 0.001), with p-ACCSer79 content significantly increased (1.7-fold; P = 0.012) at +0 h only. There was no interaction effect (P = 0.092), indicating no significant difference in p-ACCSer79 content between the two types of exercise at +0 h (1.2-fold; ES: 0.92; −0.21, 2.05). There were no significant differences versus baseline or between the two types of exercise at +3 h (Fig. 3a).
Protein content of cytosolic p-ACCSer79 (a), and of nuclear (b) and cytosolic (c) p-p38 MAPKThr180/Tyr182 before (Pre), immediately (+0 h) and 3 h (+3 h) after the CE and SIE trials. Open circles (CE) and closed triangles (SIE) represent individual values. Primary effects were analysed using two-way ANOVA with repeated measures for time followed by Tukey’s honestly significant difference post-hoc test for pairwise comparisons. *P < 0.05 vs. Pre; ‡P < 0.05 vs. CE at the same time point. Individual data points and mean bars are plotted. n = 9 for each type of exercise.
Phosphorylation of p38 mitogen-activated protein kinase (MAPK) at threonine 180 and tyrosine 182 (p-p38 MAPKThr180/Tyr182) protein content
There was an interaction effect (P = 0.035) in the nuclear fraction, with p-p38 MAPKThr180/Tyr182 content increased 3.7-fold at +0 h following SIE (P = 0.004), but not CE (1.9-fold; P = 0.997). p-p38 MAPKThr180/Tyr182 content was 2.0-fold greater in SIE compared with CE at this time point (P = 0.003; ES: 2.88; −0.17, 5.92). There was also an interaction effect (P = 0.003) in the cytosolic fraction, with p-p38 MAPKThr180/Tyr182 content increased 3.9-fold at +0 h following SIE (P < 0.001), but not CE (1.9-fold; P = 0.966). p-p38 MAPKThr180/Tyr182 content was 2.0-fold greater in SIE compared with CE at this time point (P < 0.001; ES: 2.34; 0.39, 4.29). There were no significant differences versus baseline or between the two types of exercise at +3 h in either fraction. (Fig. 3b and c).
Gene expression
There was no change in p53 mRNA content throughout (main effect of time: P = 0.883; Fig. 4a). There was a main effect of time for PGC-1α mRNA content (P < 0.001; Fig. 4b), with a significant increase (5.6-fold, P < 0.001) at +3 h only. There was no interaction effect (P = 0.910), indicating no significant difference in PGC-1α mRNA content between the two types of exercise at +3 h (1.1-fold; ES: 0.147; −0.85, 1.13); there were no significant differences versus baseline or between the two types of exercise at +0 h (all P > 0.05). Finally, results relative to the mRNA content of apoptosis-inducing factor (AIF), dynamin-related protein 1 (DRP1), mitofusin 2 (MFN2), p21, superoxide dismutase 2 (SOD2), and cytochrome c are presented in Table 2.
p53 (a), and PGC-1α (b) mRNA content before (Pre), immediately (+0 h), and 3 h (+3 h) after the CE and SIE trials, expressed relative to control (cyclophilin, glyceraldehyde 3-phosphate dehydrogenase [GAPDH], and beta-2-microglobulin [B2M] housekeeping genes). Open circles (CE) and closed triangles (SIE) represent individual values. Primary effects were analysed using two-way ANOVA with repeated measures for time followed by Tukey’s honestly significant difference post-hoc test for pairwise comparisons. *P < 0.05 vs. Pre. Individual data points and mean bars are plotted. n = 9 for each type of exercise.
PGC-1α protein content
There was an interaction effect (P = 0.046), with nuclear PGC-1α protein content increased both at +0 h (2.3-fold; P < 0.001), and at +3 h (1.7-fold; P = 0.037), following SIE but not CE (1.4-fold; P = 0.219 at +0 h, and 1.0-fold; P = 1.000 at +3 h). PGC-1α protein content was significantly greater in SIE compared with CE at +0 h (1.7-fold; P = 0.043; ES: 1.61; 0.00, 3.22), but not at +3 h (1.6-fold; P = 0.130; ES: 1.17; 0.13, 2.21). There was an interaction effect (P = 0.036), with cytosolic PGC-1α protein content increased 1.9-fold at +3 h following SIE (P = 0.015), but not CE (1.1-fold; P = 0.702). PGC-1α protein content was significantly greater in SIE compared with CE at this time point (1.7-fold; P = 0.008; ES: 1.69; 0.21, 3.17). There were no significant differences versus baseline or between the two types of exercise at +0 h. (Fig. 5a and b).
Nuclear (a) and cytosolic (b) PGC-1α protein content before (Pre), immediately (+0 h) and 3 h (+3 h) after the CE and SIE trials. Open circles (CE) and closed triangles (SIE) represent individual values. Primary effects were analysed using two-way ANOVA with repeated measures for time followed by Tukey’s honestly significant difference post-hoc test for pairwise comparisons. *P < 0.05 vs. Pre; ‡P < 0.05 vs. CE at the same time point. Individual data points and mean bars are plotted. n = 9 for each type of exercise.
Discussion
This study reports, for the first time, that only SIE, and not CE, was associated with a post-exercise increase in p-p53Ser15 in the nuclear fraction of human skeletal muscle. Similarly, only SIE, and not CE, increased nuclear PGC-1α protein content post-exercise. Although the differential response to SIE and CE may be due to differences in the degree and time course of nuclear p53 and PGC-1α protein accumulation, our findings suggest that SIE may represent a more potent stimulus than CE to induce some of the early molecular events associated with mitochondrial biogenesis, as previously suggested31. An additional novel finding was that the exercise-induced increase in the nuclear content of p53 protein in human skeletal muscle was also accompanied by an increase in the nuclear content of PHF20, a protein that stabilises and activates p5329, but was not differentially regulated by the two types of exercise investigated.
Nuclear (and cytosolic) p53 protein content was increased immediately post-exercise, with no difference between the two types of exercise. Previous research in human skeletal muscle also reported an increase in nuclear p53 protein content after a 60-min bout of CE18; however, this was only reported after 3 hours of recovery (the only time point investigated in this previous study). Our results also agree with reports of increased nuclear p53 protein content immediately after a 60-min bout of continuous running in mouse skeletal muscle25, and 1 hour after a 20-min bout of intermittent eccentric skeletal muscle contractions in rats32. A decrease in nuclear p53 protein content has also been observed in one study after a 90-min bout of exhaustive exercise in mouse skeletal muscle26. This contrasting result is difficult to explain, but may relate to the type, age, and sex of the species investigated, and the different types of muscle analysed. We add the new finding that SIE also induced nuclear p53 protein accumulation, and that this increase was similar to that observed for CE. Our results are consistent with the well-accepted notion that cellular stress is associated with accumulation of p53 protein in the nucleus, a process that is mainly mediated by post-translational events such as nuclear/cytosolic shuttling, and/or increased p53 protein stability24,33.
p53 nuclear/cytosolic shuttling, a process requiring a tightly-regulated series of events33, cannot simply be assessed by subcellular fractionation and immunoblotting. We were therefore unable to determine the contribution of subcellular shuttling to the increase in nuclear p53 protein content observed with both types of exercise. However, some indirect measurements in the present study lend support to an exercise-induced increase in p53 stability. For example, and for the first time, we report a post-exercise increase in the nuclear content of PHF20, a multidomain protein that can bind to p53 and increase its stability29. In unstressed cells, nuclear p53 is bound to murine double minute-2 (MDM2), a p53 negative regulator inducing p53 ubiquitination and nuclear export, and subsequent cytosolic degradation by the proteasome24,34. Upon cellular stress PHF20 binds to p53, enhancing its stability by diminishing MDM2-mediated p53 degradation29. Although we were unable to directly measure the PHF20-p53 interaction, the concomitant increase in these two proteins may indicate an increased PHF20-p53 interaction induced by the cellular stress of exercise, and a subsequent increase in p53 stability29. Future research is required to confirm this hypothesis, and to better understand the molecular events regulating p53 stability, degradation, and nuclear/cytosolic shuttling following exercise.
An additional factor affecting p53 stability is phosphorylation at serine 15, which reduces the p53 interaction with its negative regulator MDM227. In the present study, nuclear p-p53Ser15 was increased immediately post-exercise only following SIE, an increase that persisted until 3 h of recovery. Cytosolic p-p53Ser15 was also increased at both time points; however, the difference between the two types of exercise did not reach significance. While exercise duration and total work were greater during CE (1.7- and 3.6-fold, respectively), maximal and mean exercise intensity were 4.7-and 3.3- fold greater during SIE (Table 1). This suggests that exercise intensity, rather than exercise duration or total work, may be an important factor affecting exercise-induced changes in nuclear p-p53Ser15. The only two previous studies investigating p-p53Ser15 following endurance exercise with humans reported whole-muscle p-p53Ser15 to increase only after 3 h of recovery, regardless of exercise type (continuous or intermittent exercise at the same average intensity)28 or pre-exercise carbohydrate availability35. The earlier increase in nuclear p-p53Ser15 in our study (+0 h), compared with that in whole-muscle p-p53Ser15 in the two studies above (+3 h), raises the possibility that, similar to PGC-1α14, an increase in nuclear p-p53Ser15 may contribute to the initial phase of the exercise-induced adaptive response.
While multiple mechanisms probably contribute, the signalling kinases AMP-activated protein kinase (AMPK)36 and p38 MAPK37 have been reported to phosphorylate p53 at serine 15. In the present study, cytosolic phosphorylation of ACC, a downstream target and commonly used marker of AMPK activation38,39, was increased similarly between the two types of exercise immediately post-exercise. No ACC was detected in nuclear fractions, consistent with previous research16. Conversely, only SIE induced a significant increase in the phosphorylation of p38 MAPK in the nucleus, immediately post-exercise, consistent with the increase in nuclear p-p53Ser15 only following SIE. This may suggest that signalling through p38 MAPK may be more closely associated with the exercise-induced modulation of p-p53Ser15.
PHF20 can also act as a transcription factor, and has been shown to activate p53 gene expression30. Nonetheless, despite an increase in nuclear PHF20 protein content following both types of exercise, there was no significant change in p53 mRNA content within 3 h from the end of the bout. Studies in cells indicate that upregulation of p53 mRNA by PHF20 takes place only after 6 or 12 h30, suggesting that the 3 h post-exercise time point chosen in the present study might have been too early to detect a significant increase in p53 mRNA. Consistent with this, a small increase (~1.3-fold) has been reported following 3 h of recovery in human skeletal muscle with no difference between the three exercise intensities investigated40. Conversely, greater increases (2- to 2.5-fold) have been observed 4.5 and 7.5 h after the termination of the first running session of a twice a day exercise model41. The reasons for these divergent results may relate to the fact that participants engaged in eccentric exercise and that were fed before the post-exercise biopsies. Future research is required to clarify the controversial literature on this topic.
p53 is an inducible transcription factor that directly regulates the transcription of, amongst others, PGC-1α, DRP1, MFN2, AIF, p21 and SOD2 - a series of genes implicated in mitochondrial biogenesis19, mitochondrial remodelling21,22, cell survival42,43 and oxidative stress44. We report an exercise-induced increase in the mRNA content of these genes, although no significant differences between the two types of exercise were found. This is somewhat surprising considering the larger increase in nuclear p-p53Ser15 (and PGC-1α) after SIE compared with CE. Of note however, despite not reaching significance due to the large individual variability, the fold-change in p21 mRNA was almost twice as high following SIE compared to CE.
We observed an increase in nuclear PGC-1α protein content immediately after the termination of SIE (2.3-fold) and after 3 hours of recovery (1.7-fold). The increase we report after 3 h of recovery is similar to that observed in previous research in humans following an identical bout of SIE (1.7-fold)15; however, in the present study this increase was also observed immediately post-exercise. In contrast to SIE, CE (24 min at 63% of WPeak) did not provide a sufficient stimulus to induce a significant increase in nuclear PGC-1α protein content immediately post-exercise (1.4-fold), or after 3 hours of recovery (1.0-fold). Although these results may partially depend on the relatively short duration of the CE trial, they are consistent with the non-significant change (1.1-fold) in nuclear PGC-1α protein content reported immediately after 60 min of CE at 74% of  O2Peak17, the 1.5-fold increase observed immediately after a 90-min bout of CE at 65% of
O2Peak17, the 1.5-fold increase observed immediately after a 90-min bout of CE at 65% of  O2Peak16, and the non-significant change (0.70-fold) recorded 3 hours after a 60-min bout of CE at 70% of
O2Peak16, and the non-significant change (0.70-fold) recorded 3 hours after a 60-min bout of CE at 70% of  O2Peak18. Taken collectively, these results seem to indicate that CE at 60–75% of
O2Peak18. Taken collectively, these results seem to indicate that CE at 60–75% of  O2Peak does not induce a large increase in nuclear PGC-1α protein content. However, by directly comparing participants of similar fitness levels within the same study, we report the novel finding that SIE is associated with larger increases in nuclear PGC-1α protein content than CE.
O2Peak does not induce a large increase in nuclear PGC-1α protein content. However, by directly comparing participants of similar fitness levels within the same study, we report the novel finding that SIE is associated with larger increases in nuclear PGC-1α protein content than CE.
Similar to the exercise-induced increases in nuclear p-p53Ser15, and for the same reasons, the present results suggest that exercise intensity may also be an important factor affecting exercise-induced changes in nuclear PGC-1α protein content. Results from the cytosolic pool are also consistent with an exercise-intensity effect on the regulation of PGC-1α protein. Similar to the nuclear fraction, only SIE induced an increase in cytosolic PGC-1α protein content; however, this increase took place only after 3 h of recovery. The delayed increase in cytosolic compared with nuclear PGC-1α protein content provides further evidence that the initial phase of the exercise-induced adaptive response may indeed take place in the nucleus14.
The increase in the nuclear content of PGC-1α protein has previously been attributed to the exercise-induced translocation of PGC-1α from the cytosol to the nucleus14,15,16,45. While translocation remains possible, an alternative or additional explanation for the concomitant increase in nuclear and cytosolic PGC-1α protein content in the present study may relate to an increase in PGC-1α stability. An increase in p38 MAPK activity has previously been reported immediately post-exercise in humans15,16,17,28, and has been linked with greater PGC-1α stability46. The greater increase in the phosphorylation of p38 MAPK following SIE compared with CE in both subcellular fractions (both by 2.0-fold) occurred concomitantly with a greater increase in both nuclear and cytosolic PGC-1α protein content (both by 1.7-fold). It is therefore possible that exercise intensity may modulate PGC-1α protein content via an increase in PGC-1α stability, which may be mediated, at least in part, by greater phosphorylation of p38 MAPK46.
The PGC-1α protein has been shown to activate its own promoter through a feed-forward loop47; as a result, increased content of nuclear PGC-1α protein should further enhance PGC-1α transcriptional activity before degradation45. However, despite a greater increase in nuclear PGC-1α protein content following SIE, when compared to CE, there was no difference for the increase in PGC-1α mRNA content following the two exercise types. This highlights a potential dissociation between exercise-induced increases in PGC-1α nuclear protein content and PGC-1α mRNA (and the mRNA of cytochrome c, a downstream target of PGC-1α48). This may be related to other factors that influence PGC-1α transcriptional activity such as AMPK, a signalling protein inducing PGC-1α activation39,49, and its downstream target and commonly used biomarker ACC38,39, the phosphorylation of which was similarly increased following both exercise types. The similar increase in PGC-1α mRNA between the two types of exercise is also consistent with previous research comparing exercise at intensities below and above WPeak40,50, and is in agreement with the notion that the reported exercise intensity-dependent regulation of PGC-1α mRNA51 is limited to submaximal (i.e., <WPeak) exercise intensities40.
Although the between-subject design represents a potential limitation of this study, our findings add new insight into the early molecular events that regulate skeletal muscle remodelling in response to a single bout of exercise, and the role of exercise intensity in mediating these events. We report that a single bout of exercise induces nuclear accumulation of p53 protein, an increase that may relate to greater p53 stability, as suggested by the concomitant increase in PHF20 protein content. In addition, nuclear p-p53Ser15, a post-translational event also associated with enhanced p53 stability, and nuclear PGC-1α protein content, increased only following SIE, suggesting that exercise intensity may play an important role in the exercise-induced adaptations mediated by both p53 and PGC-1α. Results from the present study also indicate that increases in nuclear p-p53Ser15, as well as nuclear PGC-1α protein content, may represent important early events in the adaptive response to exercise. Our findings indicate that “all-out” SIE represents a valuable and possibly superior option to moderate-intensity CE for promoting the early molecular events leading to exercise-induced mitochondrial biogenesis, in a time-efficient manner.
Methods
Participants
Twenty healthy men aged 18–35 years, who were non-smokers, free of medications, moderately-trained (i.e., engaging in less than 3–4 hours per week of moderate, unstructured aerobic activity for 6 months prior to the study), and not regularly engaged in cycling-based sports, volunteered to participate in this research. Following medical screening participants were informed of the study requirements, benefits, and risks, before giving written informed consent. Approval for all the experimental protocols and the study’s procedures, which conformed to the standards set by the latest revision of the Declaration of Helsinki, was granted by the Victoria University Human Research Ethics Committee. All experiments and procedures were performed in accordance with the relevant guidelines and regulations set by the above Human Research Ethics Committee.
Study design and testing
The experimental protocol consisted of two tests - a graded exercise test (GXT), and an exercise/biopsy trial. Participants were familiarised with both tests (with the exclusion of muscle biopsies) and were required to refrain from any strenuous exercise for the 72 h preceding each test, from alcohol and any exercise for 24 h before testing, and from food and caffeine consumption for 3 h before each test. After baseline testing, participants were ranked based on their WLT and assigned in reversed counterbalanced order (ABBA) to the CE or SIE group (both, n = 10), in a between-subjects study design. The between-subject design was necessary as this study was part of a longer training study in which participants then repeated their assigned exercise trial for four weeks, as previously described12. Nineteen participants completed the study, with one participant (SIE group) withdrawing due to time constraints. Participants’ baseline physiological parameters are described in Table 1.
GXT
A discontinuous graded exercise test was performed on an electronically-braked cycle ergometer (Lode Excalibur, v2.0, The Netherlands) to determine the HRPeak,  O2Peak, WPeak, WLT (using the modified DMax method52), and the exercise intensity for the biopsy trial, as previously described12 (Table 1). The WPeak was determined as the power of the last completed stage plus 7.5 W for every additional minute completed. Expired air was continuously analysed for O2 and CO2 concentrations via a pre-calibrated gas analyser (Moxus 2010, AEI technologies, USA), and
O2Peak, WPeak, WLT (using the modified DMax method52), and the exercise intensity for the biopsy trial, as previously described12 (Table 1). The WPeak was determined as the power of the last completed stage plus 7.5 W for every additional minute completed. Expired air was continuously analysed for O2 and CO2 concentrations via a pre-calibrated gas analyser (Moxus 2010, AEI technologies, USA), and  O2 values were recorded every 15 s. The average of the two highest consecutive 15-s values was recorded as a participant’s
O2 values were recorded every 15 s. The average of the two highest consecutive 15-s values was recorded as a participant’s  O2Peak. Glass capillary tubes were used to collect ~50 μL of blood prior to the test, and after each 4-min stage. Capillary blood [La−] was determined using a pre-calibrated blood-lactate analyser (YSI 2300 STAT Plus, YSI, USA).
O2Peak. Glass capillary tubes were used to collect ~50 μL of blood prior to the test, and after each 4-min stage. Capillary blood [La−] was determined using a pre-calibrated blood-lactate analyser (YSI 2300 STAT Plus, YSI, USA).
Biopsy trial
All trials were performed in the morning to avoid variations caused by circadian rhythms. To minimise variability in muscle gene and protein expression attributable to diet, participants were provided with a standardised dinner (55 kJ·kg−1 body mass (BM), providing 2.1 g carbohydrate·kg−1 BM, 0.3 g fat·kg−1 BM, and 0.6 g protein·kg−1 BM) and breakfast (41 kJ·kg−1 BM, providing 1.8 g carbohydrate·kg−1 BM, 0.2 g fat·kg−1 BM, and 0.3 g protein·kg−1 BM), to be consumed 15 and 3 h prior to the biopsy trials, respectively. While resting in the supine position, and after injection of a local anaesthetic (1% xylocaine) into the skin and fascia of the vastus lateralis muscle, three small incisions were made about 2–3 cm apart. A resting muscle biopsy (Pre) was obtained using a biopsy needle with suction before participants began either the CE or SIE protocol an electronically-braked cycle ergometer (Velotron, RacerMate, USA). A second muscle biopsy (+0 h) was obtained immediately upon completion of the exercise bout (<5 s). A third muscle biopsy (+3 h) was obtained after 3 hours of recovery in the supine position (with no access to food and access to water ab libitum). Once obtained, muscle samples were immediately cleaned of excess blood, fat, and connective tissue, were rapidly frozen in liquid nitrogen, and stored at −80 °C for subsequent analyses. Capillary blood samples to measure [La−] were collected at rest, and immediately after the completion of exercise.
CE trial
Exercise consisted of 24 min of continuous cycling at a fixed power equivalent to 90% of WLT. This was preceded by a warm-up involving cycling for 6 min at 66% of WLT followed by 2 min of rest. Exercise intensity was set relative to WLT rather than WPeak as metabolic and cardiac stresses are similar when individuals of differing fitness levels exercise at a percent of the WLT, but can vary significantly when exercising at a percent of WPeak53. Participants received consistent verbal encouragement for the duration of the exercise bout. The overall duration of the CE exercise protocol (32 min of exercise inclusive of warm-up) was chosen based on the physical activity guidelines set by the ACSM position stand8.
SIE trial
Following the same warm-up procedure for CE, SIE consisted of 4 × 30-s “all-out” cycling bouts against a resistance set at 0.075 kg·kg−1 BM, interspersed with 4 min of rest (2 min of total exercise; 22 min of total session duration inclusive of warm-up). Participants received consistent verbal encouragement to keep the cadence as high as possible during the entire duration of the bout.
Skeletal muscle analyses
Subcellular fractionation
Nuclear and cytosolic fractions were prepared from 40–60 mg of wet muscle using a commercially-available nuclear extraction kit (NE-PER, Pierce, USA). Muscle samples were homogenised in CER-I buffer containing a protease/phosphatase inhibitor cocktail (Cell Signaling Technology [CST], 5872). Following centrifugation the supernatant was taken as the crude cytosolic fraction. Pellets containing nuclei were washed five times in PBS to remove cytosolic contamination, before nuclear proteins were extracted by centrifugation in high-salt NER buffer supplemented with the same inhibitors cocktail following manufacturers’ instruction. Sufficient muscle was available to prepare subcellular fractions from nine participants in each group. Verification of subcellular enrichment is presented in the Results section.
Immunoblotting
Protein concentration was determined in triplicate using a commercial colorimetric assay (Bio-Rad Protein Assay kit-II, Australia). Muscle lysates (10–50 μg) were separated by electrophoresis using SDS-PAGE gels (8–15%) as previously described12. The following primary antibodies were used (supplier, catalogue number): histone H3 (CST, 9715), LDHA (CST, 2012), p53 (CST, 2527), PGC-1α (Calbiochem, st-1202), p-ACCSer79 (CST, 3661), p-p38 MAPKThr180/Tyr182 (CST, 9211), p-p53Ser15 (CST, 9284), and PHF20 (CST, 3934). An internal standard was loaded in each gel, and each lane was normalised to this value, to reduce gel-to-gel variability. Whole-lane Coomassie blue staining, and immunoblots for H3 (nuclear) and GAPDH (cytosolic) were performed to verify correct loading and equal transfer between lanes54. Representative blots and loading control images are presented in Fig. 1.
Total RNA isolation
Total RNA was isolated from approximately 15–25 mg of muscle tissue using the RNeasy® Mini kit (Qiagen, Canada) according to the manufacturer’s instructions. Muscle samples were homogenised using the TissueLyser II (Qiagen, Canada), and total RNA was isolated from the aqueous phase following precipitation with 600 μL of 70% ethanol using RNeasy® Mini kit. On-column DNA digestion was performed. RNA concentration was determined by spectrophotometry (Nanodrop ND1000, Thermo Fisher Scientific, USA) by measuring the absorbance at 260 nm (A260) and 280 nm (A280), with A260/A280 ratios above 1.8 indicating high-quality RNA. Sufficient muscle was available to isolate and analyse total RNA from nine participants in each group.
Real-time RT-PCR
First-strand cDNA synthesis from 500 ng of total RNA was performed with random hexamer primers using a high-capacity cDNA reverse transcription kit (Applied Biosystems, USA), according to manufacturer’s directions. All samples and reverse transcriptase (RT) negative controls were run together to prevent technical variation. Forward and reverse primers for the target and housekeeping genes (Table 3) were designed based on NCBI RefSeq using NCBI Primer-BLAST (www.ncbi.nlm.nih.gov/BLAST/). Specificity of the amplified product was confirmed by melting point dissociation curves. The mRNA expression of AIF, cyt c, DRP1, MFN2, p21, p53, PGC-1α and SOD2 were quantified by quantitative real-time RT-PCR (Mastercycler® RealPlex2, Eppendorf, Germany), using a 10 μL PCR reaction volume and SYBR Green chemistry (iTaqTM Universal SYBR® Green Supermix, Bio-Rad, USA). All samples were run in duplicate simultaneously with template free controls, using an automated pipetting system (epMotion 5070, Eppendorf, Germany). The following PCR cycling patterns were used: initial denaturation at 95 °C (3 min), 40 cycles of 95 °C (15 s) and 60 °C (60 s). Relative changes in mRNA content were calculated using the normalised relative quantities (NRQs) method55. To account for the efficiency of RT and initial RNA concentration, the mRNA expression of four housekeeping genes was quantified, and their stability was determined using the BestKeeper software56. Cyclophilin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and beta-2-microglobulin (B2M) were classified as stable, whereas TATA-binding protein (TBP) was reported as unstable and was therefore excluded. These results were confirmed by the Normfinder algorithm57.
Statistical analysis
All values are reported as mean ± SD unless otherwise specified. Unpaired t-tests were used to assess differences between SIE and CE for Pre values in immunoblot analyses, and for age, height, body mass, HRPeak, WLT, WPeak,  O2Peak, as well as 1-s max and mean exercise intensity, and total work during the biopsy trial. To investigate the influence of exercise type and time, and the interaction between both of these variables, two-way ANOVA with repeated measures for time were used. Where no interaction effects were observed, pooled values for time are reported. Significant interactions and main effects were further analysed using Tukey’s honestly significant difference post-hoc test. Sigma Stat software (Jandel Scientific, USA) was used for all statistical analyses. The level of statistical significance was set a priori at P < 0.05. To assess the magnitude of effects, effect sizes (ES), assessed using Cohen’s d, and 95% confidence intervals (95% CI), were also calculated and are reported as (ES; 95% CI) of the between-group difference (CE vs. SIE) scores.
O2Peak, as well as 1-s max and mean exercise intensity, and total work during the biopsy trial. To investigate the influence of exercise type and time, and the interaction between both of these variables, two-way ANOVA with repeated measures for time were used. Where no interaction effects were observed, pooled values for time are reported. Significant interactions and main effects were further analysed using Tukey’s honestly significant difference post-hoc test. Sigma Stat software (Jandel Scientific, USA) was used for all statistical analyses. The level of statistical significance was set a priori at P < 0.05. To assess the magnitude of effects, effect sizes (ES), assessed using Cohen’s d, and 95% confidence intervals (95% CI), were also calculated and are reported as (ES; 95% CI) of the between-group difference (CE vs. SIE) scores.
Additional Information
How to cite this article: Granata, C. et al. Sprint-interval but not continuous exercise increases PGC-1α protein content and p53 phosphorylation in nuclear fractions of human skeletal muscle. Sci. Rep. 7, 44227; doi: 10.1038/srep44227 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Perry, C. G. R. et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 588, 4795–4810 (2010).
Bishop, D. J., Granata, C. & Eynon, N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochimica et Biophysica Acta-General Subjects 1840, 1266–1275 (2014).
Gibala, M. J., Little, J. P., Macdonald, M. J. & Hawley, J. A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 590, 1077–1084 (2012).
Granata, C., Oliveira, R. S. F., Little, J. P., Renner, K. & Bishop, D. J. Mitochondrial adaptations to high-volume exercise training are rapidly reversed after a reduction in training volume in human skeletal muscle. The FASEB Journal 30, 3413–3423 (2016).
Holloszy, J. O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 242, 2278–2282 (1967).
Spina, R. J. et al. Mitochondrial enzymes increase in muscle in response to 7-10 days of cycle exercise. J. Appl. Physiol. 80, 2250–2254 (1996).
Pedersen, B. K. & Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Spor. 16, 3–63 (2006).
Garber, C. E. et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 43, 1334–1359 (2011).
Stutts, W. C. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 50, 499–507 (2002).
Bartlett, J. D. et al. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 29, 547–553 (2011).
Burgomaster, K. A. et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 586, 151–160 (2008).
Granata, C., Oliveira, R. S. F., Little, J. P., Renner, K. & Bishop, D. J. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. 30, 959–970 (2016).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Wright, D. C. et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J. Biol. Chem. 282, 194–199 (2007).
Little, J. P., Safdar, A., Bishop, D., Tarnopolsky, M. A. & Gibala, M. J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1303–1310 (2011).
Little, J. P., Safdar, A., Cermak, N., Tarnopolsky, M. A. & Gibala, M. J. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R912–R917 (2010).
McGee, S. L. & Hargreaves, M. Exercise and Myocyte Enhancer Factor 2 Regulation in Human Skeletal Muscle. Diabetes 53, 1208–1214 (2004).
Tachtsis, B., Smiles, W., Lane, S., Hawley, J. & Camera, D. M. Acute endurance exercises induces nuclear p53 abundance in human skeletal muscle. Front. Physiol. 7 (2016).
Saleem, A., Carter, H. N., Iqbal, S. & Hood, D. A. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc. Sport Sci. Rev. 39, 199–205 (2011).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Li, J. et al. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 6 (2010).
Wang, W. et al. Mitofusin-2 is a novel direct target of p53. Biochem. Biophys. Res. Commun. 400, 587–592 (2010).
Saleem, A., Adhihetty, P. J. & Hood, D. A. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol. Genomics 37, 58–66 (2009).
Oren, M. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 274, 36031–36034 (1999).
Philp, A. & Schenk, S. Unraveling the complexities of sirt1-mediated mitochondrial regulation in skeletal muscle. Exerc. Sport Sci. Rev. 41, 174–181 (2013).
Saleem, A. & Hood, D. A. Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J. Physiol. 591, 3625–3636 (2013).
Shieh, S. Y., Ikeda, M., Taya, Y. & Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 (1997).
Bartlett, J. D. et al. Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J. Appl. Physiol. 112, 1135–1143 (2012).
Cui, G. et al. PHF20 is an effector protein of p53 double lysine methylation that stabilizes and activates p53. Nat. Struct. Mol. Biol. 19, 916–924 (2012).
Park, S. et al. Identification of Akt interaction protein PHF20/TZP that transcriptionally regulates p53. J. Biol. Chem. 287, 11151–11163 (2012).
Psilander, N., Wang, L., Westergren, J., Tonkonogi, M. & Sahlin, K. Mitochondrial gene expression in elite cyclists: Effects of high-intensity interval exercise (European Journal of Applied Physiology. Eur. J. Appl. Physiol., doi: 10.1007/s00421-010-1544-1, 110, 607 (2010).
Chen, Y. W. et al. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J. Physiol. 545, 27–41 (2002).
Marchenko, N. D. et al. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-α3 binding. Cell Death Differ. 17, 255–267 (2010).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Bartlett, J. D. et al. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R450–458 (2013).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
She, Q. B., Bode, A. M., Ma, W. Y., Chen, N. Y. & Dong, Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61, 1604–1610 (2001).
Chen, Z. P. et al. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52, 2205–2212 (2003).
Jäger, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 104, 12017–12022 (2007).
Edgett, B. A. et al. Dissociation of Increases in PGC-1α and Its Regulators from Exercise Intensity and Muscle Activation Following Acute Exercise. PLoS ONE 8 (2013).
Hammond, K. M. et al. Postexercise high-fat feeding suppresses p70S6K1 activity in human skeletal muscle. Med. Sci. Sports Exerc. 48, 2108–2117 (2016).
Gorospe, M., Wang, X. & Holbrook, N. Functional role of p21 during the cellular response to stress. Gene Expression 7, 377–385 (1998).
Stambolsky, P. et al. Regulation of AIF expression by p53. Cell Death Differ. 13, 2140–2149 (2006).
Zelko, I. N., Mariani, T. J. & Folz, R. J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biol. Med. 33, 337–349 (2002).
Anderson, R. M. et al. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 7, 101–111 (2008).
Puigserver, P. et al. Cytokine Stimulation of Energy Expenditure through p38 MAP Kinase Activation of PPARγ Coactivator-1. Mol. Cell 8, 971–982 (2001).
Handschin, C., Rhee, J., Lin, J., Tarr, P. T. & Spiegelman, B. M. An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc. Natl. Acad. Sci. USA 100, 7111–7116 (2003).
Scarpulla, R. C. Nucleus-encoded regulators of mitochondrial function: Integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1819, 1088–1097 (2012).
McGee, S. L. & Hargreaves, M. AMPK-mediated regulation of transcription in skeletal muscle. Clin. Sci. 118, 507–518 (2010).
Wang, L., Psilander, N., Tonkonogi, M., Ding, S. & Sahlin, K. Similar expression of oxidative genes after interval and continuous exercise. Med. Sci. Sports Exerc. 41, 2136–2144 (2009).
Egan, B. et al. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor γ coactivator-1α mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 588, 1779–1790 (2010).
Bishop, D. J., Jenkins, D. G., McEniery, M. & Carey, M. F. Relationship between plasma lactate parameters and muscle characteristics in female cyclists. Med. Sci. Sports Exerc. 32, 1088–1093 (2000).
Baldwin, J., Snow, R. J. & Febbraio, M. A. Effect of training status and relative exercise intensity on physiological responses in men. Med. Sci. Sports Exerc. 32, 1648–1654 (2000).
Welinder, C. & Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 10, 1416–1419 (2011).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8 (2007).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper - Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515 (2004).
Andersen, C. L., Jensen, J. L. & Ørntoft, T. F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 64, 5245–5250 (2004).
Acknowledgements
We thank the participants for their time, effort and commitment to this study. The authors would like to acknowledge Mr. Andrew Ronacher and Mr. Maarten Missinne for their valuable help in data collection and biochemical analyses, respectively. This study was funded by a grant from the ANZ-MASON Foundation provided to D.J.B. and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to J.P.L.
Author information
Authors and Affiliations
Contributions
C.G. and D.J.B. designed the research; C.G. and R.S.F.O. conducted the research; C.G., R.S.F.O., J.P.L., K.R. and D.B. analysed and interpreted data; C.G. wrote the manuscript; C.G., R.S.F.O., J.P.L., K.R. and D.J.B. critically revised and contributed to the manuscript; C.G. and D.J.B. have primary responsibility for final content. Data collection took place at Victoria University. Data analysis took place at Victoria University and the University of British Columbia Okanagan. All persons designated as authors qualify for authorship, and all those qualifying for authorship are listed. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Granata, C., Oliveira, R., Little, J. et al. Sprint-interval but not continuous exercise increases PGC-1α protein content and p53 phosphorylation in nuclear fractions of human skeletal muscle. Sci Rep 7, 44227 (2017). https://doi.org/10.1038/srep44227
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44227
This article is cited by
-
Exercise-Regulated Mitochondrial and Nuclear Signalling Networks in Skeletal Muscle
Sports Medicine (2024)
-
Identification of the best model to predict optical properties of water
Environment, Development and Sustainability (2023)
-
Factors Influencing AMPK Activation During Cycling Exercise: A Pooled Analysis and Meta-Regression
Sports Medicine (2022)
-
Effect of acute swimming exercise at different intensities but equal total load over metabolic and molecular responses in swimming rats
Journal of Muscle Research and Cell Motility (2022)
-
Acute RyR1 Ca2+ leak enhances NADH-linked mitochondrial respiratory capacity
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.