Abstract
Implementation studies are recommended to assess the feasibility and effectiveness of programmes. In Nigeria, little is known about the burden of diabetes mellitus (DM) among tuberculosis (TB) patients. The objective of this study was to determine screening efficacy, prevalence of DM and determinants of DM among TB patients. We report on a multi-centre implementation study carried-out in 13 health facilities in six States of Southern Nigeria. All newly diagnosed TB patients registered from March to October 2015 were screened for DM using current World Health Organisation guidelines. Overall, 2094 TB patients were evaluated, 196 (9.4%) were found to have DM. The prevalence of newly diagnosed DM was 5.5% (115/2094). DM prevalence varied according to age group; occurring in 2.2% of patients aged ≤ 25 years and 16.9% in patients aged (56–65) years. The additional yield of DM was 59% while the number needed to screen to detect a new case of DM was 18. Factors associated with DM were; age >40 years (aOR2.8, CI 2.1–3.9), rural residence (aOR2.3, 1.6–3.2), private health facility care (aOR2.0, 1.4–2.7), and having an occupation that engages in vigorous activity (aOR0.6, 0.4–0.9). The burden of DM among TB patients is high. Prioritization of DM screening for TB patients is indicated.
Similar content being viewed by others
Introduction
Containing the burden of tuberculosis (TB) and Diabetes mellitus (DM) remains a global health challenge. In 2015, it was estimated that 10.4 million incident cases of TB occurred globally, while 1.4 million persons died from it1. In the same year, the corresponding estimated prevalence and mortality due to DM were 415 million and 5 million, respectively2. If current trends continue, it is projected that by 2040, 642 million persons will be affected by DM2, 80% of whom will be residing in low- and middle-income countries where TB burden is highest2. DM is an important risk factor for developing TB. Systematic reviews have shown that the overall risk of TB in persons with DM is three times higher than in the general population3,4,5. In addition, persons with dual TB-DM disease have worse TB treatment outcomes with increased delays to sputum culture conversion, higher risk of relapse, death and drug resistance5,6,7. Conversely, TB and its treatment can impair glycaemic control – impacting the clinical management of DM5,8.
In recognition of the burden of DM and TB, the World Health Organization (WHO) and the International Union against Tuberculosis and Lung Disease (The Union) launched the Collaborative Framework for Care and Control of Tuberculosis and Diabetes to guide policy makers and programme managers in combating the TB-DM epidemic9. However, recent studies have shown that DM prevalence among TB cases is variable. It ranges from about 50% reported by some studies in India, about 30% in Mexico, less than 10% in the United States, to even lower values in certain African countries, Spain and China10,11,12,13,14,15,16. As reported levels of co-morbidity vary highly, National TB Control Programmes (NTPs) will need to decide whether such DM screening is needed. Where necessary, the most cost-effective approach for each setting, for example targeted screening or screening of all patients should be determined17.
Nigeria ranks third among 30 countries with estimated highest burden of TB in the world as well as third among African countries with highest burden of DM1,2. Considering that two thirds of DM patients in Africa are not aware that they have the disease, screening for DM among TB patients may contribute to early diagnosis2. In Nigeria, some research has been conducted on the prevalence of DM among TB patients18,19. However, as these studies were limited to hospitals located in a single urban area, it is unlikely to reflect the disease burden in rural areas or other geographical and cultural settings in the country. As WHO/The Union have called for operational and clinical research to build up and strengthen local evidence base for action in combating the dual epidemic17, quality data to guide this process in Nigeria is crucial but lacking. The aim of this study was to investigate the epidemiology of DM among TB patients in Nigeria. The specific objectives were; to determine 1) the proportion of TB patients with DM, 2) the additional yield of newly diagnosed DM cases, 3) the number needed to screen (NNS) to find a new case of DM, and 4) the determinants of DM among TB patients in Southern Nigeria.
Results
General characteristics
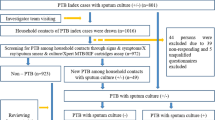
A total 2132 new and previously-treated TB patients (aged ≥ 15 years) were registered for treatment during the study period. However, 38 (1.8%) who were previously-treated TB patients were excluded from the study because they were not eligible. All eligible 2094 newly diagnosed TB patients completed the screening. The socio-demographic characteristics of the patients are shown in Table 1. The mean age was 40.7 years (standard deviation [SD] ±15.7). There were more males (56.4%) than females (43.6%), and majority resided in urban areas (51.9%). Furthermore, 95.3% of the patients had pulmonary tuberculosis (PTB), and 62.8% received care at a public facility. Among patients with PTB, 533 (26.7%) had smear-negative TB while 1463 (73.3%) had smear-positive/bacteriologically-confirmed TB. History of current smoking was elicited in 4.8% while 19.5% of patients had TB/HIV co-infection. In terms of level of physical activity according to occupation, 49.8% of patients had occupations that were largely sedentary, 32.9% had moderate activity and 17.3% had occupation associated with vigorous activity.
Prevalence of diabetes mellitus
The overall prevalence of DM among TB patients studied stratified by age, sex, residence, type of TB, physical activity and other factors, are as shown in Table 2. Of the 2094 patients, 196 (9.4%, 95% CI 8.2–10.7) were found to have DM. Of these, 81 (3.9%) were previously known to have DM, while 115 (5.5%) were newly diagnosed. The prevalence of DM was 2.2% (95% CI 1.1–4.4) among those aged ≤ 25 years compared with 15.1% (95% CI 11.5–19.5) and 16.9% (95% CI 12.6–22.3) among those aged (46–55) and (56–65) years, respectively (P < 0.001). The prevalence of DM did not differ according to type of TB, smoking status, HIV co-infection or gender. However, the prevalence of DM was 13.3% (95% CI 11.4–15.5) among rural residents compared to 5.7% (95% CI 4.5–7.2) among urban residents (P < 0.001). Similarly, the prevalence of DM among TB patients who received care at a public facility was 6.6% (95% CI 5.4–8.1) compared to 14.0% (95% CI 11.8–16.6) among patients who received care at a private facility (P < 0.001). The prevalence of DM among patients with sedentary occupation was 10.2% (95% CI 8.5–12.2). This was slightly higher in those engaged in occupation involving moderate/vigorous physical activity, but the difference was not statistically significant (p = 0.3).
Additional yield of diabetes mellitus and number needed to screen (NNS)
Of the 2094 TB patients, 196 (9.4%) were found to have DM, of whom 115 (5.5%) were newly-diagnosed cases. The additional yield of DM cases on screening was 59%. The NNS to detect one new case of DM was 18. Among patients aged 25 years or less, the NNS was 119, and among those aged (56–65) years the NNS was 9 (Table 3). The additional yield of males was similar to that of females (60% versus 60%). The NNS to diagnose one male DM patient was 18 compared to 19 for females. The additional yield of screening patients resident in rural areas for DM was 60% versus 58% for patients in urban areas. However, the NNS to diagnose one DM case among patients resident in the rural area was approximately 13 compared to 30 for urban residents. Regarding HIV status, additional yield among HIV-negative DM patients was 60.7% versus 48.5% for HIV-positive DM patients. However, the NNS to diagnose one HIV-negative DM patient was 17 compared to 26 for HIV-positive DM patients.
Risk factors associated with DM
The results of multivariable logistic regression analysis are presented in Table 4. After adjusting for potential confounders, risk factors such as age >40 years (aOR 2.8, CI 2.1–3.9), rural residence (aOR 2.3, 1.6–3.2), and private facility care (aOR 2.0, 1.4–2.7), were found to be independently associated with increased risk of DM among TB patients. However, having an occupation that engages in vigorous activity (aOR 0.6, 0.4–0.9) was found to be independently associated with decreased risk of DM among TB patients.
Discussion
This study found a DM prevalence of 9.4% among newly diagnosed TB patients. The additional yield of DM was 59% while the NNS to detect a new case of DM was 18. The prevalence of DM among patients who were older than 40 years was far higher than among younger persons; sex on the other hand was not associated with the occurrence of DM. The additional yield of DM was greater among older patients, persons who sought care at a public facility, rural residents and HIV-negative persons. In all, factors favouring occurrence of DM among TB patients in the study group were older age (over 40 years), private facility care and rural residence. Conversely, patients engaged in occupations with vigorous activity were less likely have DM.
The DM prevalence of 9.4% among newly diagnosed TB patients found in this study is much higher than was observed in a pilot study in a Nigerian city as well as in the general population, but was lower than a prevalence of 12.3% observed in some health facilities in the same city2,18,19. Our finding was also comparable with the results of studies carried-out in Ethiopia (8.3%), Uganda (8.5%) and USA (11.4%)11,12,20. However, DM prevalence among TB patients reported in this study was higher than the rates reported from China (6.3%), Spain (5.9%), Guinea-Bissau (2.8%) and Benin (1.9%); but lower than the rates reported from Taiwan (29.5%), Southern-Mexico (29.3%) and India (25.3%)13,14,15,16,21,22,23. Reason/s for the differences in prevalence might be due to disparities in patient background and disease burden. In Guinea-Bissau and Benin where low DM prevalence was observed, the burden of TB in both countries is low21,22. In addition, the low DM prevalence observed in these settings may also be explained by the high burden of TB among younger persons, who in general have a lower incidence of DM than those aged (40 years or over)24. Furthermore, differences in dietary habits and the screening methods used for DM diagnosis might contribute to the variation in prevalence of DM among TB patients in various regions13,14,15,16,21,22,23.
DM screening was conducted in large public and private facilities across Southern Nigeria under programme conditions with minimal additional training to the health facility workers. This suggests that the active screening strategy of TB patients for DM is feasible and acceptable. This finding may indicate the possibility of DM screening for TB patients at primary health care (PHC) facilities, and the need to establish its referral linkages in order to ensure quality DM services to patients diagnosed with DM. This would however require the establishment of a functional referral system to ensure access to quality DM services at appropriate secondary and tertiary health facilities.
The NNS to detect a new case of DM among TB patients was 18. This number is higher than the findings in India which showed large variations in NNS to detect DM among TB patients16. These differences may be due to the high prevalence of DM in India, the quality of screening test used and the countrywide standard procedure applied for DM screening in India25. In addition, we found that NNS needed to detect one case of DM among TB patients decreases as the age increases. Owing to the differences between the various studies, it is crucial to understand the relationship between TB and DM in different regions around the world in order to establish appropriate priorities and adapt the needs of National TB Control Programmes. In high TB burden countries with weak public health systems, the current recommendation is to rule out TB among diabetics and vice versa15,17,26. Given the low NNS noted in our setting to detect a DM case, and the need to sustain DM-TB screening programmes in Nigeria, the most cost-effective approach would be to prioritise older TB patients for targeted screening.
The strength of this study is that we were able to implement this screening programme in a routine programme setting across multiple regions and facilities with minimal additional costs and training. The study has a few limitations, however. First, it was carried out at high-end performing TB centres, therefore, the results may not be representative of all TB treatment settings e.g. PHC settings in Southern Nigeria. Secondly, we utilised FBG for diagnosis; FBG testing has low sensitivity and may fail to detect some persons with DM. However, FBG has been recommended as the initial DM screening test in resource-limited settings5. The HbA1c has also been recently recommended for the diagnosis of DM among TB patients; however, it is limited by being expensive, cumbersome to perform and inappropriate for screening individuals within routine general health services17. Third, we excluded previously-treated TB patients in this study. Although it could be argued that previously-treated TB patients are a risk group for the development of co-morbid TB-DM, we excluded them because in some of the facilities where the survey was carried-out, these patients were still being investigated for drug-resistant TB. Also, because the optimal time for DM screening among TB patients is not clear3, we excluded previously-treated TB patients because it will be difficult to ascertain if the DM diagnosed in this group is as a result of persistent TB disease or due to previous exposures to TB medications both of these situations has been shown to cause persistent hyperglycemia3,27,28,29. Besides, we wanted to establish the burden of DM in treatment-naive TB patients receiving first-line anti-TB treatment in order to provide evidence to advocate for systematic DM screening among the largest burden of adult TB patients in Nigeria. Finally, we did not undertake anthropometric measurements (e.g., body mass index, waist circumference, etc.) of the respondents. A recent study in Pakistan found anthropometric heterogeneity among TB patients with/without DM – those with known or newly-diagnosed DM being more likely to be obese compared with those without DM30. Thus, such anthropometric measurements need to be considered in future studies in our setting.
This study has potential major policy implications. First, DM screening among TB patients is important for early diagnosis of DM – especially in Nigeria where two thirds of DM cases may be unaware of their status. The current screening strategy can improve early diagnosis of DM and facilitate necessary interventions. Second, it is especially important to screen older TB patients (≥40 years). Third, the study highlights the need for similar studies in PHC settings to estimate the burden of DM and the feasibility of decentralizing DM-TB services in rural and remote under-resourced settings.
Conclusions
The burden of DM among TB patients in Nigeria is high. Screening can detect more than half of undiagnosed DM among newly diagnosed TB patients. In this and similar under-resourced settings where prioritization for DM screening is necessary, screening TB patients who are aged >40 years may yield the greatest benefits. Furthermore, screening among TB patients residing in rural areas and those engaged in occupations with sedentary and moderate physical activity is expected to increase the additional yield of DM cases.
Materials
Study design
This was a cross-sectional implementation study conducted under programme conditions in six States in Southern Nigeria. The study was conducted over an eight-month period from March to October 2015.
Setting and study population
Nigeria is divided into two regions (north and south), and six geo-political zones (three per region) with each having between five to seven States. The study was conducted in the three zones in Southern Nigeria. Patients were recruited through a two-stage sampling technique. First, two States were selected from each of the three geo-political zones in southern Nigeria through simple random sampling. The study states include Edo and Cross River (south-south zone), Enugu and Ebonyi (south-east zone), and Ogun and Ondo (south-west zone). In each selected State, the top-two (three in Ondo State) health facilities notifying and treating the highest proportion of TB patients were identified and selected for the survey. In all, 13 health facilities which offer both TB and diabetes services were selected for the survey.
Eligibility
The inclusion criteria were all adults (≥15 years) with newly-diagnosed TB starting treatment. Patients were ineligible if they had previously-treated TB.
Screening procedure
All newly-diagnosed adult TB patients aged (≥15 years) attending the study health facilities were interviewed on their first visit for commencement of TB treatment. DM status was assessed using patients self-reported history regarding DM. All eligible patients irrespective of known diagnosis of DM were investigated as follows: Random blood glucose (RBG) test was performed – if the level was less than 110 mg/dl, no further action was taken. If the RBG was ≥110 mg/dl, then the patient was asked to return for fasting blood glucose (FBG) test the next day. A glucometer (ACCU-CHEK Active Glucose Monitoring System, Roche Diabetes Care Limited, Burgess Hill, UK) was used for screening DM. Standard WHO diagnostic criteria were used for making a diagnosis of diabetes mellitus, i.e., fasting blood glucose (FBG) value of ≥126 mg/dl19. Patients who were found to be diabetic were referred to the diabetes clinic of their facility for further evaluation and management. Moreover, known DM patients were educated on the need to continue follow-up care for DM.
A standardized proforma was used to collect patients’ socio-demographic and clinical variables from the TB registers as well as the screening results17. Physical activity was assessed based on patient’s occupation and classified as ‘sedentary’, ‘moderate’ and ‘vigorous’ according to WHO classification31. Diagnosis of TB was made in line with the WHO/NTP guidelines32. Patients found positive for Mycobacterium tuberculosis on smear microscopy or based on MTB RIF Xpert test were considered to have bacteriological-positive TB. Smear-negative TB was defined by negative smear (or Xpert) results with radiology, clinical and/or histology findings consistent with active TB, followed by a clinicians’ decision to treat with a full course of anti-TB chemotherapy. Extra-pulmonary TB (EPTB) was based on histological or strong clinical evidence consistent with active EPTB, and a clinicians’ decision to treat with a full course of TB chemotherapy. HIV counseling and testing was provided to all persons with presumed TB during submission of sputum specimen for microscopy.
Statistical analysis
Data were double-entered, validated for consistency and analysed using Epi Info. Dataset available as a supplementary information (SI). Patients’ demographic and clinical characteristics were described in terms of frequencies and percentages. The main outcomes for analysis were the number and proportion of TB patients with known and/or newly diagnosed DM, further stratified by age, sex, type of TB, residence and physical activity. Bivariate analysis was performed to assess the association of these factors with DM prevalence. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to describe associations between groups. Multivariable logistic regression analysis was performed to estimate adjusted ORs using the full model fits. NNS was calculated for all patients whose DM status was assessed. Additional yield of new cases of DM was calculated using the following formula: (newly diagnosed DM cases × 100)/(known DM cases + newly diagnosed DM cases).
Ethics approval
An informed verbal consent was obtained from each participant before enrolment in the study. We confirm that all blood sugar testing were performed in accordance with relevant WHO guidelines and regulations in use in clinical practice in Nigeria. The Ethics and Research Advisory Board of German Leprosy and TB Relief Association, Nigeria approved the study. Approval was also obtained from the State TB Control Programme in six states selected for the project.
Additional Information
How to cite this article: Ekeke, N. et al. Screening for diabetes mellitus among tuberculosis patients in Southern Nigeria: a multi-centre implementation study under programme settings. Sci. Rep. 7, 44205; doi: 10.1038/srep44205 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
World Health Organization. Global tuberculosis report 2016. Geneva: World Health Organization; 2016 [cited 2016 Nov. 1]. http://www.who.int/tb/publications/global_report/en/.
International Diabetes Federation. IDF Diabetes, 7 ed. Brussels, Belgium: International Diabetes Federation http://www.diabetesatlas.org (2015).
Dooley, K. E. & Chaisson, R. E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 9, 737–746 (2009).
Ruslami, R., Aarnoutse, R. E., Alisjahbana, B., van der Ven, A. J. & van Crevel, R. Implications of the global increase of diabetes for tuberculosis control. Trop. Med. Int. Health 15, 1289–1299 (2010).
Pizzol, D. et al. Tuberculosis and diabetes: current state and future perspectives. Trop. Med. Int. Health. 21, 694–702 (2016).
Baker, M. A. et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC. Med. 9, 81 (2011).
Mi, F. et al. Diabetes mellitus and tuberculosis: pattern of tuberculosis, two-month smear conversion and treatment outcomes in Guangzhou, China. Trop. Med. Int. Health 18, 1379–1385 (2013).
Van-Cromphaut, S., Vanhorebeek, I. & Van-den-Berghe, G. Glucose metabolism and insulin resistance in sepsis. Curr. Pharm. Des. 14, 1887–1899 (2008).
World Health Organization and the International Union Against Tuberculosis and Lung Disease. Collaborative Framework for Care and Control of Tuberculosis and Diabetes. WHO/HTM/TB/2011.15. Geneva, Switzerland: WHO, 2011.
Viswanathan, V. et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLOS. ONE 7, e41367 (2012).
Magee, M. J. et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009–2012. Ann. Epidemiol. 24, 369–375 (2014).
Kibirige, D., Ssekitoleko, R., Mutebi, E. & Worodria, W. Overt diabetes mellitus among newly diagnosed Ugandan tuberculosis patients: a cross sectional study. BMC. Infect. Dis. 13, 122–129 (2013).
Jiménez-Corona, M. E. et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 68, 214–220 (2013).
Wang, Q. et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: a community based cohort study. PLOS. One. 18, e82660 (2013).
Moreno-Martínez, A. et al. Factors associated with diabetes mellitus among adults with tuberculosis in a large European city, 2000–2013. Int. J. Tuberc. Lung Dis. 19, 1507–1512 (2015).
Shidam, U. G., Roy, G., Sahu, S. K., Kumar, S. V. & Ananthanarayanan, P. H. Screening for diabetes among presumptive tuberculosis patients at a tertiary care centre in Pondicherry, India. Int. J. Tuberc. Lung Dis. 19, 1163–1168 (2015).
Harries, A. D. et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int. J. Tuberc. Lung Dis. 19, 879–886 (2015).
Olayinka, A. O., Anthonia, O. & Yetunde, K. Prevalence of diabetes mellitus in persons with tuberculosis in a tertiary health centre in Lagos, Nigeria. Indian J. Endocrinol. Metab. 17, 486–489 (2013).
Ogbera, A. O. et al. Undiagnosed diabetes mellitus in tuberculosis: A Lagos report. Indian J. Endocrinol. Metab. 18, 475–479 (2014).
Workneh, M. H., Bjune, G. A. & Yimer, S. A. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLoS. One. 11, e0147621 (2016).
Ade, S. et al. Low prevalence of diabetes mellitus in patients with tuberculosis in Cotonou, Benin. Public Health Action. 5, 147–9 (2015)
Haraldsdottir, T. L. et al. Diabetes mellitus prevalence in tuberculosis patients and the background population in Guinea-Bissau: a disease burden study from the capital Bissau. Trans. R. Soc. Trop. Med. Hyg. 109, 400–407 (2015)
Chang, J. T. et al. Effect of type-2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergency of multi drug resistance. J. Formos. Med. Assoc. 110, 372–381 (2011).
World Health Organization, Benin Ministry of Health. Final report of the STEPS survey in Benin. Geneva, Switzerland: WHO, 2007. http://www.who.int/chp/steps/2007_STEPS_Report_Benin.pdf Accessed January 2017.
Members of tuberculosis-diabetes study group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop. Med. Int. Health. 18, 636–645 (2013).
World Health Organization. Screening for type 2 diabetes. Report of a World Health Organization and International Diabetes Federation Meeting, 2003. WHO/NMH/MNC/03.1. Geneva, Switzerland: WHO, 2003. http://www.who.int/diabetes/publications/en/screening_mnc03.pdf Accessed March 2016.
Baghaei, P., Marjani, M., Javanmard, P., Tabarsi, P. & Masjedi, M. R. Diabetes mellitus and tuberculosis facts and controversies. J. Diabetes Metab. Disord. 12, 58 (2013).
Zack, M. B., Fulkerson, L. L. & Stein, E. Glucose intolerance in pulmonary tuberculosis. Am. Rev. Respir. Dis. 108, 1164–1169 (1973).
Atkin, S., Masson, E., Bodmer, C., Walker, B. & White, M. Increased insulin requirement in a patient with type 1 diabetes on rifampicin. Diab. Med. 10, 202 (1992).
Aftab, H. et al. High prevalence of diabetes and anthropometric heterogeneity among tuberculosis patients in Pakistan. Trop. Med. Int. Health. (in press), doi: 10.1111/tmi.12842.
Joint FAO/WHO/UNU. Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, Rome, 17–24 Oct. 2001. Rome, FAO/WHO/UNU, 2004.
National Tuberculosis and Leprosy Control Programme, Revised Workers’ Manual, Sixth ed., Federal Ministry of Health, Abuja, Nigeria, 2015.
Acknowledgements
This work was funded by the German Leprosy & TB Relief Association, Enugu, Nigeria. We acknowledge the support of the TB patients who consented for the study and the administrative head of the hospitals surveyed who gave permissions for the survey to be performed in their facility.
Author information
Authors and Affiliations
Contributions
N.E., K.N.U., A.O.M., J.N.C., C.C.N., D.C.O., N.O.M., M.C.A. and C.A. conceived the study; N.E., J.N.C. and K.N.U. designed the study protocol; N.E., K.N.U., J.N.C., C.C.N., A.O.M., E.E.E., F.O.S., I.A., I.A., S.A., O.I., J.B.O., N.O.M., C.A., M.C.A., O.K.M. and D.C.O. collected data, performed data entry and carried out the data analysis and interpretation; K.N.U., N.E., A.O.M., D.C.O., J.N.C., N.O.M. and C.C.N., drafted the manuscript; N.E., K.N.U., J.N.C., C.C.N., A.O.M., E.E.E., F.O.S., I.A., I.A., S.A., O.I., J.B.O., N.O.M., C.A., M.C.A., O.K.M. and D.C.O. critically revised the manuscript for intellectual content. N.E., K.N.U., J.N.C., C.C.N., A.O.M., E.E.E., F.O.S., I.A., I.A., S.A., O.I., J.B.O., N.O.M., C.A., M.C.A., O.K.M. and D.C.O. read and approved the final manuscript. J.N.C. and K.N.U. are guarantors of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ekeke, N., Ukwaja, K., Chukwu, J. et al. Screening for diabetes mellitus among tuberculosis patients in Southern Nigeria: a multi-centre implementation study under programme settings. Sci Rep 7, 44205 (2017). https://doi.org/10.1038/srep44205
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44205
This article is cited by
-
The collaborative framework for the management of tuberculosis and type 2 diabetes syndemic in low- and middle-income countries: a rapid review
BMC Public Health (2024)
-
Tuberculosis and diabetes mellitus comorbidity in an adult Ugandan population
BMC Infectious Diseases (2024)
-
Gradient association between pulmonary tuberculosis and diabetes mellitus among households with a tuberculosis case: a contact tracing-based study
Scientific Reports (2022)
-
Prevalence of cardiovascular risk factors in active tuberculosis in Africa: a systematic review and meta-analysis
Scientific Reports (2022)
-
Diabetes mellitus among patients attending TB clinics in Dar es Salaam: a descriptive cross-sectional study
BMC Infectious Diseases (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.