Abstract
DNA transposons and retroviruses are versatile tools in functional genomics and gene therapy. To facilitate their application, we conducted a genome-wide insertion site profiling of the piggyBac (PB), Tol2 and Sleeping Beauty (SB) transposons and the murine leukemia virus (MLV) in mouse embryonic stem cells (ESCs). PB and MLV preferred highly expressed genes, whereas Tol2 and SB preferred weakly expressed genes. However, correlations with DNase I hypersensitive sites were different for all vectors, indicating that chromatin accessibility is not the sole determinant. Therefore, we analysed various chromatin states. PB and MLV highly correlated with Cohesin, Mediator and ESC-specific transcription factors. Notably, CTCF sites were correlated with PB but not with MLV, suggesting MLV prefers smaller promoter–enhancer loops, whereas PB insertion encompasses larger chromatin loops termed topologically associating domains. Tol2 also correlated with Cohesin and CTCF. However, correlations with ESC-specific transcription factors were weaker, suggesting that Tol2 prefers transcriptionally weak chromatin loops. Consistently, Tol2 insertions were associated with bivalent histone modifications characteristic of silent and inducible loci. SB showed minimum preference to all chromatin states, suggesting the least adverse effect on adjacent genes. These results will be useful for vector selection for various applications.
Similar content being viewed by others
Introduction
DNA transposons and retroviruses have been widely used as invaluable tools in various fields of life science, including gene therapy1, cancer gene discovery2,3,4, and insertional mutagenesis5,6. Retroviruses have been the most popular vector for application in mammalian cells for the last few decades because of their high efficiency of infection. Compared to retroviruses, the use of DNA transposons has been hampered for many years because most DNA transposons are inactivated during evolution and active DNA transposons have not been available. However, resurrection of the Sleeping Beauty (SB) transposon from the salmonid fish genome7 ignited the development of a series of active transposons. Currently, there are several available DNA transposon vectors such as piggyBac (PB) from cabbage looper moth, Trichoplusia ni8 and Tol2 from medaka fish9.
We have extensively utilized DNA transposons and murine leukemia virus (MLV) for functional genomics and gene transfer. We developed a method of transposon-tagged germline mutagenesis in mice using the SB transposon and generated more than 300 mutant mouse lines10,11,12. We also utilized MLV, Tol2 and PB for insertional mutagenesis of mouse embryonic stem cells (ESCs) and generated more than 1,000 mutant cell lines5. During these experiments, we observed that the same genes were repeatedly inserted by the same vectors, indicating the substantial bias of vector insertion sites. From this, we realized that information regarding vector insertion preference is necessary when choosing an appropriate vector in various experimental settings. However, the number of mutant mice and the cell lines we generated were insufficient for genome-wide in-depth analyses. Furthermore, precise evaluation of the insertion preference was hampered by bias during mutagenesis such as the identification of gene hits by reporter gene expression.
Here, we report a large-scale genome-wide characterization of insertion site profiles of MLV, PB, Tol2 and SB. We carefully designed our experiments to minimize any selection bias during the detection of insertion sites. We utilized high-throughput DNA sequencing technologies to obtain a large number of insertion sites that are sufficient for statistical analyses. We used mouse ESCs as host cells because of the availability of a large dataset of genomic and epigenomic information such as gene expression, histone modifications, chromatin binding sites of various transcriptional regulators, and higher-order chromatin structures. Our analyses revealed that all vectors have a distinct insertion site preference. These results will be useful to determine the efficient utilization of each vector in functional genomics and gene therapy studies.
Results
Experimental design and consideration for unbiased analyses
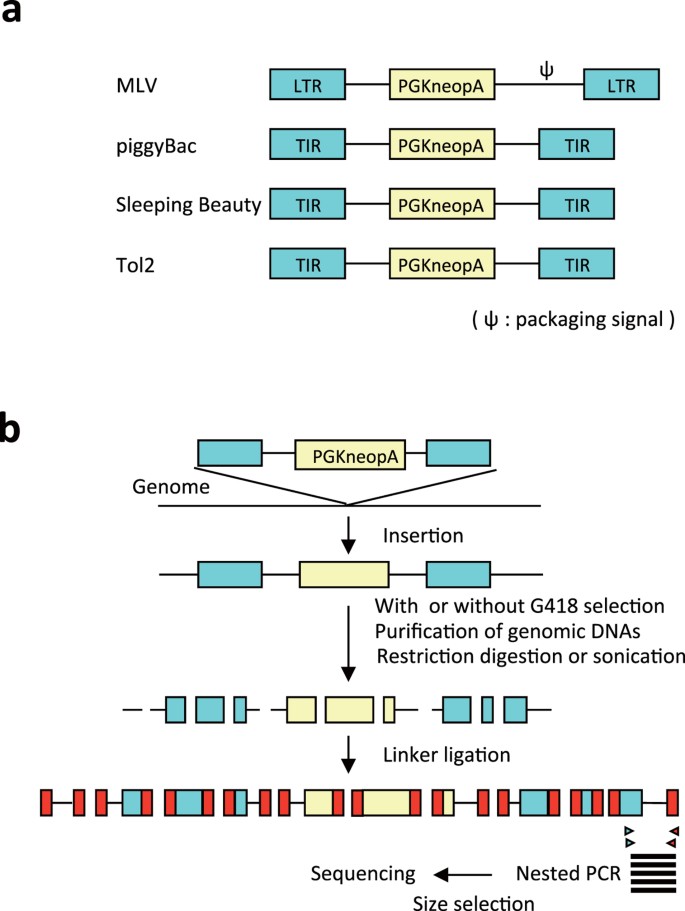
Figure 1 shows an overview of the analyses. The vector structures are shown in Fig. 1a and the experimental scheme to determine the insertion sites are presented in Fig. 1b. All vectors contained a neo gene expression cassette driven by a phosphoglycerate kinase-1 promoter13, which has widely been used in mouse ESCs.
To characterize insertion site profiles as precisely as possible, we listed the possible factors that may introduce bias during the analysis and considered solutions to this (Supplementary Table S1). First, we utilized high-throughput DNA sequencing methods to obtain a large dataset. We employed two complementary platforms, Roche GS FLX (Supplementary Table S2) and Illumina GA2 (Supplementary Table S3). We used both platforms in some experiments in order to assess whether any platform-specific bias existed. The dataset obtained with Roche GS FLX was used more often in the present study because its long read length allowed us to achieve accurate mapping on the mouse genome. Second, we tried to avoid the effect of transcriptional silencing of the neo selection marker. Neo selection markers can be silenced in some insertion sites such as heterochromatin regions, in which case such insertion sites would be lost during G418 selection and excluded from the downstream analysis. To avoid this bias, we set up a condition for multiple vector insertion per cell so that silencing of the neo gene in a given locus would be rescued by the expression of the neo gene in other loci. Southern blot analysis of G418-resistant ESC clones showed that the mean number of vector DNA per cell was 1.4, 4.4, 2.6, 3.0 in MLV, PB, Tol2, SB, respectively (Supplementary Fig. S1). We used Southern blot analysis to verify that vector DNA was not present in G418-sensitive clones (Supplementary Fig. S1), further confirming that the loss of vector-inserted clones was a rare event under our experimental conditions. As an alternative to avoid selection bias, we omitted G418 selection in some experiments and compared the results with and without selection. This evaluation was necessary, especially for MLV, because a high copy number of MLV insertions was hard to achieve in ESCs (Supplementary Fig. S1). Third, remobilization of integrated transposons can lead to the misinterpretation of insertion preference because DNA transposons hop locally as we and others reported previously10,11,14,15,16,17,18. In cases where transposons hopped locally after cell division, original insertion sites and re-insertion sites could be close to each other and misinterpreted as a hotspot for insertion. To avoid this, we conducted three independent transfections and assessed the insertion sites between different experiments. Fourth, PB and SB are inserted into TATA and TA sequences7,8, respectively, which inevitably constrains the distribution of insertion sites. Therefore, we also introduced this constraint in in silico-generated controls. Fifth, distribution of restriction sites used for genomic DNA fragmentation in Roche GS FLX may introduce bias. To avoid this, we reflected the distribution of the restriction site in in silico controls. Alternatively, we fragmented genomic DNAs with sonication in Illumina GA2 and compared the results with Roche GS FLX.
Genome-wide distribution of vector insertion sites
Following G418 selection of vector-inserted cells and restriction digestion of genomic DNAs (Fig. 1b), we determined insertion sites for MLV (n = 5,894,), PB (n = 6,368), Tol2 (n = 4,439), and SB (n = 8,641), using Roche GS FLX (Supplementary Table S2). Genome-wide distribution of the insertion sites was assessed in 1-Mb windows with exon distribution (Fig. 2a, Supplementary Fig. S2). Almost all genomic regions were inserted by all four vectors with this resolution. Of note, some genomic regions exhibited poor insertion events in all datasets including in silico controls (shown by red dotted lines in Fig. 2a). This observation indicates that comparison with the in silico control is essential for the precise evaluation of insertion site distribution. Although genome-wide insertion was observed by 1-Mb windows, insertion sites were not uniformly distributed and seemed biased toward exon rich regions (Fig. 2a, Supplementary Fig. S2). To validate the insertion preference toward exon-rich regions statistically, we divided the entire genome into 500-kb regions and categorized them into equal sized five groups by exon density: lowest, low, mid, high, and highest (Supplementary Fig. S3). Vector insertions were more frequently observed in regions with higher exon density compared to the in silico controls (Fig. 2b), demonstrating insertion bias toward exon-rich regions. We also calculated the correlation coefficients between the number of insertion sites and exon density for all vectors (Fig. 2c, range from 0.45 to 0.56). Matched control inserts showed markedly lower correlation coefficients (Fig. 2c, range from 0.09 to 0.20). To assess the insertion bias further, we analysed the distribution of the inter-insertion distances, distance between adjacent insertion sites (Fig. 2d,e). MLV showed significant enrichment of insertion sites at reduced inter-insertion distances compared to other vectors. (Fig. 2d,e, see Supplementary Table S4 for the inter-insertion distance and the statistical comparison between vectors). The number of less than 10-kb inter-insertion distances were 9.73-fold higher than in the matched control in MLV (Fig. 2e), suggesting MLV insertions are clustered within narrow genomic regions. We further assessed the distribution bias by quantifying hotspots of vector insertion (Fig. 2f, see Methods for the definition of hotspot). Consistent with the results of the inter-insertion distance (Fig. 2d,e), MLV showed the highest number of hotspots, followed by PB and Tol2, whereas SB showed the lowest number of hotspots (Fig. 2f, see Supplementary Table S5 for the number of hotspots and statistical comparison between vectors). Importantly, clusters of vector insertions in most hotspot regions derived from independent transfections (Supplementary Fig. S4). Therefore, we concluded that the local hopping feature of the transposons did not affect the determination of hotspot regions.
(a) Representative distribution of insertion sites on mouse chromosome 11. Horizontal stripes in the vertical bar indicate exon density. Insertion frequencies of MLV (red), PB (blue), Tol2 (green) and SB (yellow) are plotted on the left side of each vertical bar, and insertion frequencies of in silico control are shown on the right side. Red dotted lines indicate regions with poor insertion frequency for vectors and in silico controls. The data are presented in 1-Mbp windows. (b) Fraction of vector insertion by exon density. Genomic regions were divided into 500-kb bins and grouped into five ranks (lowest, low, mid, high, and highest) according to exon density. Insertion frequency was calculated from the observed data (left) and the in silico control data (right). (c) Pearson’s correlation coefficient (r) between exon density (total exon size in 500-kb window) and insertion density (number of inserts in 500-kb window). We compared the correlation coefficients of observed data to those of in silico control data using bootstrap method. All vectors showed significant increase of correlation coefficient (more inserts in exon rich regions) in observed data (P < 0.001). (d) Distribution of the distance between insertion sites. We sampled 4,000 insertions sites from each vector dataset 1,000 times by permutation and measured the distance between adjacent insertion sites. The X-axis shows bins of inter-insertion distance presented as log10 scale and the Y-axis indicates the number of cases in each bin. Mean and standard deviation (SD) are shown as log10 scale. Inter-insertion distances of 10-kb are indicated by a vertical line. Insertions to the left of this line are quantitated in (e). (e) Frequency of inter-insertion distances within 10-kb. (f) Hotspot insertion sites. Hotspots are defined as genomic regions fulfilling the following criteria: 2 hits within a 30-kb window (left), 3 hits within a 50-kb window (middle), or 4 or more hits within a 100-kb window (right). We sampled 4,000 insertion sites from each dataset 1,000 times and determined the mean number of hotspots as shown in the Y-axis. In (e,f), P-values were calculated by bootstrapping. *P < 0.001.
Because we observed insertion bias toward exon rich regions (Fig. 2a–c), we compared vector insertions by the features of genes near to insertion sites. First, we compared the insertion frequency inside RefSeq genes irrespective of their expression levels in ESCs. All vectors inserted inside genes with a similar frequency (Fig. 3a) including SB that showed minimum insertion bias in Fig. 2. However, substantial differences were observed when we classified genes by their expression levels in ESCs (Fig. 3b). MLV was highly biased to highly expressed genes, and PB showed a similar preference (Fig. 3b, see Supplementary Table S6 for statistical comparison between vectors). In contrast, gene expressions were weaker at Tol2 and SB insertion sites (Fig. 3b, Supplementary Table S6). The difference between PB and Tol2 was unexpected because the frequency of hotspots was similar between them (Fig. 2f, Supplementary Table S5). We also analysed the distribution of insertion sites relative to the transcribed region of the RefSeq genes (Fig. 3c). MLV, PB and Tol2 showed a preference for TSS, whereas SB insertion did not correlate with TSS and was slightly enriched throughout transcribed regions (Fig. 3c). We therefore conducted high-resolution analysis around TSS and revealed the bimodal distribution of MLV, PB, Tol2 insertions: the peak of the distribution was not exactly at the TSS but rather upstream and downstream of TSS (Fig. 3d). Notably, the insertion frequency of MLV at TSS was lower than for PB and Tol2 and was close to the basal level (Fig. 3d). These results indicate that the vector insertion profile was influenced by factors other than gene expression levels. These findings were not biased by G418 selection because similar results were obtained without G418 selection (Supplementary Table S2, Supplementary Fig. S5). We also confirmed that the Illumina GA2 analyses (Wt in Supplementary Table S3) gave similar results to the Roche GS FLX (Supplementary Fig. S6). Because genomic DNAs were fragmented by sonication in Illumina GA2 instead of restriction digestion, this also indicates that DNA fragmentation methods did not introduce bias to the analyses of vector insertion preference.
(a) Relative insertion frequency inside RefSeq genes. The Y-axis shows the ratio of the number of insertion sites inside RefSeq genes to that of the corresponding matched control. P-values were calculated by binomial statistics. *P < 0.001. (b) Insertion frequency relative to gene expression levels. The X-axis shows bins of increasing expression ranks in ESCs from lowest to highest. (c) Relative distance between TSS and vector insertion sites. The distance from TSS was divided by gene size and in silico control data was subtracted. TES, transcription end site. (d) Distribution of insertion sites ± 5-kb regions relative to TSS. In silico control data were subtracted from these values.
The results in Fig. 3 indicated that each vector was influenced by different factors such as gene expression levels and TSS, suggesting that the insertion of each vector is regulated by different mechanisms.
Distribution of vector insertions at histone modifications
Recent studies demonstrated that gene expression profiles are largely determined by histone modifications19. Whereas mRNA levels reflect the current state of gene expression, histone modification patterns provide further information such as the inducibility of genes upon stimulation or the underlying mechanisms of gene activities. Therefore, we considered that the analysis of histone modification might provide mechanistic insights into the correlation of vector insertions with gene expression profiles. We analysed the enhancer markers H3K4me1 and H3K27ac (Fig. 4a) and found that MLV insertion was highly enriched in the regions with both markers, followed by PB and Tol2. In contrast, SB showed no significant enrichment in regions with either marker. Recent studies revealed a new class of enhancer called a “super-enhancer”, which is a large cluster of enhancers characterized by an unusually high level of Mediator binding20. MLV and PB insertions were significantly enriched in both conventional enhancers and super-enhancers compared to in silico-generated matched controls (Fig. 4b, left and middle, see Supplementary Table S7 for statistical comparison between vectors), and the degree of enrichment in super-enhancers was greater than that in conventional enhancers (Fig. 4b, right). In contrast, the degree of enrichment of Tol2 and SB insertions in super-enhancers was similar to that in conventional enhancers (Fig. 4b, right). These results indicate a preference of MLV and PB for strong enhancers.
(a) Line plots of enhancer type histone modification in ± 5-kb regions relative to insertion sites. The panels show the mean density values after the subtraction of control values. (b) Enrichment of vector insertion sites at enhancers or super-enhancers. The number of insertion sites located inside enhancer or super-enhancer regions were compared to the expected number of insertion sites obtained from matched controls. P-values were calculated by binomial test and adjusted by FDR for multiple comparisons. *P < 10−4, **P < 10−14, ***P < 10−71. (c) Line plots of histone modifications representing transcriptional status in ± 5-kb regions relative to insertion sites. (d) Bar plots of histone marks relative to insertion sites. We used public datasets of histone modification-enriched regions to calculate the fraction of insertion sites located in each region as shown in the Y-axis (see Methods for details). P-values were calculated by binomial test and adjusted by FDR for multiple comparisons. *P < 10−5, **P < 10−8, ***P < 10−15, ****P < 10−21.
Next, we investigated the relationship of vector insertion with histone modifications that are highly associated with transcriptional status (H3K4me3, H3K36me3, and H3K27me3) (Fig. 4c,d). H3K4me3 is enriched at active TSS, H3K36me3 accumulates in transcribed regions, and H3K27me3 is a repressive mark introduced by the Polycomb repressive complex21. Substantial enrichment of H3K4me3 was observed for MLV and PB (Fig. 4c,d, see Supplementary Table S8 for statistical comparison between vectors). This result is consistent with the preference of MLV and PB for TSS regions (Fig. 3c,d). Accumulation of H3K36me3 was observed at the distal region of MLV and PB insertion sites (Fig. 4c). This can be explained by the insertion preference of MLV and PB near transcriptionally active TSSs. In contrast, H3K4me3 deposition was not observed at SB insertion sites (Fig. 4c,d). This result is consistent with the observation that SB insertion is not skewed to TSS (Fig. 3c,d). Similar results were obtained with and without G418 selection (Supplementary Fig. S5), indicating G418 selection did not introduce bias. H3K36me3 was not enriched at SB insertion sites (Fig. 4c), consistent with the weak relationship of SB insertion with gene expression (Fig. 3b). By contrast, Tol2 insertion sites exhibited a unique association pattern with histone modifications. Although H3K4me3 was accumulated at Tol2 insertion sites, no apparent accumulation was observed for H3K36me3 (Fig. 4c). This suggests that TSSs around Tol2 insertion are active for transcription initiation but inactive for transcription elongation. The most intriguing observation was the enrichment of Tol2 insertion sites at H3K27me3-modified regions (Fig. 4c,d). It has been reported that substantial fractions of genes are marked by both H3K4me3 and H3K27me3 in ESCs19,21. This bivalent modification is considered to represent a poised state of gene expression, which indicates that these genes are silent in undifferentiated ESCs and induced upon differentiation19,21. To investigate whether Tol2 insertion sites are enriched for bivalent modification of H3K4me3 and H3K27me3, we used a previously reported dataset of the bivalent regions19. Tol2 insertion was most highly enriched in bivalent regions among the four vectors (Fig. 4d, Supplementary Table S8), strongly suggesting that Tol2 has a preference for the transcriptionally poised chromatin state.
Insertion preference at developmentally regulated genes
Distinct distribution patterns of the four vectors relative to the status of histone modifications (Fig. 4) suggest that the target genes of each vector might exhibit different expression profiles during development. To address this, we conducted Gene Set Enrichment Analysis (GSEA)22 using a microarray dataset of ESCs and NPCs (Fig. 5a). Genes at the insertion hotspots were grouped as a gene set and rank-ordered by expression specificity in ESCs and NPCs (Fig. 5a). Gene sets frequently observed in MLV and PB insertion sites were enriched with ESC-specific genes (Fig. 5b). This result is consistent with the observation that both MLV and PB insertions were enriched in ESC-super-enhancer regions (Fig. 4b) that determine the cell identity of ESCs20,23. In contrast, gene sets frequently observed in Tol2 and SB insertion sites were enriched with NPC-specific genes (Fig. 5c), indicating that the insertion sites of Tol2 and SB are enriched with genes that are silent or weakly expressed in undifferentiated ESCs and induced upon differentiation into NPCs. Notably, this association was most strongly observed for Tol2. This result is consistent with the observation that Tol2 insertion sites are enriched with bivalent histone modification (Fig. 4d), which is a marker of the transcriptionally poised state and is often observed in inducible genes21. These results demonstrate that each vector prefers a distinct class of developmentally regulated genes.
Distinct distribution of insertion sites relative to open chromatin
Vector insertion profiles were highly correlated with gene expression levels (Figs 3b and 5). Because expressed genes are considered to be present in open chromatin regions, we investigated the correlation between insertion sites and DNase I hypersensitive sites (DNase I HSs). Surprisingly, distributions of DNase I HSs were different for all vectors (Fig. 6a). Although both MLV and PB preferred highly expressed genes (Figs 3b and 5), the distribution of DNase I HSs was bimodal in MLV, whereas a single peak was seen at the PB insertion site (Fig. 6a). Both Tol2 and SB showed weak association with ESC-expressed genes (Figs 3b and 5). However, a sharp narrow peak of DNase I HSs was observed at the Tol2 insertion site, whereas almost no correlation was seen in SB (Fig. 6a). These results indicate that open chromatin is not the sole determinant of vector insertion sites.
(a,b) Line plots of DNase I HSs (a) and the density of transcriptional regulatory proteins (b) in ± 5-kb regions relative to vector insertion sites. The panels show the means of density values after subtraction of control values. P-values were calculated by binomial test and adjusted by FDR for multiple comparisons. *P < 10−3, **P < 10−6, ***P < 10−10, ****P < 10−30. (c) Density map of regulatory proteins in ± 5-kb regions relative to the insertion sites of MLV (left) and PB (right). Each row represents one insertion site, and the insertion sites are located at the centre of these plots. We determined whether the insertion sites overlap with the regulatory protein binding sites. Areas overlapping or not with the binding sites, are shown in black or white, respectively. The insertion sites are sorted by the total width of peak regions of Med12 within ± 5-kb from the insertions site.
Distinct distribution of insertion sites relative to transcriptional regulators involved in 3D chromatin structures
Because open chromatin was only partially associated with insertion site preference, we investigated the relationship of insertion sites with 3D chromatin structures. Recent studies revealed the hierarchy of chromatin structure and its effect on gene expression24,25,26,27. Large megabase-sized chromatin loops, termed topologically associating domains (TADs), are formed by CTCF and Cohesin. Within TADs, there are smaller loops formed by Cohesin and Mediator, which contribute to enhancer–promoter interactions. Within enhancer–promoter loops, general transcription apparatus or cell-type specific transcription factors are clustered. Therefore, we analysed the relationship of insertion sites and DNA-binding sites of these regulatory proteins. The following proteins were analysed: general transcription apparatus (Pol2, TBP), Mediator (Med1, Med12), Cohesin (Smc1, Smc3, Nipbl), insulator protein (CTCF), enhancer binding protein (P300), ESC-specific transcription factors (Nanog, Oct4, Sox2) and Brd4. Brd4 is a member of the BET (bromodomain and extra-terminal domain) family. Brd2, Brd3 and Brd4 were recently reported to interact with retrovirus integrase and to recruit the retroviral preintegration complex to the insertion site28,29.
These analyses revealed a relationship of vector insertion with various factors involved in 3D chromatin structures (Fig. 6b, see Supplementary Table S9 for statistical comparison between vectors). PB insertion was highly enriched at the DNA-binding sites of Cohesin, Mediator, and CTCF, which play critical roles in the formation of anchor sites of TADs (Fig. 6b). PB was also highly enriched at the DNA-binding sites of general transcription apparatus and ESC-specific transcription factors (Fig. 6b), suggesting that PB-inserted chromatin loops are transcriptionally active. MLV insertion was also associated with general transcription apparatus, ESC-specific transcription factors, Cohesion, and Mediator (Fig. 6b). However, a marked difference was observed in CTCF: There was a sharp peak of CTCF-enrichment at the PB insertion site, whereas such a peak was not observed in MLV (Fig. 6b), suggesting MLV prefers smaller-size chromatin loops representing enhancer-promoter interactions24,25,26,27. Tol2 insertions were highly enriched at Cohesin (Smc1 and Smc3) and CTCF as in the case of PB. There was no difference between the enrichment at CTCF, Smc1, and Smc3 binding sites between PB and Tol2 (Fig. 6b, Supplementary Table S9, P-values were Smc1 = 0.160, Smc3 = 0.351, and CTCF = 0.549 by Fisher’s exact test). The result indicates insertion preference for the anchor sites of chromatin loops in PB and Tol2. In contrast, correlations of Tol2 with Mediator and ESC-specific transcription factors were weaker than those of PB (Fig. 6b, Supplementary Table S9, P-values comparing the difference in enrichment between Tol2 and PB were <10−9 for Med1, Med12, Nanog, Oct4, and Sox2 binding sites by Fisher’s exact test), suggesting that Tol2-inserted chromatin loops harbour transcriptionally poised or weakly expressed genes. This idea is consistent with the preference of Tol2 to bivalent histone modifications that are present at silent and inducible loci (Fig. 4d). Of note, SB showed almost no or only poor correlation with all chromatin states examined (Fig. 6b, Supplementary Table S9).
Interestingly, chromatin occupancy of regulatory proteins was highest at the insertion sites of PB and Tol2. In contrast, MLV showed a bimodal distribution pattern (Fig. 6b) as in DNase I HSs (Fig. 6a). This bimodal distribution was also observed in Brd4 (Fig. 6b), which is reported to be an interactor of retroviral integrase28,29. These results suggest that MLV is inserted at a distant location after being recruited by Brd4.
One of the intriguing observations in Fig. 6b is that most transcriptional regulatory proteins showed similar distribution patterns. This suggests that the chromatin binding sites of these proteins may form a cluster. To investigate this, we aligned ChIP-seq signals of the regulatory proteins side-by-side around 5-kb regions of each insertion site (Fig. 6c). All regulatory proteins showed a similar distribution pattern: bimodal distribution was observed in MLV, whereas the signals were strongest near the insertion sites in PB, indicating that the DNA-binding sites of the regulatory proteins were indeed clustered.
Perturbation of chromatin states and its effect on vector insertion profiles
The results described above suggest that chromatin states are major determinants of vector insertion profiles. To validate this idea, we perturbed the chromatin states of ESCs and investigated its effect on vector insertion distribution. Among various chromatin states analysed in this study, we focused on H3K27me3 because the insertion preference of Tol2 in H3K27me3-modified regions was one of the most notable and unexpected findings (Fig. 4c,d). The trimethylation of H3K27 is catalysed by Polycomb repressive complex 2 including Eed as an essential component30. We therefore introduced Tol2 or PB transposons into a homozygous Eed-mutant (Eedm/m) ESC line, which is present in our previously reported mutant ESC bank5. After confirming the loss of H3K27me3 modification in Eedm/m ESCs (Fig. 7a–c), we determined the distribution patterns of Tol2 and PB in Eedm/m ESCs using the Illumina platform (Supplementary Table S3) and compared them with those in wild-type (Wt) ESCs (Fig. 7d–f) with regards to chromatin states. As a reference for the genome-wide distribution pattern of histone modifications (H3K27me3 and H3K4me3), we used public ChIP-seq datasets from Wt ESCs19. As expected, homozygous knockout of Eed decreased the enrichment of Tol2 insertions in the regions corresponding to the H3K27me3 marks in the Wt genome (Fig. 7d,f, left, P = 0.0357 by Fisher’s exact test), which is consistent with the idea that the H3K27me3 mark is an important determinant of Tol2 insertion. In contrast, the frequency of PB insertion at H3K27me3-modified regions did not decrease in Eedm/m ESCs (Fig. 7d,f, left), supporting the specific effect of H3K27me3 on Tol2 insertion. Of note, this insertion site preference was not completely abolished in Eedm/m ESCs (Fig. 7d), indicating that H3K27me3 itself is not the direct target of Tol2 insertion. Other epigenetic modifications associated with H3K27me3 could remain and enhance Tol2 insertion in their corresponding regions even after the disruption of Eed. Unexpectedly, we observed significant reduction of Tol2 and PB insertions in the H3K4me3-modified regions in Eedm/m ESCs (Fig. 7e,f, middle), which was more evident in Tol2 than in PB. One possible explanation for this observation is that the homozygous knockout of Eed disrupted the H3K4me3 and H3K27me3 bivalent modification, which is more strongly associated with Tol2 insertion rather than with PB insertion (Fig. 4c,d). However, no significant reduction of Tol2 and PB enrichment was observed in the bivalent regions in Eedm/m ESCs (Fig. 7f, right). It has been reported that the elimination of H3K27me3-modification by Eed-knockout alters the expression levels of a large number of developmental regulators in mouse ESCs30. We therefore hypothesize that secondary or additional changes in chromatin states associated with the loss of H3K27me3 could also affect the insertion preferences of Tol2 and PB in the H3K4me3-modified regions.
(a) Disruption of Eed by gene trap. Eedm indicates mutant allele. For the gene trap vector, only relevant elements for gene disruption are presented. Wt, wild-type; m, mutation; SA, splice acceptor; hyg, hygromycin-resistance gene; pA, polyadenylation signal. (b) qRT-PCR of Eed expression for wild-type ESCs (Wt) and homozygous mutant ESCs (Eedm/m). Locations of the PCR primers are shown in (a). Error bars indicate standard deviation. (c) Immunostaining of H3K27me3. (d,e) Line plots of histone modifications in ± 5-kb regions relative to insertion sites. The panels show the means of density values after the subtraction of control values. (f) Bar plots of histone marks relative to insertion sites. We compared the number of insertion sites located within ± 2-kb from histone modification-enriched regions between Wt and Eedm/m ESCs by Fisher’s exact test and adjusted by FDR for multiple comparisons. In (d-f), public datasets of histone modification-enriched regions of Wt ESCs were used as in the case of Fig. 4c and d. P-values were calculated by Fisher’s exact test and adjusted by FDR for multiple comparisons.
These data from Eedm/m ESCs suggest that chromatin state affects vector insertion profiles. Based on the various chromatin states investigated in the present study, we summarized the characteristic features of each vector insertion in Fig. 8 (see Discussion for details). We also summarized the observations in the present study and previous reports in Supplementary Table S10 to clarify our novel findings.
Discussion
Vector insertion preference has been investigated extensively in previous studies (Supplementary Table 10) 17,31,32,33,34,35,36,37,38,39,40,41,42,43,44. The main difference between our study and previous studies is that we analysed four vectors systematically under the same experimental conditions. Furthermore, we interpreted our results based on various factors involved in the 3D organization of chromatin (Fig. 8).
We observed a distinct association between vector insertion preference and chromatin structural proteins, e.g., Cohesin and CTCF24,25,26,27 (Fig. 8). Cohesin is a ring form protein complex and cooperates with a DNA-binding protein CTCF to make a megabase-sized DNA loop called a topologically associating domain, TAD. There are smaller DNA loops within TADs that represents the promoter–enhancer interaction of embedded genes. Cohesin forms this smaller-size promoter–enhancer interacting loop without CTCF24,25,26,27. Both PB and MLV insertion sites showed a correlation with Cohesin (Fig. 6b). Considering the significant insertion preference of PB and MLV into TSS and enhancer regions (Figs 3c,d and 4a,b), it is highly likely that the correlation with Cohesin represents an insertion preference for the anchor areas of the promoter–enhancer loops (Fig. 8). However, correlation of PB with CTCF was much higher than MLV (Fig. 6b, Supplementary Table S9, P-value between PB and MLV = 2.41 × 10−18 by Fisher’s exact test), suggesting PB has a preference for the anchor areas of both promoter–enhancer loops and TADs, whereas MLV prefers promoter–enhancer loops (Fig. 8). This observation is consistent with the result that MLV had more hotspots than other vectors (Fig. 2f). A recent report indicated similarities between insertion site distributions of PB and MLV34. Our result is consistent with their report and further revealed a substantial difference between PB and MLV in the light of 3D chromatin structure.
Tol2 insertion correlated with both Cohesin and CTCF to a similar high level as observed for PB (Fig. 6b), suggesting that Tol2 prefers anchor areas of both promoter–enhancer loops and TADs (Fig. 8). However, Tol2 showed a weak correlation with Mediator and ESC-specific transcription factors (Fig. 6b). This result strongly suggests that Tol2-inserted loops are transcriptionally weak (Fig. 8). Transcriptionally active and inactive genes are often clustered in the genome, and TADs delineate the boundaries of the clusters25. It was reported that transcriptionally silent TADs are enriched by Polycomb repressive complexes25. Consistent with this idea, Tol2 insertion was associated with the bivalent modification of H3K4me3 and H3K27me3 (Fig. 4d). We speculate that PB and MLV prefer transcriptionally active chromatin loops, whereas Tol2 prefers transcriptionally weak chromatin loops (Fig. 8).
The results of Tol2 insertion preference need to be interpreted carefully. First, the preference of Tol2 for H3K27me3 in ESCs does not indicate insertion into heterochromatin regions. We speculate that the Tol2 preference for H3K27me3 is caused by coexisting modification with H3K4me3 in ESCs. Indeed, Tol2 insertion was reported to be inversely correlated with H3K27me3 modification in HeLa cells31. H3K27me3-modified regions are probably heterochromatinised in HeLa cells and antagonize Tol2 insertion. Second, Tol2 insertion is not restricted to bivalent regions because Tol2 also inserted into expressed genes although the expression levels were weaker than for PB and MLV (Fig. 3b).
Interestingly, most transcriptional regulators showed similar correlation patterns (Fig. 6b). This result is consistent with previous reports that a large protein complex of transcriptional regulators is present at the anchor site of TADs and promoter–enhancer loops24,25,26,27. These transcriptional regulators showed a binding peak at the insertion site of PB and Tol2, whereas the binding peak relative to the MLV insertion site was bimodal and several hundred bases away from the insertion site (Fig. 6b). Recent reports have demonstrated that Brd proteins interact with MLV integrase and define the insertion site28,29. However, our results indicated that the Brd4 binding peak was also bimodal similar to other transcriptional regulators and did not coincide with the MLV insertion site (Fig. 6b). We speculate that MLV integrase cannot replace the large protein complex at the anchor region and insert into adjacent less crowded regions. A recent report demonstrated that the PB transposase also interacts with Brd proteins34. It would be interesting to investigate whether the Tol2 transposase also interacts with Brd proteins because both PB and Tol2 correlated with Brd4 in our analysis (Fig. 6b).
To validate the role of chromatin states on vector insertion profiles, we investigated the distribution of vector insertion sites in Eed-homozygous mutant ESCs and demonstrated that vector insertion profiles changed in the absence of H3K27me3 mark (Fig. 7). To further assess the effect of chromatin states on vector insertion profiles, it would be interesting to induce redistribution of chromatin marks (not the loss of chromatin marks as in the case of Eed-homozygous mutant ESCs) and examine its effect on vector insertions. ESCs acquire a naive ground state when cultured in 2i media (serum-free medium with Gsk3/Mek inhibitors)45 and undergo substantial chromatin remodeling46,47,48. Chromatin states will also be altered when ESCs are differentiated into various cell lineages. Analysis of vector insertions in these culture conditions will provide further insights into the relationship between chromatin states and vector insertion.
Interestingly, SB insertion showed almost no relationship with any genomic and epigenomic features (Figs 2, 3, 4, 5, 6 and 8). Furthermore, SB insertion showed no correlation with DNase I HSs (Fig. 6a). This result suggests that SB can access tightly packed chromatin regions. This idea is consistent with our previous observation that SB transposase can efficiently excise the SB transposon from heterochromatin regions49,50. A previous study demonstrated that the SB insertion site was affected by sequence-dependent local DNA conformation32. This report together with our current results indicates the unique characteristics of SB when interacting with the host genome. It should be noted that SB transposase has been improved by extensive mutagenesis51. We utilized HSB52 in the present analyses. We speculate that other versions of SB transposases will also, if not all, have similar properties because a recent report demonstrated minimum insertion bias of the highly active SB100X transposase51 in human CD4+ T cells compared to MLV, PB and HIV34.
The current results provide valuable information for the application of each vector in various experimental settings. All vectors have been used as a mutagen for insertional mutagenesis4,5,11,12. The current results indicate that each vector targets distinct sets of genes suggesting that the combination of different vectors will expand the number of target genes. PB and MLV effectively target highly expressed genes, whereas Tol2 and SB would be appropriate for targeting moderately expressed or silent genes. This idea is supported by our previous experience in the construction of a mouse mutant ESC library in which the coverage of the mutation was expanded by the combination of retrovirus and Tol25. A neutral feature of SB insertion will be essential if unbiased vector insertion is required. We previously utilized an enhancer trap-type SB vector for the saturated tagging of cis-regulatory elements of the Pax1 gene in mouse ESCs53. Although expression of the Pax1 gene is extremely low in ESCs, cis-regulatory elements were successfully tagged by the SB vector, indicating the advantage of the neutral feature of SB insertion.
Retroviral vectors are the most widely used vector for gene therapy when long-term gene expression is needed. Non-viral vectors are emerging as a promising alternative because of the simplicity of clinical grade sample preparation and their cost-effectiveness compared with viral vectors1. Indeed, all three transposon vectors analysed in the present study have been used successfully for the introduction of a chimeric antigen receptor into human T cells to redirect their antigen specificity toward cancer cells1,54,55. One of the major factors for the selection of gene therapy vectors is their insertion site preference. A recent report of clonal expansion of HIV-infected lymphocytes in human patients suggested that the adverse effect of vector insertion needs to be carefully monitored in gene therapy56. The safety issue of vector insertion has been addressed mainly by the insertion site proximity to transcriptional units1. The relative location of the vector insertion site to regulatory regions analysed in this study will help further assess safety during vector selection.
In summary, the distinct features of vector insertion profiles revealed in this study provide valuable information for the application of transposon and retroviral vectors for use in genetic engineering and gene therapy.
Methods
Vector construction
To construct the retroviral vector pCMT-PGKneo, a PacI-XhoI fragment containing the floxed PGK-neo-pA cassette was excised from pMulti-ND-1.057 and cloned into the PacI-XhoI sites of pCMT-SAhygpA5.
The PB transposon vector pPB-PGKneo was constructed as follows. First, the 3′ terminal repeat of the PB transposon was amplified by PCR from pPB-SB-SA-bgeo58 using primers PB-P1 (CGACTCACTATAGGGCGAATTGGAGCTAGG) and PB-P5 (GCCGATATCAGATCTCTCGAGGAATTCGTTTAAACGGGCCCTTTGTTACTTTATAGAAGAAATTTTGAG). The PCR product was digested with AscI and EcoRV and ligated to the AscI-EcoRV fragment of pPB-SB-SA-bgeo containing the 5′ terminal repeat of the PB transposon and the plasmid backbone, resulting in PB-MCS-P5. Next, the PmeI–XhoI fragment of the pMulti-ND-1.0 containing the floxed PGK-neo-pA cassette was inserted into the PmeI-XhoI sites of the pPB-MCS-P5, resulting in pPB-PGKneo.
To construct the Tol2 transposon vector pTL2-PGKneo, the PacI (blunt)-XhoI fragment containing the floxed PGK-neo-pA cassette was released from pMulti-ND-1.0 and cloned into the BglII (blunt)-XhoI sites of pT2AL200R150G59.
To construct the SB transposon vector pSB-PT2-PGKneo, the PmeI-NotI fragment containing the floxed PGK-neo-pA cassette was excised from pMulti-ND-1.0 and cloned into the EcoRV-NotI sites of pT2/HB14.
To construct the PB transposase expression vector pCAGGS-mPB, an EcoRI linker was inserted into the unique XhoI site of mPB58. The PB transposase-containing fragment was then released by EcoRI digestion and cloned into the EcoRI site of pCAGGS-EGFP60, resulting in pCAGGS-mPB.
To construct the SB transposase expression vector pCAGGS-HSB2, an EcoRI linker was inserted into the unique BamHI site of pCMV-HSB252. The SB transposase-containing fragment was then released by EcoRI digestion and cloned into the EcoRI site of pCAGGS-EGFP, resulting in pCAGGS-HSB2.
Cell culture, transfection of transposons and infection of retrovirus
The V6.5 F1 hybrid mouse ESC line (C57BL/6 × 129S4/SvJae)61 was cultured in Knockout Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 20% foetal bovine serum, 0.1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml L-glutamine and 1,000 U/ml leukemia inhibitory factor (EMD Millipore, Darmstadt, Germany) on mitomycin C-treated mouse embryonic fibroblast (MEF) feeder cells. Eed-homozygous mutant ESCs were generated from vdR2-4, a derivative of V6.5, and the details for the method of derivation was described previously5.
We conducted three independent transfections for each transposon. On day 0, 2 × 106 ESCs were transfected with 10 μg of the transposon vector and 10 μg of the transposase expression vector using 120 μl of TransFast reagent (Promega, Madison, WI, USA), and plated onto one 6-cm dish. The following combinations of transposon and transposase vectors were utilized: pPB-PGKneo and pCAGGS-mPB, pTL2-PGKneo and pCAGGS-T2TP9, and pSB-PT2-PGKneo and pCAGGS-HSB2. In case ESCs were selected by G418, each cell population was passaged to two 10-cm dishes on day 2 and cultured in the presence or absence of G418 (150 μg/ml). ESCs were passaged at a ratio of 1:2 when they reached confluence. On day 9, MEFs were removed by plating ESCs on a gelatin-coated dish for 30 min and unattached cells were transferred onto a fresh dish. On day 11, ESCs were lysed and genomic DNAs were extracted. In case ESCs were not selected by G418, MEFs were removed on day 2 and ESCs were lysed on day 3. To infect ESCs with the MLV vector, Plat-E packaging cells62 were transfected with the MLV vector using Lipofectamine 2000 (Thermo Fisher Scientific). Two days later, viral supernatants were filtered through a 0.45-μm pore-size membrane and used to infect ESCs. The culture protocol following infection was same as that used for the transfection of transposon vectors.
Quantitative RT-PCR
Total RNAs were extracted with TRIzol (Thermo Fisher Scientific) and treated with TURBO DNA-free kit (Thermo Fisher Scientific) to remove contaminating genomic DNAs. Eight hundreds ng of total RNAs were reverse transcribed with SuperScript IV (Thermo Fisher Scientific) using random primers (Promega). Expression levels of mRNAs encoding Eed and β-actin were quantified by real-time PCR using LightCycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Mannheim, Germany) and a LightCycler (Roche Diagnostics). The primer pairs were 5′-AACATGTCCGAGAGGGAAGTGT-3′ and 5′-TATTTGCATTTCTTTGACTTCCATT-3′ for Eed and 5′-CAGGGTGTGATGGTGGGAATGGGTCAGAAG-3′ and 5′-TACGTACATGGCTGGGGTGTTGAAGGTCTC-3′ for β-actin. The amplification conditions for Eed were 95 °C for 10 min for one cycle, followed by 40 cycles of 95 °C denaturation for 10 s, 52 °C annealing for 5 s and 72 °C extension for 10 s. The same PCR conditions were used for β-actin except that the annealing temperature was 55 °C and the extension time was 20 s. The quantity of each transcript was measured from a standard curve, and the amounts of Eed transcript were normalized to β-actin transcript levels.
Immunostaining
Cells grown on coverslips were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in 250 mM HEPES (pH 7.4) for 10 min, permeabilized with 1% Triton-X (Nacalai Tesque, Kyoto, Japan) in phosphate-buffered saline (PBS) for 20 min, and subjected to blocking with 100% Blocking One-P (Nacalai Tesque) for 20 min at room temperature. Cells were incubated in 2 μg/ml Cy3-conjugated anti-H3K27me3 antibody (CMA323)63 and 10 ng/ml DAPI (Thermo Fisher Scientific) in PBS containing 10% Blocking One-P and 0.5% Triton-X for 2 h at room temperature. After washing three times with PBS, coverslips were mounted using Prolong-Gold (Thermo Fisher Scientific).
Library construction for the sequencing of vector insertion sites
Oligonucleotide sequences of linker DNA, PCR primers and sequencing primers are shown in Supplementary Table S11. For sequencing with Roche GS FLX, we digested 10 μg of genomic DNAs with HaeIII and ligated it with splinkerette linker DNA64. In case ESCs were not selected with G418, it is highly likely that transfected plasmid DNAs were co-purified with genomic DNAs because genomic DNAs were extracted only 3 days after transfection. We therefore digested linker-ligated DNAs with DpnI that cleaves Dam-methylated GATC site to avoid PCR-amplification of plasmid-derived DNAs. The junction between the vector DNA and flanking genomic region was amplified by nested PCR using vector-specific primers and linker-specific primers. To avoid reduction of PCR-amplification efficiency by overloading template DNA per reaction, we divided 10 μg of the splinkerette-ligated DNA into 8 tubes and conducted the first PCR reaction separately. The first PCR products were pooled from 8 tubes into one tube, mixed well, and an aliquot of the first PCR product was used as a template for the second PCR reaction. We observed that amplification of the transposon vector backbone derived from transposase-independent vector insertions markedly inhibited amplification of the transposon-inserted genomic regions. We also observed amplification of the internal region of the MLV vector because the MLV-specific primers anneal to both upstream and downstream LTRs. To avoid amplification of the vector backbone of the transposon and the MLV-internal regions, we cleaved splinkerette-ligated DNA and the first PCR products using the following enzymes prior to PCR reaction: PvuII for MLV, XbaI for Tol2, DraI and PflMI for PB, and BamHI for SB. PCR products were size-fractionated by agarose gel electrophoresis, and 240–800-bp fragments were purified and sequenced by Roche GS FLX. For sequencing by Illumina GA2, we fragmented 10 μg genomic DNAs by ultrasound using Covaris S220 (Covaris, Woburn, MA, USA), blunt-ended, and ligated splinkerette linker DNA. Linker-ligated DNAs were digested with DpnI to avoid PCR-amplification of transfected plasmid DNAs. The first PCR products were purified with streptavidin-coupled Dynabeads (Thermo Fisher Scientific) according to the manufacture’s protocol, and an aliquot of the purified DNA was used as a template for the second PCR reaction. PCR products were size-fractionated (300–400-bp) by agarose gel electrophoresis and 76-bp regions were sequenced at each end by Illumina GA2.
Detection of insertion sites from Roche GS FLX sequence reads
The insertion sites of four vector types were determined by aligning the flanking sequence of the vector-tagging site against a mouse reference genome. Briefly, we removed the vector sequence from Roche GS FLX sequence reads using cross_match (http://www.phrap.org), and these trimmed reads with read length >25 nt were aligned against the mouse genome assembly (UCSC mm8) using BLAT65. Reads aligned to the reference genome with >90% identity and >90% coverage were extracted as candidate insertion loci. We confirmed the presence of specific motifs (such as TATA site for PB and TA site for SB) at the vector insertion sites. Reads with multiple alignment results were excluded from the analysis. Genomic coordinates of the insertion sites are deposited in the DDBJ database under the accession number DRA004513 (Analysis: DRZ007718 - DRZ007723).
Detection of insertion sites from Illumina GA2 sequence reads
Insertion sites of Tol2 and PB were determined by aligning the flanking sequence of the vector region against the mouse reference genome. We trimmed off the vector sequences and linker sequences from Illumina GA2 sequence reads using cutadapt version 1.9.166. For Tol2, reads with sequence mean base quality >30 were selected using Trimmomatic version 0.3367. This process was not conducted for PB because the presence of the consensus target sequence at the insertion sites could be used for sequence-quality check as described later. We discarded <25 bp reads to achieve high quality mapping. We used bwa-backtrack version 0.7.1368 to align the sequence reads against the mm8 reference assembly. We used ‘bwa aln’ and ‘bwa sampe -a 600’ options, and obtained chromosomal coordinates of the integration sites. We identified the reads mapped in proper pairs and uniquely aligned to the genome with high-mapping quality (>30) using samtools version 1.369 (‘samtools view -f 66 -F 256’ option and awk command). For PB, we identified the reads with the consensus sequence of PB insertion. According to our experimental protocol, most of the insertion site sequences should be duplicated by PCR. Therefore, we used alignments supported by two or more reads using BEDTools version v2.16.270 ‘genomecov command’, resulting in the final dataset of vector insertion sites. Genomic coordinates of the insertion sites are deposited in the DDBJ database under the accession number DRA002594 (Analysis: DRZ007730, DRZ007732, DRZ007734, DRZ007736).
Control insertion site
To determine the characteristics of genomic distribution of the insertion sites, we created size-adjusted in silico control insertions71. For the Roche GS FLX sequencing datasets, we created control insertion sites by checking three criteria: distance from enzyme cutting sites, distance from insert motif (SB = TA, PB = TTAA), and presence/absence of secondary enzyme cut sites. First, we determined the enzyme cut sites (HaeIII) on the mouse reference genome and randomly selected these positions as in silico enzyme cut sites. We created the insertion sites with matched fragment size and matched strand directionality relative to experimentally obtained vector insertion sites. Then, we selected the nucleotide position of specific motifs for vectors with specific insertion motif sequences. The difference of control fragment size from the observed fragment size must be less than 20-bp. Finally, we removed insertion sites containing secondary enzyme cut sites within its fragment (PvuII for MLV, XbaI for Tol2, DraI and PflMI for PB, and BamHI for SB).
For the Illumina GA2 sequence dataset, we did not use the restriction enzymes for the genomic DNA fragmentation, and thus we did not apply the steps for enzyme cut sites. Specifically, for PB, we determined all genomic positions of the consensus sequence of PB insertion (TTAA) in the mm8 reference genome using Bowtie version 1.1.272 ‘bowtie -a -v 0’ and randomly selected these positions. For Tol2, we randomly selected genomic positions from the mm8 reference genome using BEDTools version v2.16.270 ‘random’ command because Tol2 does not have a consensus sequence for insertion. We then generated control insertion fragments having matched fragment sizes relative to the experimental dataset.
For both Roche GS FLX sequence dataset and Illumina GA2 dataset, we tested the mappability of these control insertion sites by aligning them using BWA (version 0.7.13). We aligned in silico matched control reads against the mm8 reference genome using bwa ‘aln’ and ‘samse’ with default setting, and identified the ones that were aligned to the predicted position and had high mapping quality (>30). We repeated this process 1,000 times, and obtained 1,000 datasets of in silico control insertion sites. Genomic coordinates of the matched control insertion sites for the Roche GS FLX and the Illumina GA2 sequence datasets are deposited in the DDBJ database under the accession numbers DRA004513 (Analysis: DRZ007745 - DRZ007750) and DRA002594 (Analysis: DRZ007741 - DRZ007744), respectively.
Hotspot insertion site analysis
To compare the number of common insertion sites between different vectors with different numbers of vector insertions, we sampled insertion sites from both the observed and in silico control datasets by permutation. Specifically, we sampled 4,000 insertion sites from each vector dataset and its control dataset, and then we counted the number of 2 hit (2 insertions within 30-kb), 3 hit (3 insertions within 50-kb), and 4 hit (4 or more insertions within 100-kb) hotspot insertion loci. We repeated this random sampling from each dataset 1,000 times and calculated the mean of 2 hit, 3 hit, and 4 hit loci. We also performed the same hotspot insertion site analysis for the matched control datasets.
Genome-wide distribution of insertions sites
To visualize the genome-wide distribution of insertion sites, we sampled 4,000 insertion sites from each vector dataset by permutation. We repeated this sampling 1,000 times, and we calculated the mean count of insertion sites in every 1-Mbp window. We performed this analysis for both vector inserts and their matched control dataset.
Enrichment of insertion sites in exon-rich regions
We tested whether the insertion sites were enriched in exon-rich regions by dividing the genome into 500-kb bins (5,263 bins). First, we calculated the total size of exons in each bin and calculated the exon density (total exon size bp/500-kb). Then, we ranked these bins into five equal sized groups (exon density: lowest, low, mid, high, and highest). We determined the number of insertion sites located in each group for observed insertion sites and control insertion sites. The fractions of observed insertion sites located in bins with the highest exon density ranged from 38% (SB) to 54% (MLV). The fraction of control insertion sites located in bins with the highest exon density ranged from 22% to 26%.
Inter-insertion distance calculation
We determined how closely insertion sites were clustered in each vector dataset by measuring the distance between insertion sites. Briefly, we sampled 4,000 insertions sites from each vector dataset 1,000 times by permutation. For each sampled dataset, we sorted the insertion sites by their chromosome coordinates. We measured the distance between (i + 1)th insertions site and ith insertion site as the inter-insertion distance. We calculated the descriptive statistics such as means and standard deviation of log10 transformed inter-insertion distance.
Gene expression ranking and insertion sites
To compare the gene expression level with the frequency of insertion sites, we used microarray gene expression data from mouse ESCs19. We sorted genes based on the expression levels and divided them into eight equal sized bins. For example, the top ranked bin (bin #1) contained genes with the top 12.5 percentile expression levels. We counted the number of insertions located within 50-kb from genes and calculated the frequency of insertions in each bin using the following equation: (number of insertion in genes in a given bin)/(total number of insertion in all bins). We plotted the ratio of the observed rate/control rate. We performed similar analyses for insertion sites located inside genes.
Genes and transcription start site analysis
We determined the relative location of various insertion sites to known genes using the RefSeq database. We compared our insertion sites to the NCBI RefSeq database and determined the fraction of insertion events located inside the RefSeq genes. The rate of in-gene insertion events in observed cases was divided by the rate of in-gene insertion events in in silico matched control cases. We also determined the frequency of insertion events relative to transcription start sites (TSSs) as reported by the RefSeq database. We used two approaches, gene-size scaled bins and 100-bp bin. In the first approach, we divided the insertion distances from the nearest TSSs by the size of targeted genes. In the second approach, we divided ± 5-kb TSS regions into 100-bp window bins, and we counted the number of cases inserted into each bin. The counts were divided by the total number of insertion events.
Gene Set Enrichment Analysis
We performed Gene Set Enrichment Analysis22 using genes with frequent vector insertion sites. First, we collected genes with 3, 4, 5 or more insertion sites within ± 50-kb window for each vector dataset. Then, we compared the enrichment of these genes in gene expression patterns between two cell types, ESCs and neural progenitor cells (NPCs), using the microarray gene expression dataset reported by Mikkelsen et al. (GEO: GSM198062-GSM1198067)19. We used the software developed at the Broad Institute22 for analysis and used the normalized enrichment score (NES) as an indicator for the enrichment of frequently inserted genes.
Relationship between insertion sites and distribution patterns of transcription regulators, histone modifications, DNase I hypersensitive sites and enhancer regions
We compared the distribution patterns of transcription regulators and histone modifications around vector insertion sites using the ChIP-seq dataset. To analyse the relationship between insertion sites and histone modifications, we used the ChIP-seq data from ESCs such as H3K4me3, H3K27me3, and H3K36me3 as reported by Mikkelsen et al. (NCBI GEO GSE12241)19. Specifically, we determined the mean ChIP-seq density values every 25-bp throughout ± 5-kb regions around the vector insertion sites (400 data points for each insertion site). We also used previously reported datasets of the histone modification-enriched regions (Hidden Markov Model19) to calculate the overlapping regions between H3K4me3 and H3K27me3. Furthermore, we determined the relationship between insertion sites and transcription regulators using the previously reported ChIP-seq dataset (GSM937540 for Brd4, GSM594600 and GSM594601 for P30073, and GSE2256226 for other transcription regulators). To visualize the distribution of transcription factor binding sites around insertion site, we calculated the relative distances to peak regions of transcription factor binding sites from each insertion sites within a ± 5-kb window. We analysed the correlation between insertion sites and DNase I hypersensitive sites using the ENCODE dataset of mouse ESCs on UCSC genome browser (GSM1014154). DNaseI sensitive zones were identified using the HotSpot algorithm74. Because the ENCODE dataset was based on the mouse mm9 genome assembly, we converted the coordinate of insertion sites based on mm8 genome assembly to the coordinates in mm9 genome assembly using the coordinate conversion tool, liftOver, from UCSC. We analysed the association between insertion sites and enhancers or super-enhancers by counting the number of insertion sites located inside these regions. We used the coordinates of enhancer and super-enhancer regions in mouse ESCs from Whyte et al.20. We determined the enrichment of enhancers in the observed insertions compared to those of the matched control insertions.
Statistical Analysis
We performed statistical comparisons of the numbers of insertion sites located in genes, histone-modified regions, and the binding sites of transcriptional regulators between observed insertion sites and control insertion sites using binomial statistics. We estimated the probability of success using control datasets and calculated the significance. We applied FDR multiple testing correction to P-values. For the comparison of the insertion preference between different vectors or between Wt and Eedm/m ESCs, we used Fisher’s exact test. For example, we compared the number of insertion sites located inside of ChIP-seq peaks for transcription factor binding sites between MLV and PB. We performed the comparison for six combinations (among four vectors) and adjusted the P-values for multiple testing using the FDR method. To compare sampled datasets, we used a bootstrap approach36. Specifically, we compared the number of hotspots and inter-insertion distances within each vector (observed vs. control) and between different vectors by sampling 4,000 insertion sites repeatedly. We created fragment-size matched and enzyme restriction site matched control insertion sites for each vector 1,000 times. We counted the number of control datasets with a mean value more extreme than the value observed in the experimental data 1,000 times. For example, we counted the number of insertion sites with <10 kb inter-insertion distance in the experimental dataset and in the matched control dataset. We counted the number of control sets with more insertion sites located inside RefSeq genes than the corresponding experimental dataset. The sum divided by 1,000 was the P-value, and this was adjusted for multiple testing using the FDR method. The significance threshold was P = 0.05.
Southern blot analysis
Genomic DNAs of five G418-resistant and five G418-sensitive clones were digested by HindIII (MLV, PB, Tol2) or BglII (SB), separated by agarose gel, transferred to a nylon membrane, and hybridized with the neo probe using the standard protocol.
Accession codes:
Raw sequence reads, and genomic coordinates (mm8 assembly) for vector insertion sites and matched controls are deposited in the DDBJ database. Accession numbers are as follows.Roche FLX
-
Sequence dataset: DRA004513.
-
Genomic coordinates for vector insertion sites: DRA004513/Analysis: DRZ007718 (Tol2, +G418), DRZ007719 (MLV, +G418), DRZ007720 (PB, +G418), DRZ007721 (SB, +G418), DRZ007722 (MLV, −G418), DRZ007723 (PB, −G418).
-
Genomic coordinates for matched controls: DRA004513/Analysis: DRZ007745 (Tol2, +G418), DRZ007746 (MLV, +G418), DRZ007747 (PB, +G418), DRZ007748 (SB, +G418), DRZ007749 (MLV, −G418), DRZ007750 (PB, −G418).
Illumina GA2
-
Sequence dataset: DRX021632 (Wt ESCs, Tol2), DRX021634 (Eedm/m ESCs, Tol2), DRX021636 (Wt ESCs, PB), DRX021638 (Eedm/m ESCs, PB).
-
Genomic coordinates for vector insertion sites: DRA002594/Analysis: DRZ007730 (Wt ESCs, Tol2), DRZ007732 (Eedm/m ESCs, Tol2), DRZ007734 (Wt ESCs, PB), DRZ007736 (Eedm/m ESCs, PB).
-
Genomic coordinates for matched controls: DRA002594/Analysis: DRZ007741 (Wt ESCs, Tol2), DRZ007742 (Eedm/m ESCs, Tol2), DRZ007743 (Wt ESCs, PB), DRZ007744 (Eedm/m ESCs, PB).
Additional Information
How to cite this article: Yoshida, J. et al. Chromatin states shape insertion profiles of the piggyBac, Tol2 and Sleeping Beauty transposons and murine leukemia virus. Sci. Rep. 7, 43613; doi: 10.1038/srep43613 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hackett, P. B., Largaespada, D. A., Switzer, K. C. & Cooper, L. J. N. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl. Res. 161, 265–283 (2013).
Moriarity, B. S. & Largaespada, D. A. Sleeping Beauty transposon insertional mutagenesis based mouse models for cancer gene discovery. Curr. Opin. Genet. Dev. 30, 66–72 (2015).
Copeland, N. G. & Jenkins, N. A. Harnessing transposons for cancer gene discovery. Nat. Rev. Cancer 10, 696–706 (2010).
Rad, R. et al. PiggyBac transposon mutagenesis: a tool for cancer gene discovery in mice. Science 330, 1104–1107 (2010).
Horie, K. et al. A homozygous mutant embryonic stem cell bank applicable for phenotype-driven genetic screening. Nat. Methods 8, 1071–1077 (2011).
Huang, Y. et al. Isolation of homozygous mutant mouse embryonic stem cells using a dual selection system. Nucleic Acids Res. 40, e21 (2012).
Ivics, Z., Hackett, P. B., Plasterk, R. H. A. & Izsvák, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510 (1997).
Ding, S. et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 (2005).
Kawakami, K. & Noda, T. Transposition of the Tol2 element, an Ac-like element from the Japanese medaka fish Oryzias latipes, in mouse embryonic stem cells. Genetics 166, 895–899 (2004).
Horie, K. et al. Characterization of Sleeping Beauty transposition and its application to genetic screening in mice. Mol. Cell. Biol. 23, 9189–9207 (2003).
Keng, V. W. et al. Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system. Nat. Methods 2, 763–769 (2005).
Horie, K. et al. Efficient chromosomal transposition of a Tc1/mariner- like transposon Sleeping Beauty in mice. Proc. Natl. Acad. Sci. USA 98, 9191–9196 (2001).
Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 (1991).
Cui, Z., Geurts, A. M., Liu, G., Kaufman, C. D. & Hackett, P. B. Structure–function analysis of the inverted terminal repeats of the Sleeping Beauty transposon. J. Mol. Biol. 318, 1221–1235 (2002).
Dupuy, A. J., Fritz, S. & Largaespada, D. A. Transposition and gene disruption in the male germline of the mouse. Genesis 30, 82–88 (2001).
Luo, G., Ivics, Z., Izsvák, Z. & Bradley, A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 95, 10769–10773 (1998).
Li, M. A. et al. The piggyBac transposon displays local and distant reintegration preferences and can cause mutations at noncanonical integration sites. Mol. Cell. Biol. 33, 1317–1330 (2013).
Keng, V. W. et al. Efficient transposition of Tol2 in the mouse germline. Genetics 183, 1565–1573 (2009).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Whyte, W. A. et al. Master Transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Phillips-Cremins, J. E. et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153, 1281–1295 (2013).
Dowen, J. M. et al. Control of cell Identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014).
Kagey, M. H. et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467, 430–435 (2010).
Bonora, G., Plath, K. & Denholtz, M. A mechanistic link between gene regulation and genome architecture in mammalian development. Curr. Opin. Genet. Dev. 27, 92–101 (2014).
Sharma, A. et al. BET proteins promote efficient murine leukemia virus integration at transcription start sites. Proc. Natl. Acad. Sci. USA 110, 12036–12041 (2013).
De Rijck, J. et al. The BET family of proteins targets moloney murine leukemia virus integration near transcription start sites. Cell Reports 5, 886–894 (2013).
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006).
Grabundzija, I. et al. Comparative Analysis of transposable element vector systems in human cells. Mol. Ther. 18, 1200–1209 (2010).
Liu, G. et al. Target-site preferences of Sleeping Beauty transposons. J. Mol. Biol. 346, 161–173 (2005).
de Jong, J. et al. Chromatin landscapes of retroviral and transposon integration profiles. PLoS Genet. 10, e1004250 (2014).
Gogol-Döring, A. et al. Genome-wide profiling reveals remarkable parallels between insertion site selection properties of the MLV retrovirus and the piggyBac transposon in primary human CD4+ T Cells. Mol. Ther. 24, 592–606 (2016).
Vrljicak, P. et al. Genome-wide analysis of transposon and retroviral insertions reveals preferential integrations in regions of DNA flexibility. G3 (Bethesda) 6, 805–817 (2016).
LaFave, M. C. et al. MLV integration site selection is driven by strong enhancers and active promoters. Nucleic Acids Res. 42, 4257–4269 (2014).
De Ravin, S. S. et al. Enhancers are major targets for murine leukemia virus vector integration. J. Virol. 88, 4504–4513 (2014).
Huang, X. et al. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2,and PiggyBac transposons in human primary T cells. Mol. Ther. 18, 1803–1813 (2010).
Turchiano, G. et al. Genomic analysis of Sleeping Beauty transposon integration in human somatic cells. PLoS One 9, e112712 (2014).
Campos-Sa ́nchez, R., Kapusta, A., Feschotte, C., Chiaromonte, F. & Makova, K. D. Genomic landscape of human, bat, and ex vivo DNA transposon integrations. Mol. Biol. Evol. 31, 1816–1832 (2014).
Larue, R. C. et al. Bimodal high-affinity association of Brd4 with murine leukemia virus integrase and mononucleosomes. Nucleic Acids Res. 42, 4868–4881 (2014).
Wu, X., Li, Y., Crise, B. & Burgess, S. M. Transcription start regions in the human genome are favored targets for MLV integration. Science 300, 1749–1751 (2003).
Mitchell, R. S. et al. Retroviral DNA integration:ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2, e234 (2004).
Hackett, C. S., Geurts, A. M. & Hackett, P. B. Predicting preferential DNA vector insertion sites: implications for functional genomics and gene therapy. Genome Biol. 8, S12 (2007).
Ying, Q.-L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Marks, H. et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590–604 (2012).
Marks, H. & Stunnenberg, H. G. Transcription regulation and chromatin structure in the pluripotent ground state. Biochim. Biophys. Acta 1839, 129–137 (2014).
Galonska, C., Ziller, M. J., Karnik, R. & Meissner, A. Ground state conditions induce rapid reorganization of core pluripotency factor binding before global epigenetic reprogramming. Stem Cell 17, 462–470 (2015).
Yusa, K., Takeda, J. & Horie, K. Enhancement of Sleeping Beauty transposition by CpG methylation: possible role of heterochromatin formation. Mol. Cell. Biol. 24, 4004–4018 (2004).
Ikeda, R. et al. Sleeping Beauty transposase has an affinity for heterochromatin conformation. Mol. Cell. Biol. 27, 1665–1676 (2007).
Mátés, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 (2009).
Tolar, J. et al. Real-time imaging of stem cells following transgenesis by transposition. Mol. Ther. 12, 42–48 (2005).
Kokubu, C. et al. A transposon-based chromosomal engineering method to survey a large cis-regulatory landscape in mice. Nat. Genet. 41, 946–952 (2009).
Dolnikov, A. et al. Antileukemic potency of CD19-specific T cells against chemoresistant pediatric acute lymphoblastic leukemia. Exp. Hematol. 43, 1001–1014.e5 (2015).
Tsukahara, T. et al. The Tol2 transposon system mediates the genetic engineering of T-cells with CD19-specific chimeric antigen receptors for B-cell malignancies. Gene Ther. 22, 209–215 (2015).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014).
Inoue, N., Ikawa, M., Isotani, A. & Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234–238 (2005).
Cadinanos, J. & Bradley, A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 35, e87 (2007).
Urasaki, A., Morvan, G. & Kawakami, K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174, 639–649 (2006).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS letters 407, 313–319 (1997).
Eggan, K. et al. Hybrid vigor, fetal overgrowth, and viability of micederived by nuclear cloning and tetraploid embryo complementation. Proc. Natl. Acad. Sci. USA 98, 6209–6214 (2001).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000).
Hayashi-Takanaka, Y. et al. Tracking epigenetic histone modifications in single cells using Fab-based live endogenous modification labeling. Nucleic Acids Res. 39, 6475–6488 (2011).
Devon, R. S., Porteous, D. J. & Brookes, A. J. Splinkerettes-improved vectorettes for greater efficiency in PCR walking. Nucleic Acids Res. 23, 1644–1645 (2005).
Kent, W. J. BLAT-The BLAST-Like Alignment Tool. Genome Res. 12, 656–664 (2002).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2124 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Ciuffi, A. et al. Methods for integration site distribution analyses in animal cell genomes. Methods 47, 261–268 (2009).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 107, 21931–21936 (2010).
John, S. et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat. Genet. 43, 264–268 (2011).
Acknowledgements
We thank K. Kawakami for providing the Tol2 vectors, A. Bradley for the PB vectors, P. B. Hackett, Z. Ivics, and Z. Izsvák for the SB vectors, H. Kimura for ani-H3K27me3 antibody, X. Xu, H. Ozaki and I. Nikaido for assisting with bioinformatics analyses, and V. W. Keng for proofreading the manuscript. Computational support was mostly provided by Ohio Supercomputer Center (KA #PAS0425). Some of the computations were performed on the National NIG supercomputer at ROIS National Institute of Genetics. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 22116509, 24116709 and 221S0002 to C. K.) and Precursory Research for Embryonic Science and Technology (PRESTO) program from the Japan Science and Technology Agency (to K.H.).
Author information
Authors and Affiliations
Contributions
J.Y. and K.H. conceived the study. J.Y. and K.H. performed all experiments with the assistance of C.K. in sample preparation for DNA sequencing. K.A. and R.M. conducted bioinformatics analysis. J.Y., K.A. and K.H. wrote the paper with input from C.K. and J.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yoshida, J., Akagi, K., Misawa, R. et al. Chromatin states shape insertion profiles of the piggyBac, Tol2 and Sleeping Beauty transposons and murine leukemia virus. Sci Rep 7, 43613 (2017). https://doi.org/10.1038/srep43613
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43613
This article is cited by
-
Sleeping Beauty transposon mutagenesis in mouse intestinal organoids identifies genes involved in tumor progression and metastasis
Cancer Gene Therapy (2024)
-
Application of transposon systems in the transgenesis of bovine somatic and germ cells
BMC Veterinary Research (2022)
-
Current strategies employed in the manipulation of gene expression for clinical purposes
Journal of Translational Medicine (2022)
-
CRISPR and transposon in vivo screens for cancer drivers and therapeutic targets
Genome Biology (2020)
-
Simplified platform for mosaic in vivo analysis of cellular maturation in the developing heart
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.