Abstract
Hydrogen peroxide (H2O2) is present ubiquitously in marine surface waters where it is a reactive intermediate in the cycling of many trace elements. Photochemical processes are considered the dominant natural H2O2 source, yet cannot explain nanomolar H2O2 concentrations below the photic zone. Here, we determined the concentration of H2O2 in full depth profiles across three ocean basins (Mediterranean Sea, South Atlantic and South Pacific Oceans). To determine the accuracy of H2O2 measurements in the deep ocean we also re-assessed the contribution of interfering species to ‘apparent H2O2’, as analysed by the luminol based chemiluminescence technique. Within the vicinity of coastal oxygen minimum zones, accurate measurement of H2O2 was not possible due to interference from Fe(II). Offshore, in deep (>1000 m) waters H2O2 concentrations ranged from 0.25 ± 0.27 nM (Mediterranean, Balearics-Algeria) to 2.9 ± 2.2 nM (Mediterranean, Corsica-France). Our results indicate that a dark, pelagic H2O2 production mechanism must occur throughout the deep ocean. A bacterial source of H2O2 is the most likely origin and we show that this source is likely sufficient to account for all of the observed H2O2 in the deep ocean.
Similar content being viewed by others
Introduction
H2O2 is found ubiquitously in natural surface waters1,2,3. Photochemistry4,5,6, biological activity7,8,9, and physical mixing1,10, are considered to be the dominant processes controlling H2O2 concentration in the surface ocean. H2O2 concentrations from 20–800 nM are reported in surface marine waters with a diurnal oscillation typically observed11,12,13. H2O2 is a reactive intermediate in the biogeochemical cycling of a range of elements in surface waters14,15. It also has direct effects on surface dwelling microorganisms as ambient H2O2 concentrations are sufficient to trigger oxidative stress in many marine phytoplankton species16,17.
Intriguingly, measurable concentrations of up to 6 nM H2O2 have been reported for the deep North Pacific18, far below the photic zone. The distribution of H2O2 throughout the deep ocean is however poorly constrained and thus the possible effects of H2O2 on biogeochemical cycles in these waters are presently difficult to assess. If H2O2 is found at low nM concentrations throughout the deep ocean this also raises questions about its origin. A photochemical source of H2O2 at these depths is not plausible so the foremost hypothesis is a dark biological source19,20 associated with bacterially mediated remineralisation of organic matter.
Here we report full depth profiles of H2O2 in three different ocean basins (Mediterranean Sea, South Atlantic and South Pacific Oceans) to determine whether measurable concentrations of H2O2 are found ubiquitously in the deep ocean. We also re-assess the robustness of the widely used flow-injection luminol based chemiluminescence method12,21,22 for the analysis of low (<10 nM) H2O2 concentrations in seawater.
Results
Verifying the accuracy of H2O2 measurements
As noted by Yuan and Shiller18, at low nM concentrations it is very difficult to establish conclusively that a measured, or ‘apparent’, concentration of H2O2 is real and not an artifact of sample handling or the result of compounds other than H2O2 causing a false positive signal. Potential systematic problems with light exposure, filtration and contamination from the atmosphere or de-ionized water residues exist. All of these potential H2O2 contamination sources were however eliminated. The use of opaque sample bottles and foil shields minimized background light. The ambient lighting used in our laboratory produced only a small increase in H2O2 concentrations even when seawater was deliberately exposed to light by removal of sample bottle lids and placement of samples directly below our 330 lumens light source (<0.6 nM min−1, which is also inclusive of any contamination arising from atmospheric H2O2). Filtration was not used for normal sampling purposes and all sampling apparatus was pre-conditioned with seawater to ensure no contamination from de-ionized water derived H2O2.
With respect to the possible detection of species other than H2O2, the H2O2 luminol based FIA method is expected to be robust under most circumstances22. Organic peroxidases typically produce a low luminol response relative to the equivalent molar concentration of H2O2 and are only present at <1 nM in surface seawater23. Yuan and Shiller18 for example found that the response of methyl hydroperoxide solution was 11% that of H2O2 (and this signal may have actually arisen from a H2O2 impurity). Some trace metal ions also cause a positive interference (Table 1)22. However, in oxygenated offshore seawaters the anticipated interferences should be minimal. Dissolved Fe (DFe) concentrations in the Atlantic for example rarely exceed 1 nM24,25 which is equivalent to an apparent H2O2 signal of <0.5 nM. The response of Fe(II) is however stronger than that of Fe(III) (Table 1). At low O2 concentrations the oxidation rate of Fe(II) to Fe(III) is reduced such that high (nM) concentrations of Fe(II) can be found in the water column26. For example, in the Southern Ocean Sarthou et al.27 measured Fe(II) half-lives with respect to oxidation of 3–11 min in surface seawater (at pH 8.0–8.1 and O2 234–353 μM), whereas Scholz et al.28 determined that the apparent Fe(II) half-life in bottom water of the Peruvian oxygen minimum zone (OMZ) was 16–18 h (at pH 7.6, O2 <0.5 μM). Outside OMZs the half-life of Fe(II) in seawater is thus expected to be sufficiently short that the delay between sample collection and analysis (typically 10 min − 1 h) should be sufficient for Fe(II) to decay to undetectable (low pM) concentrations29. Furthermore, as an apparent H2O2 signal arising from mainly non-H2O2 components is deducted from our measured H2O2 concentrations (see below), this should include any residual concentration of Fe(II). However, accounting for Fe(II) remains problematic in OMZs where nM Fe(II) concentrations are present during analysis.
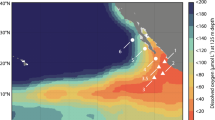
It is also particularly difficult to assess the effect of dissolved V (DV) on apparent H2O2 concentrations as less data are presently available on the distribution of reduced V species in the marine environment. Vanadium exhibits a relatively uniform concentration profile in the ocean with typical dissolved concentrations of 30–40 nM and a dominant oxidation state of V(V)30,31. Yet under reducing conditions a fraction of DV may exist as V(IV). Nanomolar concentrations of V(IV) have indeed been observed in sub-oxic and coastal waters32. As is the case for Fe(II) and Fe(III), any apparent H2O2 signal arising from V(IV) in oxic seawater (as per Table 1) should be accounted for by the deduction of an aged seawater blank from all measurements. But, as is the case for Fe(II), V(IV) interference may still be problematic in OMZs due to potentially enhanced concentrations which are subject to change as the sample is handled and analyzed. We present two depth profiles of DV in the S Pacific (Fig. 1) in order to assess the potential for this interference to occur within the vicinity of a coastal OMZ.
(a) Location of a shallow (Station 10) and offshore (Station 12) station within the Peruvian oxygen minimum zone (OMZ), plotted in Ocean Data View54. (b) H2O2 concentrations (after deduction of an aged seawater blank) and concentrations of potentially interfering trace metals at two stations in the OMZ. A Station 10 H2O2, dissolved (<0.2 μm) V (DV), Fe(II). B Station 10 Salinity, temperature, dissolved Fe (DFe), dissolved O2. C Station 12 H2O2, DV, Fe(II). D Station 12 Salinity, temperature, DFe, dissolved O2. (b) made in R version 3.2.3 (2015), GWDG Göttingen, Germany, http://ftp5.gwdg.de/pub/misc/cran/.
Trace metal samples were collected and analysed for Fe(II), DFe and DV at two stations (Stations 10 and 12, Fig. 1(a)) in the Peruvian OMZ. Oxygen depletion to <2 μmol kg−1 was evident in the water column at both stations (Fig. 1(b)). Consistent with results reported elsewhere along the Peruvian coastline26,33,34 low O2 waters below the surface mixed layer were enriched in DFe with the majority of DFe present as Fe(II). Up to 30 nM DFe was present at the shallow station (Station 10) and up to 2.4 nM at the offshore station (Station 12). Fe(II) concentrations were up to 12 nM and 0.77 nM at the shallow and offshore stations, respectively (Fig. 1(b)). The apparent H2O2 signal at these stations closely followed Fe(II) concentrations at all depths below 20 m, consistent with the interference demonstrated in aged seawater (Table 1). At both stations DV profiles exhibited a decline at intermediate depths associated with low O2 concentrations (Fig. 1(b)). The solubility of V(V) in seawater exceeds that of V(IV), thus a decline in DV concentration is expected under reducing conditions when V(IV) is formed35. Only a relatively small dissolved V(IV) concentration, ~1 nM, would be required to produce a nM apparent H2O2 signal (Table 1). Whilst we cannot determine the concentration of dissolved V(IV) present, it remains possible that this ion is a problematic interference with the luminol method for determination of H2O2 in coastal OMZs. It is not possible to correct for this interference as the concentration of reduced species (both Fe(II) and V(IV)) cannot be quantified with sufficient accuracy at the exact time H2O2 concentrations are measured. An aged (1–2 weeks old) seawater blank accounts for the presence of stable interfering species, but not unstable interfering species such as Fe(II) and V(IV)- the concentration of which must be known at the time H2O2 is measured if a correction is to be made. This difficulty arises, not only because of logistical constraints in coordinating the simultaneous measurement of multiple redox sensitive variables, but also because the measurement of Fe(II) via luminol itself is subject to a V(IV) interference36 and the chemiluminescence response of luminol to some ions, including Fe(II), is non-linear37. As an additional precaution we have therefore excluded all stations from our H2O2 dataset where Fe(II) exceeded 0.3 nM at multiple depths (Fe(II) was measured at all stations in the S Atlantic and S Pacific with a detection limit of ~0.2 nM, data not shown).
Some insight into what an apparent H2O2 signal is can be gained from use of the enzyme catalase, which removes H2O2 from solution. After surface seawater was aged in the dark at 6 °C for >2 weeks a variable apparent H2O2 signal of >1 nM could still be observed in all surface seawater tested. An apparent H2O2 signal of 0.4–1.9 nM was still measured after treatment with catalase at 25 °C for 1 h (Fig. 2). It is plausible that a residual, measurable concentration of H2O2 remained in seawater after catalase addition. Yet, if this residual H2O2 concentration depended primarily upon enzyme efficiency, we would expect to have observed the same residual H2O2 concentration in every catalase treated seawater sample. The residual signal however varied between water samples (Fig. 2). This indicated that the residual signal arose mainly from components other than H2O2.
As a further test to determine if the apparent H2O2 signal could actually have arisen from H2O2, we used the oxidation rate of Fe(II) in de-oxygenated seawater. Rate constants are available for the oxidation of Fe(II) via H2O2 in seawater (Equation (1)) at any environmentally relevant pH, temperature and salinity38,39. Thus, by comparing the oxidation rate of Fe(II) spiked into catalase treated and non-catalase treated aged seawater, the concentration of H2O2 present in aged seawater was estimated. The apparent H2O2 signal determined for aged S Atlantic seawater ranged from 0.43–0.96 nM after the addition of catalase (Fig. 2). When a 5 nM Fe(II) spike was added to this seawater, after de-oxygenating and treatment with catalase, the Fe(II) signal was stable for in excess of 1 h. Whereas, without catalase a 1.8 nM decrease was observed (in 1 h). Using the rate constant log k 4.17 M−1 s−1 (determined at 10 °C and pH 8.1739 matching our experimental conditions of 10.4 °C and pH 8.2) and assuming a pseudo-first order reaction (with constant [H2O2]) according to Equation (1), we determined the theoretical decline in Fe(II) concentration.

Assuming the decrease in Fe(II) concentration in non-catalase treated aged seawater could be attributed exclusively to oxidation by H2O2, the H2O2 concentration in aged S Atlantic seawater prior to catalase treatment was 7 nM. No change was observed in Fe(II) concentration after catalase treatment. This suggested that catalase effectively lowered the initial 7 nM H2O2 to no more than a few hundred pM. If H2O2 were actually present in solution at the apparent concentrations measured (0.4–1.9 nM, Fig. 2), 0.4 nM of H2O2 should have resulted in a decline of 0.1 nM Fe(II) over 1 h, and 1.0 nM of residual H2O2 should have resulted in a decline of 0.25 nM Fe(II). The absence of Fe(II) decay was consistent with our interpretation of the residual post-catalase apparent H2O2 signal (Fig. 2) as being due to components other than H2O2. For the purposes of calculating H2O2 concentration in seawater, the residual signal after aging and catalase treatment should therefore be treated as a blank. The detection limit of the method was therefore (3 standard deviations of the blank) 0.4–0.8 nM, similar to the typical blank of 0.6 nM reported elsewhere using similar apparatus5,40. This was higher than the 0.1–0.3 nM detection limit if the signal arising only from the reagent and background light/electrical noise was considered.
H2O2 concentrations in the Mediterranean Sea and South Atlantic
Depth profiles of H2O2 were collected in the Mediterranean Sea (69 stations), S Atlantic (14 stations) and S Pacific Oceans. As outlined above, H2O2 measured at all 13 S Pacific stations and 30 S Atlantic stations was unreliable because of high (>0.3 nM) Fe(II) concentrations at multiple depths associated with coastal OMZs (Fig. 1). These stations are excluded and not discussed further.
The reproducibility of the apparent H2O2 signal when sampling triplicate samplers from the same depth (Fig. 3(a)) verified that the signal was not subject to random contamination. A comparison of H2O2 measurements using equipment that was, or was not, trace metal clean (OTE and Niskin samplers with internal stainless steel components), indicated a small (mean 1.2 nM based on linear intercept, R2 = 0.94) overestimation of H2O2 concentration (Fig. 3(b)) when water was collected from Niskin samplers with metal components. The difference (n = 67 pairs) was statistically significant (P < 0.001, Wilcoxon Signed Rank Test – a paired test used here because it does not require a normally distributed population) and remained statistically significant both when the 3 highest concentrations were excluded (n = 64 pairs, P < 0.001) and when only concentrations <5.0 nM were compared (n = 40 pairs, P < 0.001).
(a) Replicate apparent H2O2 measurements using 3 separate samplers deployed at the same depth. Mean (n = 4 injections) and standard deviation of apparent H2O2 shown. Apparent H2O2 included a signal arising from components other than H2O2 in solution. (b) Comparison between H2O2 samples from two sub-surface sampling methods. Ocean Test Equipment samplers mounted on a powder coated sampling rosette with a Kevlar cable, and Niskin samplers with internal metal components on a standard stainless steel rosette (n = 67 pairs) deployed at the same depths along the South Atlantic transect (December 2015, line of best fit plotted R2 = 0.94).
Proceeding from north-east to south-west along the S Atlantic transect, H2O2 was elevated at four of the first five stations throughout the water column, separated by a station with low H2O2 concentrations (Fig. 4) which were more typical of stations observed further south. These elevated concentrations may be associated with the OMZ, but this is difficult to confirm due to high (>0.3 nM) Fe(II) concentrations within the OMZ along the shelf (at excluded stations, not shown) that interfered with the method for H2O2 analysis (Table 1). In the Mediterranean (Fig. 5) the vertical profile of H2O2 was very similar to that observed at the southern-most stations in the S Atlantic. High (>4 nM) H2O2 was normally confined to approximately the uppermost 100 m. A notable exception was however found in shallower waters (<500 m) for example off the coast of west Sicily (Fig. 5) where the gradient in H2O2 concentration declined more slowly with depth and elevated (at least 2–5 nM) H2O2 was found throughout the water column.
(a) GEOTRACES GA08 transect proceeding northeast-south in the South Atlantic, December 2015 (b) H2O2 concentrations in the South Atlantic plotted as distance along section shown (km) from northeast (left) to south (right). The first station (left) is marked on (a). Plotted in Ocean Data View54.
(a) Ocean Certain western Mediterranean transect between Sardinia and Sicily, August 2015 (b) H2O2 concentrations along the transect plotted as distance along section shown (km) from northwest (left) to southeast (right). Plotted in Ocean Data View54.
H2O2 concentrations in the surface ocean correlate strongly with irradiance5 and thus vary both diurnally and seasonally. Modest variation in temporally different reported concentrations in the same region is therefore expected. Our two most extensive datasets, for the S Atlantic (Fig. 6(a)) and the western Mediterranean (Fig. 6(b)), exhibited surface water concentrations of H2O2 with similar ranges to those reported by others5,12. Price et al.12 for example measured H2O2 concentrations ranging from 34–143 nM (mean 91 ± 29 nM) in surface waters from the Straits of Sicily, whereas here we report concentrations with a range 24–81 nM (mean 43 ± 22 nM).
(a) H2O2 across all South Atlantic stations sampled. Only samples collected using trace metal clean Ocean Test Equipment samplers (181 data points, excluding 99 below detection limit) are shown (same data as Fig. 4b). Inset H2O2 at, or below, 1000 m (83 datapoints, excluding 80 below detection limit). For stations with multiple samples beneath 1000 m, profiles are connected if H2O2 exceeded 2.9 nM at any depth. (b) H2O2 across all Mediterranean stations. All sub-surface samples were collected from Niskin samplers free from internal metal components on a stainless steel cable (total 482 data points-including those shown in Fig. 5b, excluding 199 below detection limit). Inset H2O2 at, or below, 1000 m only (60 datapoints, excluding 64 below detection limit). For stations with multiple samples beneath 1000 m, profiles are connected if H2O2 exceeded 2.9 nM at any depth.
Discussion
A sharp decline in H2O2 concentration with depth was illustrated within the datasets as a whole (Fig. 6) consistent with the confinement of the dominant H2O2 source, direct photochemistry, to the surface ocean and H2O2 concentrations thus dropping rapidly below the thermocline1. Our depth profiles of H2O2 suggested that the source of H2O2 in the deep ocean is pelagic with no obvious benthic sources evident at depth in the S Atlantic or Mediterranean (Figs 4(b) and 5(b)). A H2O2 source may be associated with the OMZ in the Angolan Basin (Fig. 4(b)), but this is difficult to confirm given our exclusion of shelf stations from the S Atlantic dataset and the uncertainty associated with false positive interferences from trace metals within the OMZ (Fig. 1).
The H2O2 concentrations observed in deep (>1000 m) waters at some stations in both the S Atlantic and Mediterranean were within the range 1–6 nM previously reported in the N Pacific18. However, the mean concentrations measured here are lower with the majority of measurements at >1000 m depth in both the Mediterranean and S Atlantic below detection (<0.4−0.8 nM). There is thereby moderate uncertainty associated with determining the mean deep (>1000 m) H2O2 concentration as this depends on how the datapoints below detection are treated. Using the most conservative approach, where we consider that datapoints below detection may take any value between 0 and the detection limit, the mean H2O2 concentration for the four transects where sufficient data points are available (Table 2) ranges from 0.25 ± 0.27 to 2.9 ± 2.2 nM. The mean H2O2 concentration >1000 m globally is therefore likely within the range 0.25–2.9 nM.
Unfortunately the rate of H2O2 decay has not been determined at in situ temperature and pressure for non-surface marine waters. Yet, when Yuan and Shiller18 incubated deep seawater (from 1500 and 5000 m in the N Pacific) at 21 °C (at which temperature most H2O2 decay experiments are reported) the observed decay constant, which is assumed to be first order, was 0.003 h−1 corresponding to a half-life of 230 h. This was slower than the decay measured in N Pacific surface waters where rate constants ranged from 0.004–0.02 h−1, corresponding to half-lives of 33–170 h18,41,42. As H2O2 decay is considered to be primarily microbially mediated43 decay constants in deep seawater would be expected to scale approximately with microbial activity18. Anticipated decay rates in the deep ocean would thereby be at least two orders of magnitude smaller than those in surface waters18,44,45. The H2O2 decay rate constant measured at 1500 and 5000 m in the N Pacific is thereby quite high (14–72% of that measured in the same region for surface waters), which may imply that an inorganic mechanism also contributes to the observed decay rate18. There are several uncertainties here as it is unknown to what extent the mechanism for H2O2 decay in the deep ocean remains primarily enzymatic, as it is in surface waters, and it is also unclear whether a rate constant determined at 1 atm pressure and 21 °C can be extrapolated to in situ conditions at >1000 m depth.
None the less, if this rate constant is applicable to the deep ocean in situ and, as we suggest here, H2O2 is found throughout the deep ocean at a mean concentration of 0.25–2.9 nM, H2O2 must be produced at a rate of 0.8–9 pM h−1 to balance the measured decay. Reported rates of dark H2O2 production vary in the surface ocean, but two studies in different environments observed very similar ranges: peak surface dark H2O2 production rates for unfiltered coastal and Sargasso seawater are reported as 0.8–2.4 nM h−1 46 and 1–3 nM h−1 19, respectively. The supposed dark production rate of H2O2 in the deep ocean would thereby have to be 0.03–1.1% of that found in surface waters. Measurements of leucine incorporation suggest that bacterial activity at 1000 m depth is approximately 0.8% of that at the surface47. So, whilst not conclusive evidence, this calculation does at least suggest that a dark production mechanism, similar to that observed in surface waters19,46, could feasibly account for all of the H2O2 present in the deep ocean.
Conclusions
Several trace metal species cause positive interferences with the widely used luminol-chemiluminescence method for H2O2 analysis in seawater. The majority of a residual 0.43–1.9 nM apparent H2O2 signal found in all aged, catalase treated seawater sampled cannot be attributed to H2O2 and should therefore be treated as a blank, which raises the detection limit of the method to 0.4–0.8 nM. Within the vicinity of OMZs, the concentration of Fe(II), and possibly also V(IV), is sufficiently high to dominate the apparent H2O2 signal in seawater below the thermocline. These interferences from transient species cannot presently be removed with great certainty.
After accounting for the apparent H2O2 signal arising from interfering ions, H2O2 appears to be present at very low nM concentrations in deep (below 1000 m) seawaters with the mean concentration varying across the different transects sampled: 2.9 ± 2.2 nM (Mediterranean, Corsica-France), 0.25 ± 0.27 nM (Mediterranean, Balearics-Algeria), 0.55 ± 1.3 nM (Mediterranean, Sardinia-Sicily) and 0.90 ± 1.2 nM (S Atlantic, Angola Basin). The source of this H2O2 remains uncertain, yet the observed distribution is consistent with a pelagic source. Estimates of deep ocean H2O2 decay rates and bacterial activity suggest that dark, bacterial production of H2O2 is sufficient to explain all H2O2 present at these depths.
Methods
Cruise work
Cruise work was conducted onboard RV Minerva Uno (Ocean Certain, August 2015, western Mediterranean Sea), FS Sonne (SFB 754, October 2015, South Pacific Ocean) and FS Meteor (GEOTRACES, November-December 2015, South Atlantic Ocean). All sample and reagent bottles for H2O2 analyses (opaque high density polyethylene (HDPE), Nalgene) were pre-cleaned by soaking sequentially in Mucasol detergent (Sigma-Aldrich) for 1 day and 1 M HCl for 1 week with 3 de-ionized water (18.2 MΩ cm−1, Milli-Q, Millipore) rinses after each stage. Bottles were then dried in a laminar flow hood and stored in re-sealable plastic bags until required. Seawater for the analysis of H2O2 was collected using various methods to assess sampling artifacts. Seawater was either collected: from surface waters using a plastic bucket on a nylon line twice rinsed with surface seawater before sample collection, from a towfish which continuously pumped surface seawater through plastic tubing into a clean laboratory on deck, from trace metal clean Ocean Test Equipment (OTE) samplers mounted on a powder coated sampling rosette with a Kevlar conducting cable complying with GEOTRACES specifications for trace metals, or from Niskin samplers (with, and without, internal metal components) mounted on a standard stainless steel sampling rosette with a stainless steel conducting cable. In all cases the HDPE sample bottles were rinsed 3 times with seawater, filled gently to overflowing, and sealed with no headspace. Samples were not filtered. For OTE and Niskin samplers, seawater was collected immediately after dissolved gas collection. Seawater was collected straight from the spigot of the samplers to minimize light exposure. All samples were analyzed within 2 h of sampling from the OTE or Niskin samplers. For surface samples, the time delay between sample collection and analysis for H2O2 was approximately 10 min.
Chemical analysis
H2O2 concentrations were determined via flow injection analysis (FIA) using the Co(II) catalyzed oxidation of luminol, which is the most commonly used analytical method in seawater12,21,22. A FIA system was assembled using two 10-port valves (Valco, Vici), a photonmultiplier tube (PMT, H9319-11, Hamamatsu), a glass flow cell with a mirrored base (Waterville Analytical Products) and a peristaltic pump (MiniPuls 3, Gilson). The flow cell, PMT and valve were arranged as per Yuan and Shiller22, but with two alternating reagent loops such that one loop was loading and one loop unloading, with the same flow rates (2.5 mL min−1 sample and 1.4 mL min−1 reagent) and target sample loop internal volume (60 μL) used22. It was verified that this configuration produced close to optimal chemiluminescence peak height responses by testing the effect of small changes to flow rate and tubing lengths (reagent loop and, separately, the length of tubing between the valve and detector) on the peak height produced for a 50 nM standard addition to aged seawater. H2O2 concentrations were derived from mean peak height and are reported as the mean (±standard deviation) of 4 injections. The peak height response was always linear in the range 0–100 nM (R2 typically > 0.99 and always > 0.98). The PMT was secured inside an electrical box to minimize background light, and all reagent and sample tubing was opaque (black PTFE, Global FIA, 0.8 mm internal diameter) except peristaltic pump tubing (PVC, Gradko).
The H2O2 reagent was prepared by adding the following reagents to aged de-ionized water (stored at room temperature for >24 h in the dark) and making up to a total volume of 2 L: 0.54 g luminol (98%, ROTH), 44 g potassium carbonate (reagent grade, ROTH), 7.8 mL Co(II) solution (1000 ppm standard in 2% by volume HNO3, ROTH) and approximately 15 mL HCl (Trace Metal grade, Fisher Scientific) to adjust the pH of solution to 10.222. Once mixed, H2O2 reagent was stored at approximately 6 °C for in excess of 24 h (to maximize the luminol response22) and then allowed to warm to room temperature for 6–12 h before use. Reagent remaining unused after 3 days was discarded. Between stations/experiments the FIA apparatus was rinsed with 0.1 M HCl followed by de-ionized water. To ensure the complete removal of acid and de-ionized water, an aged seawater blank was always run prior to re-commencing analysis. Calibrations were undertaken between stations, and with new reagent batches, using at least 6 standard additions of H2O2 (TraceSelect, Fluka) to aged (unfiltered) seawater obtained from >1000 m depth (stored at room temperature in opaque HDPE bottles). H2O2 was sequentially diluted to create stock solutions of 100 mM and 100 μM using de-ionized water. The use of narrow-necked, opaque HDPE sample bottles, aluminium foil shields over the apparatus and reduced lighting in the laboratory minimized the potential for undesired photochemistry during sample analysis.
Experiments to verify the accuracy of H2O2 analysis
Mediterranean, Atlantic and Pacific seawaters were stored in the dark at 6 °C for >2 weeks for assessment of the baseline signal from a mixture of luminol reagent and seawater in the absence of high H2O2 concentrations. This aged seawater was then spiked with a freshly prepared solution of bovine catalase (Sigma) to a final enzyme concentration of 1–3 units mL−1, and allowed to stand with gentle mixing at 25 °C (to optimize enzyme performance) for 1 h. H2O2 spikes of 4 and 8 nM were also added to Atlantic seawater and treated identically to verify the effectiveness of the enzyme addition. The oxidation rate of a 5 nM Fe(II) spike in low O2 seawater was used to estimate the concentration of residual H2O2 in aged, catalase treated seawater. Fe(II) was spiked into aged seawater with low O2 in the presence and absence of catalase under a N2 atmosphere. After seawater was aged in the dark at 6 °C for 3 days, N2 was bubbled through seawater for >2 h. An Fe(II) spike was then added and the concentration of Fe(II) measured continuously for a period of >1 h. Fe(II) concentration was measured via FIA using luminol48,49,50 with a dual Fe(II)/H2O2 system51 assembled using the same components as the H2O2 system (see above). FIA was conducted in a laminar flow hood using the same procedures for handling reagent and seawater solutions as per the H2O2 analysis. Fe(II) reagent solution was made with 0.26 g luminol and 1.06 g potassium carbonate in 10 mL de-ionized water and stored overnight at 6 °C to ensure complete dissolution. This was then added to a 2 L solution of de-ionized water containing 80 mL ammonium hydroxide (Trace Metal grade, Fisher Scientific), to which approximately 22 mL HCl (Trace Metal grade, Fisher Scientific) was added to adjust the final pH to 10.1. The mixed reagent was allowed to stand for >24 h prior to use to maximize the luminol response37.
The interference with the H2O2 luminol chemiluminescence method from Fe(II), Fe(III) and V(IV) was investigated by spiking solutions of these ions into S Atlantic seawater that had been aged in the dark. Fe(II) additions were made into both low (aged, no H2O2 spike) and high (aged, spiked to 42 nM) H2O2 Atlantic seawater as the luminol response to some interferences may be non-linear36,37. Fe(II), V(IV) and Fe(III) stock solutions were made from ammonium Fe(II) hexahydrate (99%, Sigma Aldrich), vanadyl sulfate hydrate (99.99%, Sigma Aldrich) and Fe(III) chloride hexahydrate (>99.99%, Sigma Aldrich), respectively. Primary stocks were acidified using 1 mL HCl (Trace Metal grade, Fisher Scientific) per L solution and used on the day of preparation to minimize trace metal oxidation state changes.
To assess the potential for in-situ metal concentrations to interfere with H2O2 measurement in samples from OMZs, samples for trace metal analysis were collected from OTE samplers on a Kevlar wire adhering to GEOTRACES standards for trace metal sample collection (FS Sonne, October 2015, South Pacific). Sub-sampling was conducted under N2 gas (purity > 99.999%) in a metal free clean container where the air was filtered. Samples for Fe(II) analysis were collected first and analysed immediately (analysis complete within 22 min of sub-sampling OTE samplers) without filtration as above. Dissolved seawater samples for trace metals (filtered with 0.2 μm, Acropack 500) were collected in 125 mL low density polyethylene (Nalgene) bottles (pre-cleaned according to GEOTRACES protocols: 1 day in Mucasol detergent, 1 week in 1.2 M HCl, 1 week in 1.2 M HNO3 with 3 de-ionized water rinses after each stage) and stored double sealed in plastic bags. Samples were acidified in a laminar flow hood within 1 day of collection (140 μL Optima grade HCl, Fisher Scientific) and then stored acidified for >6 months. Samples were buffered to pH 6.4 using a 1.5 M ammonium acetate buffer before loading onto a WAKO resin52 for off-line pre-concentration (SeaFAST, Elemental Scientific Inc.). The buffer was prepared using ammonium hydroxide solution (Optima grade, Fisher Scientific) and acetic acid (Optima grade, Fisher Scientific) in de-ionized water adjusted to pH 8.5. Elution was performed in 1 M distilled HNO3 (distilled using a sub-boiling PFA distillation system (DST-1000, Savillex) from SPA grade HNO3 (Romil)). Pre-concentrated samples were analysed by high resolution inductively coupled plasma-mass spectrometry (HR-ICP-MS, ELEMENT XR, ThermoFisherScientific) using isotope dilution for Fe and standard addition for V53. Measurement of SAFe GEOTRACES reference water S produced concentrations of V 33.6 ± 1.1 nM (no consensus value available) and Fe 0.091 ± 0.004 nM (consensus value: 0.093 ± 0.008 nM).
Additional Information
How to cite this article: Hopwood, M. J. et al. Hydrogen peroxide in deep waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci. Rep. 7, 43436; doi: 10.1038/srep43436 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Miller, W. L. & Kester, D. R. Peroxide variations in the Sargasso Sea. Mar. Chem. 48, 17–29 (1994).
Moore, C. A., Farmer, C. T. & Zika, R. G. Influence of the Orinoco River on hydrogen peroxide distribution and production in the eastern Caribbean. J. Geophys. Res. 98, 2289 (1993).
Van Baalen, C. & Marler, J. E. Occurrence of hydrogen peroxide in sea water. Nature 211, 951 (1966).
O’Sullivan, D. W., Neale, P. J., Coffin, R. B., Boyd, T. J. & Osburn, C. L. Photochemical production of hydrogen peroxide and methylhydroperoxide in coastal waters. Mar. Chem. 97, 14–33 (2005).
Steigenberger, S. & Croot, P. L. Identifying the processes controlling the distribution of H2O2 in surface waters along a meridional transect in the eastern Atlantic. Geophys. Res. Lett. 35, L03616 (2008).
Shaked, Y., Harris, R. & Klein-Kedem, N. Hydrogen peroxide photocycling in the Gulf of Aqaba, Red Sea. Environ. Sci. Technol. 44, 3238–44 (2010).
Fujiwara, K., Ushiroda, T., Takeda, K., Kumamoto, Y. & Tsubota, H. Diurnal and Seasonal Distribution of Hydrogen-Peroxide in Seawater of the Seto Inland Sea. Geochem. J. 27, 103–115 (1993).
Milne, A., Davey, M. S., Worsfold, P. J., Achterberg, E. P. & Taylor, A. R. Real-time detection of reactive oxygen species generation by marine phytoplankton using flow injection-chemiluminescence. Limnol. Oceanogr. 7, 706–715 (2009).
Wong, G. T. F., Dunstan, W. M. & Kim, D. B. The decomposition of hydrogen peroxide by marine phytoplankton. Oceanol. Acta 26, 191–198 (2003).
Johnson, K. S., Willason, S. W., Wiesenburg, D. A., Lohrenz, S. E. & Arnone, R. A. Hydrogen peroxide in the western Mediterranean Sea: a tracer for vertical advection. Deep Sea Res. Part A, Oceanogr. Res. Pap. 36, 241–254 (1989).
Yuan, J. & Shiller, A. The distribution of hydrogen peroxide in the southern and central Atlantic ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 2947–2970 (2001).
Price, D., Mantoura, R. F. C. & Worsfold, P. J. Shipboard determination of hydrogen peroxide in the western Mediterranean sea using flow injection with chemiluminescence detection. Anal. Chim. Acta 377, 145–155 (1998).
Gerringa, L. J. A., Rijkenberg, M. J. A., Timmermans, R. & Buma, A. G. J. The influence of solar ultraviolet radiation on the photochemical production of H2O2 in the equatorial Atlantic Ocean. J. Sea Res. 51, 3–10 (2004).
Voelker, B. M. & Sulzberger, B. Effects of Fulvic Acid on Fe(II) Oxidation by Hydrogen Peroxide. Environ. Sci. Technol. 30, 1106–1114 (1996).
Moffett, J. W. & Zika, R. G. Reaction kinetics of hydrogen peroxide with copper and iron in seawater. Environ. Sci. Technol. 21, 804–810 (1987).
Baltar, F., Reinthaler, T., Herndl, G. J. & Pinhassi, J. Major Effect of Hydrogen Peroxide on Bacterioplankton Metabolism in the Northeast Atlantic. PLoS One 8, e61051 (2013).
Morris, J. J., Johnson, Z. I., Szul, M. J., Keller, M. & Zinser, E. R. Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the ocean’s surface. PLoS One 6, e16805 (2011).
Yuan, J. & Shiller, A. M. Hydrogen peroxide in deep waters of the North Pacific Ocean. Geophys. Res. Lett. 31, L01310 (2004).
Palenik, B. & Morel, F. M. M. Dark production of H2O2 in the Sargasso Sea. Limnol. Oceanogr. 33, 1606–1611 (1988).
Vermilyea, A. W., Hansard, S. P. & Voelker, B. M. Dark production of hydrogen peroxide in the Gulf of Alaska. Limnol. Oceanogr. 55, 580–588 (2010).
Price, D., Worsfold, P. J., Fauzi, R. & Mantoura, C. Determination of hydrogen peroxide in sea water by flow-injection analysis with chemiluminescence detection. Anal. Chim. Acta 298, 121–128 (1994).
Yuan, J. C. & Shiller, A. M. Determination of subnanomolar levels of hydrogen peroxide in seawater by reagent-injection chemiluminescence detection. Anal. Chem. 71, 1975–1980 (1999).
Zika, R. G., Moffett, J. W., Petasne, R. G., Cooper, W. J. & Saltzman, E. S. Spatial and temporal variations of hydrogen peroxide in Gulf of Mexico waters. Geochim. Cosmochim. Acta 49, 1173–1184 (1985).
Rijkenberg, M. J. A. et al. The distribution of dissolved iron in the west atlantic ocean. PLoS One 9, e101323–e101323 (2014).
Hatta, M. et al. An overview of dissolved Fe and Mn distributions during the 2010–2011 US GEOTRACES north Atlantic cruises: GEOTRACES GA03. Deep Sea Res. Part II Top. Stud. Oceanogr. 116, 117–129 (2015).
Hong, H. & Kester, D. R. Redox state of iron in the offshore waters of Peru. Limnol. Oceanogr. 31, 512–524 (1986).
Sarthou, G. et al. Labile Fe(II) concentrations in the Atlantic sector of the Southern Ocean along a transect from the subtropical domain to the Weddell Sea Gyre. Biogeosciences 8, 2461–2479 (2011).
Scholz, F. et al. Nitrate-dependent iron oxidation limits iron transport in anoxic ocean regions. Earth Planet. Sci. Lett. 454, 272–281 (2016).
Santana-Casiano, J. M., Gonzaalez-Davila, M. & Millero, F. J. Oxidation of nanomolar levels of Fe(II) with oxygen in natural waters. Environ. Sci. Technol. 39, 2073–2079 (2005).
Collier, R. W. Particulate and dissolved vanadium in the North Pacific Ocean. Nature 309, 441–444 (1984).
Jeandel, C., Caisso, M. & Minster, J. F. Vanadium behaviour in the global ocean and in the Mediterranean sea. Mar. Chem. 21, 51–74 (1987).
Wang, D. & Sañudo Wilhelmy, S. A. Vanadium speciation and cycling in coastal waters. Mar. Chem. 117, 52–58 (2009).
Chever, F. et al. Total dissolvable and dissolved iron isotopes in the water column of the Peru upwelling regime. Geochim. Cosmochim. Acta 162, 66–82 (2015).
Vedamati, J., Goepfert, T. & Moffett, J. W. Iron speciation in the eastern tropical South Pacific oxygen minimum zone off Peru. Limnol. Oceanogr. 59, 1945–1957 (2014).
Sadiq, M. Thermodynamic solubility relationships of inorganic vanadium in the marine environment. Mar. Chem. 23, 87–96 (1988).
Ussher, S. J. et al. Investigation of iron(II) reduction and trace metal interferences in the determination of dissolved iron in seawater using flow injection with luminol chemiluminescence detection. Anal. Chim. Acta 652, 259–265 (2009).
King, D. W., Lounsbury, H. A. & Millero, F. J. Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ. Sci. Technol. 29, 818–824 (1995).
Millero, F. J. & Sotolongo, S. The oxidation of Fe(II) with H2O2 in seawater. Geochim. Cosmochim. Acta 53, 1867–1873 (1989).
González-Davila, M., Santana-Casiano, J. M. & Millero, F. J. Oxidation of iron (II) nanomolar with H2O2 in seawater. Geochim. Cosmochim. Acta 69, 83–93 (2005).
Croot, P. L., Streu, P., Peeken, I., Lochte, K. & Baker, A. R. Influence of the ITCZ on H2O2 in near surface waters in the equatorial Atlantic Ocean. Geophys. Res. Lett. 31 (2004).
Roe, K. L., Schneider, R. J., Hansel, C. M. & Voelker, B. M. Measurement of dark, particle-generated superoxide and hydrogen peroxide production and decay in the subtropical and temperate North Pacific Ocean. Deep. Res. Part I Oceanogr. Res. Pap. 107, 59–69 (2016).
Yuan, J. C. & Shiller, A. M. Distribution of hydrogen peroxide in the northwest Pacific Ocean. Geochemistry Geophys. Geosystems 6, Q09M02 (2005).
Petasne, R. G. & Zika, R. G. Hydrogen peroxide lifetimes in South Florida coastal and offshore waters. Mar. Chem. 56, 215–225 (1997).
Karner, M. B., DeLong, E. F. & Karl, D. M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409, 507–510 (2001).
Teira, E., Lebaron, P., van Aken, H. M. & Herndl, G. J. Distribution and activity of Bacteria and Archaea in the deep water masses of the North Atlantic. Limnol. Oceanogr. 51, 2131–2144 (2006).
Moffett, J. W. & Zajiriou, O. C. An investigation of hydrogen peroxide chemistry in surface waters of Vineyard Sound with H2 18O2 and 18O2 . Limnol. Oceanogr. 35, 1221–1229 (1990).
Giering, S. L. C. et al. Reconciliation of the carbon budget in the ocean’s twilight zone. Nature 507, 480–483 (2014).
Seitz, W. R. & Hercules, D. M. Determination of Trace Amounts of Iron(II) Using Chemiluminescence Analysis. Anal. Chem. 44, 2143–2149 (1972).
O’Sullivan, D. W., Hanson Jr., A. K. & Kester, D. R. Stopped flow luminol chemiluminescence determination of Fe(II) and reducible iron in seawater at subnanomolar levels. Mar. Chem. 49, 65–77 (1995).
Rose, A. L. & Waite, T. D. Chemiluminescence of luminol in the presence of iron(II) and oxygen: Oxidation mechanism and implications for its analytical use. Anal. Chem. 73, 5909–5920 (2001).
Jones, M. R., Nightingale, P. D., Turner, S. M. & Liss, P. S. Adaptation of a load-inject valve for a flow injection chemiluminescence system enabling dual-reagent injection enhances understanding of environmental Fenton chemistry. Anal. Chim. Acta 796, 55–60 (2013).
Kagaya, S. et al. A solid phase extraction using a chelate resin immobilizing carboxymethylated pentaethylenehexamine for separation and preconcentration of trace elements in water samples. Talanta 79, 146–152 (2009).
Milne, A., Landing, W., Bizimis, M. & Morton, P. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Anal. Chim. Acta 665, 200–207 (2010).
Schlitzer, R. Ocean Data View. (2004).
Acknowledgements
This work was supported by the European Commission (OCEAN-CERTAIN, FP7-ENV-2013-6.1-1; no: 603773) and the Collaborative Research Centre 754 (SFB 754) “Climate-Biogeochemistry Interactions in the Tropical Ocean” funded by the German Research Foundation (DFG). We also thank the DFG for funding the M121 GEOTRACES cruise. The authors thank the complement and embarked scientists from the RV Minerva Uno, FS Meteor and FS Sonne. Labview software for operating the H2O2/Fe(II) FIA systems was designed by P Croot, M Heller, C Neill and W King. Statistics were performed in SigmaPlot.
Author information
Authors and Affiliations
Contributions
M.H. and E.A. designed the study. M.H., C.S. and I.R. performed cruise and analytical work. M.H. and I.R. wrote the initial manuscript draft and then all authors contributed to its revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hopwood, M., Rapp, I., Schlosser, C. et al. Hydrogen peroxide in deep waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci Rep 7, 43436 (2017). https://doi.org/10.1038/srep43436
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43436
This article is cited by
-
Reactive oxygen species affect the potential for mineralization processes in permeable intertidal flats
Nature Communications (2023)
-
Mechanisms of silver nanoparticle toxicity to the coastal marine diatom Chaetoceros curvisetus
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.