Abstract
Thyroid is a one of the most important endocrine organs. Understanding the molecular mechanism underlying thyroid development and function, as well as thyroid diseases, is beneficial for the clinical treatment of thyroid diseases and tumors. Through genetic linkage analysis and exome sequencing, we previously identified an uncharacterized gene C14orf93 (RTFC, mouse homolog: 4931414P19Rik) as a novel susceptibility gene for familial non-medullary thyroid carcinoma, and demonstrated its function in promoting thyroid tumor. However, the role of RTFC in thyroid development and function remains unexplored. In this study, we found that knockout of Rtfc compromises the in vitro thyroid differentiation of mouse embryonic stem cells. In contrast, Rtfc−/− mice are viable and fertile, and the size and the morphology of thyroid are not affected by Rtfc knockout. However, female Rtfc−/− mice, but not male Rtfc−/− mice, display mild hypothyroidism. In summary, our data suggest the roles of Rtfc in in vitro thyroid differentiation of embryonic stem cells, and in vivo thyroid function.
Similar content being viewed by others
Introduction
Thyroid is one of the most important endocrine organs in vertebrate. It plays important roles in development, growth, and metabolism through secreting thyroid hormones1,2. Thyroid hormones regulate many developmental processes, including neural, bone, and reproductive organ development3,4,5. It is well known that lack of sufficient thyroid hormone in early human development stages results in cretinism. In adults, the primary effects of thyroid hormones are regulating metabolism such as protein, carbohydrate, lipid, and vitamin metabolism6.
Thyroid is the first emerging endocrine organ during embryogenesis, formed around the 22nd day post conception in humans7. Onset of thyroid hormone synthesis represents the accomplishment of thyroid development8. Thyroid transcription factors (TTFs), including NK2 homeobox 1 (NKX2-1), forkhead box protein E1 (FOXE1), paired box protein 8 (PAX8) and haematopoietically expressed homeobox (HHEX), are expressed in thyroid precursor cells, and also important for the functional differentiation of the gland in late development and postnatally9,10,11,12,13,14. NKX2-1, FOXE1 and PAX8 cooperate to activate genes that drive thyroid hormone synthesis, such as TG, TPO, and SLC5a515,16,17. Given the importance of TTFs in development, cell proliferation, and differentiation, mutations in these genes are associated with thyroid disorders. For example, mutations in NKX2-1, FOXE1 and PAX8, as well as polymorphisms in FOXE1, are associated with thyroid dysgenesis18,19,20,21,22,23,24,25,26. Moreover, genetic alterations of NKX2-1, PAX8, and FOXE1, have been reported to be related to thyroid cancer27,28,29,30.
Through genetic linkage analysis and exome sequencing, we previously identified C14orf93 (also named as Regulator of Thyroid Function and Cancer, RTFC) as a novel susceptibility gene for familial nonmedullary thyroid cancer (FNMTC)31. The oncogenic functions of R115Q, V205M, and G209D RTFC mutants are demonstrated by cell surviving assay, migration assay, and colony forming assays. Moreover, RTFC has been identified as a potential antigen associated with the pathogenesis of peripheral T-cell lymphomas, not otherwise specified (PTCL, NOS)32. Two in vitro biochemical screens suggested that RTFC might have RNA and phosphopeptide (pSer/pThr-X-X-X-pSer/pThr) binding activities33,34. Yet, the role of RTFC in normal development, as well as the molecular function of RTFC, remain unexplored.
In this study, we investigated the function of Rtfc (originally named 4931414P19Rik) in mouse thyroid development and thyroid function. We found that knockout of Rtfc compromises the thyroid differentiation of mouse embryonic stem cells (ESCs), while overexpression of RTFC promotes mouse ESCs to differentiate toward the thyroid lineage. In contrast, Rtfc−/− mice are viable and fertile, and the size and the morphology of thyroid are not affected by Rtfc knockout. However, female Rtfc−/− mice, but not male Rtfc−/− mice, display mild hypothyroidism. In summary, our data demonstrated the roles of RTFC in in vitro thyroid differentiation of ESCs, and in vivo thyroid function.
Results
Rtfc is involved in thyroid differentiation of mouse ESCs
Rtfc expression dynamics during human and mouse development were looked up from NCBI UniGene EST profiles (https://www.ncbi.nlm.nih.gov/unigene/) and EMBL-EBI database (http://www.ebi.ac.uk/), respectively (Fig. S1). It is notable that Rtfc is expressed at low levels during embryo development, and its expression increases and hits the peak at the juvenile stage, implying a functional role of Rtfc in development.
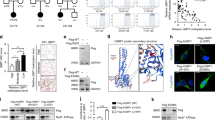
To investigate the role of Rtfc in the normal development of thyroid, we took advantage of an in vitro thyroid differentiation system of mouse ESCs by overexpression of Nkx2.1 and Pax835. An “Nkx2.1-IRES-Pax8” cassette driven by a tetracycline-inducible promoter was integrated into the engineered ColA1 locus through FLPe recombinase–mediated recombination in KH2 ESCs, resulting in a Dox-inducible Nkx2.1 and Pax8 ESC line (iN/P ESCs). When iN/P ESCs were induced to differentiate into thyroid cells, the expression level of Rtfc slightly increases, similar to its expression dynamics during in vivo embryogenesis (Fig. S2). A pair of transcription activator-like effector nucleases (TALENs) were designed and constructed to disrupt the Rtfc gene in iN/P ESCs (Fig. 1a). Two Rtfc knockout iN/P ESC lines were established, and sequencing of the TALENs targeting sites validated the disruption of Rtfc alleles (Fig. 1b). When Rtfc knockout and WT iN/P ESCs were differentiated toward the thyroid lineage, the up-regulation of endogenous thyroid genes, including Nkx2.1, Pax8, Foxe1, Tg, Duox2, and Duoxa2, are reduced in Rtfc knockout cells, compared to WT cells (Fig. 1c). Conversely, overexpression of human WT and V205M (a mutation identified in a FNMTC pedigree31) RTFC enhance the activation of thyroid genes during ESC differentiation (Fig. 1d and e). These data suggest that Rtfc contributes to in vitro thyroid differentiation of ESCs. Moreover, V205M RTFC mutant appears to be more potent in promoting thyroid differentiation (Fig. 1e), implying that this mutation enhances the function of RTFC, consistent with our previous data that V205M increases the colony forming capacity of thyroid cancer cells31.
(a) The design of TALENs to knock out the Rtfc gene. Boxes represent exons of the Rtfc gene. The coding regions are shown in black. (b) Sequences at the TALENs targeting site in two Rtfc knockout (KO) clones. (c) Knockout of Rtfc reduces the thyroid differentiation efficiency of mouse ESCs. WT ESCs and two Rtfc KO ESC lines were differentiated toward the thyroid lineage. The expression levels of thyroid markers were measured in day 7 differentiated cells (D7) by quantitative RT-PCR. Day 0 undifferentiated WT ESCs (WT, D0) were included as a control to show the activation of thyroid markers after differentiation. Data are shown as mean ± SD (n = 3). (d) Western blot to detect the overexpression of FLAG tagged GFP, WT and V205M RTFC proteins in mouse ESCs. Stable mouse ESC lines overexpressing FLAG tagged GFP, WT and V205M RTFC were established and subjected to Western blot. A triangle indicates the band of FLAG-GFP, and an asterisk marks the band of FLAG-RTFC. Cropped blots are shown, and full-length blots are presented in Supplementary Figure S5. (e) Overexpression of WT and V205M RTFC promote the thyroid differentiation of mouse ESCs. ESCs overexpressing GFP, FLAG tagged WT and V205M RTFC, were differentiated toward the thyroid lineage. The expression of thyroid markers in day 7 differentiated cells (D7), as well as in day 0 undifferentiated GFP ESCs (GFP, D0), were analyzed. Data are shown as mean ± SD (n = 6, including 2 independent clones and 3 technical replicates).
Rtfc−/− mice are viable and fertile
To further demonstrate the function of Rtfc in thyroid development in vivo, one Rtfc homozygous and eight Rtfc heterozygous knockout founder mice were generated by zygotic injection of TALEN mRNA (Fig. 2a). Sequencing result showed a 1-bp deletion (Δ1) of the Rtfc gene in the Rtfc homozygous knockout (Rtfc−/−) founder mouse (Fig. 2b). And this founder mouse was used for subsequent mating and experiments. Mating between female and male Rtfc+/− mice yielded Rtfc+/+, Rtfc+/−, and Rtfc−/− progenies, and the ratio of three genotypes is close to the expected Mendelian ratio of 1:2:1 (Table 1). Moreover, live pups were born by Rtfc−/− parents. Thus, Rtfc−/− mice are viable and fertile.
(a) Genotyping of the founder mice. Only one allele of Rtfc was modified in founder mice 1, 3, 5, 6, 7, 9 and 10, while both Rtfc alleles were modified in founder mouse 8. It appears that there were three Rtfc alleles in founder mouse 4. Neither Rtfc allele was modified in founder mouse 2. (b) Sanger sequencing result of the representative homozygous knockout mouse reveals a 1-bp deletion in two Rtfc gene alleles. The red box marks the deleted G in the Rtfc−/− (Δ1) mouse.
Female Rtfc−/− mice display mild hypothyroidism
Next, we examined the weight and morphology of liver, spleen, heart, kidney, as well as body weight of Rtfc−/− mice, and found them indistinguishable from those of Rtfc+/+ and Rtfc+/− mice (Fig. 3a–c). In addition, no difference in thyroid size and morphology was observed among Rtfc+/+, Rtfc+/−, and Rtfc−/− mice of both genders (Fig. 4a). Tissue section and HE staining revealed normal follicle size and structure in the thyroid of Rtfc−/− mice (Fig. 4b). Furthermore, knockout of Rtfc does not affect the expression of seven thyroid-related genes (Nkx2-1, Pax8, Foxe1, Tshr, Duox2, Duoxa2 and Nis) in the thyroid (Fig. S3).
(a) Body weight of mouse is not affected by Rtfc knockout. (b) Images of heart, spleen, kidney and liver, demonstrate that the size and the morphology of these organs are similar in Rtfc+/+, Rtfc+/− and Rtfc−/− mice. (c) Rtfc knockout does not affect the relative weight of heart, spleen, kidney and liver (normalized to the body weight).
(a) Thyroid morphology of representative 3-month old Rtfc+/+, Rtfc+/− and Rtfc−/− male and female mice. White and yellow arrows indicate the upper and lower edges of thyroid, respectively. Scale bars: 1 mm. (b) HE staining of thyroid tissue sections of 3-month old Rtfc+/+, Rtfc+/− and Rtfc−/− male and female mice. Scale bars: 200 μm. (c) Serum total T3, total T4 and TSH levels of 3-month old Rtfc+/+, Rtfc+/− and Rtfc−/− male and female mice, measured by radioimmunoassay. Each dot represents the value in a mouse, and bars are averages. (d) Expression of Tg and Tpo in the thyroid of 3-month old Rtfc+/+ and Rtfc−/− male and female mice. Each dot represents the value in a mouse, and bars are averages.
As the size and the morphology of thyroid are not affected by Rtfc knockout, we further tested the function of thyroid, by measuring the levels of total T3, total T4 and TSH in serum. Knockout of Rtfc does not affect the serum T3, T4, and TSH levels in male mice. Rather, it reduces the serum T4 level only in female mice, and the serum T4 level in female Rtfc−/− mice is the lowest (Fig. 4c). Compared with female Rtfc+/+ mice, the serum T3 and TSH levels of female Rtfc−/− mice slightly decreases and increases, respectively, even though the differences are statistically insignificant (Fig. 4c). These data suggest that Rtfc knockout compromises the function of thyroid and results in mild hypothyroidism in female mice, but not in male mice.
To understand how thyroid function is compromised in female Rtfc−/− mice, we examined the expression of two critical genes Tg and Tpo for the production of thyroid hormones, in the thyroid of female Rtfc−/− and Rtfc+/+ mice. Tg encodes thyroglobulin, which is used by thyroid to produce the thyroid hormones T3 and T4. Tpo encodes thyroperoxidase, which catalyzes both the iodination of tyrosine residues and the coupling of iodotyrosine residues in thyroglobulin, for the production of T3 and T436. Our results showed that the expression of Tpo, but not Tg, is reduced in female Rtfc−/− mice, in comparison to female Rtfc+/+ mice (Fig. 4d). Thus, the reduced level of Tpo in the thyroid of Rtfc−/− female mice, might account for the decrease of serum T4. Consistent with the unchanged serum T3, T4 and TSH levels in Rtfc−/− male mice, the expression levels of Tg and Tpo are not affected by Rtfc knockout in male mice (Fig. 4d). Nevertheless, it remains possible that other factors, such as a pituitary effect of Rtfc, contribute to the decreased thyroid hormones and the relatively steady level of TSH.
Discussion
Previously, we have demonstrated the role of RTFC in thyroid cancer31. In this study, we investigated the role of Rtfc in the development of thyroid. Knockout of Rtfc reduces the thyroid differentiation efficiency of mouse ESCs, suggesting the role of Rtfc in thyroid development. However, Rtfc−/− mice are viable and fertile, and the size and the morphology of thyroid in Rtfc−/− mice are indistinguishable from WT mice. This discrepancy might be due to the more permissive and optimized environment for thyroid development in vivo. When ESCs differentiate into the thyroid lineage in vitro, some extrinsic cues, such as growth factors and signaling molecules, might be missing or compromised. Thus, the effect of Rtfc KO on thyroid can only be detected in the in vitro ESC differentiation system. Nevertheless, female Rtfc−/− mice, but not male Rtfc−/− mice, display mild hypothyroidism. These data validate the in vivo role of Rtfc in regulating the function of thyroid. Lack of any thyroid phenotype in male Rtfc−/− mice might be due to a more robust regulatory mechanism in male, thus compensating the loss of Rtfc. Consistently, thyroid diseases are more prevalent in women than in men, which might be caused by gonadal hormones and/or genes encoded by sexual chromosomes37,38. Even though only mild thyroid defects were identified in female Rtfc−/− mice, it remains possible that Rtfc knockout might have subtle effects in other tissues, which requires further investigation.
The molecular function of RTFC remains elusive. RTFC is widely expressed in a variety of tissues in both mouse and human (Fig. S4) and the RTFC proteins are highly conserved among different species, suggesting the functional importance of the RTFC gene. For example, human and mouse RTFC proteins share 87.5% of identity. Through analyzing the protein sequence, no structural domain is identified, except for a putative signal peptide at the N-terminus of RTFC. However, a pilot study to characterize the subcellular distribution of human proteins reveals that RTFC is mainly localized in the nucleus and the membrane39. It is interesting that overexpression of RTFC in mouse ESCs suppresses endogenous Rtfc expression (Fig. 1e), suggesting that Rtfc protein may function as a transcriptional regulator, negatively regulating the transcription of its own gene. Whether RTFC is secreted remains to be proved. In addition, RTFC has been identified in two screens for RNA binding proteins and for phosphopeptide binding proteins33,34. However, these biochemical properties of RTFC have not been further validated and linked to any biological process.
In summary, our data revealed the biological function of Rtfc in regulating the development and the function of thyroid. The role of Rtfc in other organs and the molecular functions of Rtfc require further investigation.
Methods
Ethics statement
All animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The use of mice for this research is approved by Nankai Animal Care and Use Committee (Approval number: 20140001).
Cell culture
KH2 mouse ESCs were cultured in growth medium consisting of 85% DMEM (high glucose, Invitrogen), 15% fetal bovine serum (FBS, Hyclone), 2 mM L-glutamine, 5000 units/ml penicillin and streptomycin, 0.1 mM nonessential amino acids (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma), and 1000 units/ml LIF (ESGRO, Chemicon).
Construction of cell lines
Inducible Nkx2.1-IRES-Pax8 (iN/P) ESCs were constructed from the parent cell line, KH2. In brief, the Nkx2.1-IRES-Pax8 cassette was inserted into the flp-in vector pgkATGfrt. The resulting vector and an Flpe expression vector were co-electroporated into KH2 cells. Twenty-four hours after electroporation, the cells were selected with 140 μg/ml hygromycin (Roche) for 10 days. The remaining hygromycin resistant colonies were individually picked. The correct recombination events were verified by genomic DNA PCR. Quantitative RT-PCR was also carried out to confirm the induced expression of Nkx2.1 and Pax8 upon doxycycline (Dox, 5 μg/ml) treatment.
A pair of TALEN vectors targeting the exon 2 of mouse Rtfc gene (Fig. 1a) were constructed. Recognition sequences of TALENs are 5′-CTGGCTGAGGCCGCCCT-3′ (left arm) and 5′-TTTAACAACTCATTGTT-3′ (right arm). Mouse ESCs were transfected with two TALEN vectors using Lipofectamine™ 2000 (Invitrogen). Forty-eight hours after transfection, transfected ESCs were re-seeded in a 6-well plate at 500 cells per well. After 5–8 days, individual colonies were picked. Disruption of the HinP1I site in the Rtfc locus was first screened by PCR amplification and HinP1I (NEB) digestion. Then sequencing analysis was carried out to confirm the frame-shift mutations in mutated ESC clones.
To construct stable GFP, WT and V205M RTFC overexpression iN/P ESCs, plasmids overexpressing GFP, WT, and V205M RTFC were transfected into iN/P ESCs using Lipofectamine™ 3000 (Invitrogen). Twenty-four hours after transfection, the transfected cells were selected with 500 μg/ml G418 (Gibco) for 9 days. The remaining G418 resistant colonies were individually picked. The integration of exogenous DNA was verified by genomic DNA PCR. Quantitative RT-PCR and Western blot were also carried out to validate the overexpression of WT and V205M RTFC.
Thyroid differentiation of ESCs
Embryoid bodies (EBs) were formed by culturing ESCs in hanging drops (1, 000 cells per 25 μl drop) in EB differentiation medium (mouse ESC growth medium without LIF, but supplemented with 50 μg/ml ascorbic acid (Sigma)). On day 4, EBs were harvested and plated in gelatin-coated 6-well plates (20 EBs/well) in EB differentiation medium supplemented with 5 μg/ml Dox (Sigma) for 3 days to allow further differentiation.
Generation of Rtfc knockout mice
The same TALENs were used to knockout Rtfc in mouse ESCs and in mice. TALEN mRNAs were transcribed in vitro using the Sp6 mMESSAGE mMACHINE Kit (Ambion). TALEN mRNAs (10 ng/μl) were injected into the cytoplasma of fertilized eggs of C57BL/6 mice. The injected embryos were cultured in KSOM with amino acids at 37 °C under 5% CO2 in air for 24 hours, and then transferred into pseudopregnant mice. One-cell embryo injection and embryo transfer were carried out by Cyagen biosciences (Guangzhou, China).
Genotyping of Rtfc alleles
Genomic DNA was extracted from mice toes or cells using phenol-chloroform method, and PCR amplification was performed with primers, 5′-TGGAGCACTTGTGAAGCGACCTAT-3′ and 5′-AGTTGCTGGCTTCTCCTGGACTT-3′. PCR products were then digested by HinP1I at 37 °C. DNA fragments were separated in 2% agarose gel by electrophoresis. For WT Rtfc allele, the PCR products were completely digested into two fragments of 304 bp and 162 bp. For mutated Rtfc allele, the amplified DNA cannot be cut by HinP1I, resulting in a single ~466 bp band.
Western Blot Analysis
Cells were lysed in lysis buffer (Beyotime), and protein concentration was measured using a BCA protein assay kit (Beyotime) to ensure equal loading. The samples were resolved by SDS-PAGE, followed by transferring onto a PVDF membrane (Millipore). Membranes were probed with anti-C14orf93 (Sigma), anti-FLAG (Sigma), anti-tubulin (Huada, Beijing). Bound primary antibodies were recognized by HRP-linked secondary antibodies (GE Healthcare). Immunoreactivity was detected by ECL Plus (Beyotime). Digital images were taken by the automatic chemiluminescence imaging analysis system (Tanon).
Quantitative RT–PCR
Total RNA was extracted from cells using RNeasy Mini kit (Qiagen). cDNA synthesis was performed using Transcriptor First Strand cDNA Synthesis kit (Roche) according to manufacturer’s instruction. PCR reactions were performed with FastStart Universal SYBR Green Master (Roche) in a BioRad iQ5 system. PCR cycling conditions were: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 30 s, and then a melting curve of the amplified DNA was acquired. Quantification of target genes was normalized with β-Actin. Primer information was listed in Supplementary Table S1.
Thyroid dissection
Three-month old mice were sacrificed by cervical dislocation, and then thyroids were taken out by dissecting of necks. Images were acquired with Leica M165 FC Fluorescent Stereo Microscope.
Hematoxylin and eosin (HE) staining
Dissected thyroids were fixed using 4% PFA at 4 °C for 24 h, dehydrated through xylenes and alcohols, and embedded in paraffin. Sections were cut at 5 μm and stained with haematoxylin and eosin for histological examination. Images were taken with Leica DM3000 Microscope.
Measurement of serum thyroid hormones and TSH
Serum total thyroxine (T4), total 3,3′,5-Triiodothyronine (T3) and thyroid stimulating hormone (TSH) were measured with Iodine [125I] Thyroxine Radioimmunoassay Kit, Iodine [125I] 3,3′,5-Triiodothyronine Radioimmunoassay Kit, and Iodine [125I] Human Thyroid Stimulating Hormone Radioimmunoassay Kit (Beijing north institute of biological technology, Beijing), respectively. These radioimmunoassays were performed by Beijing Kemeixinyun Biotechnology Company.
Statistical analysis
All data were analyzed by Student’s t-test. Statistically significant p values were indicated in figures as follows: ***p < 0.001, **p < 0.01, *p < 0.05. Additional information of methods is provided in online supplementary file.
Additional Information
How to cite this article: Yu, Y. et al. Rtfc (4931414P19Rik) Regulates in vitro Thyroid Differentiation and in vivo Thyroid Function. Sci. Rep. 7, 43396; doi: 10.1038/srep43396 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Brent, G. A. Mechanisms of thyroid hormone action. J Clin Invest 122, 3035–3043 (2012).
Cheng, S. Y., Leonard, J. L. & Davis, P. J. Molecular aspects of thyroid hormone actions. Endocr Rev 31, 139–170 (2010).
Thompson, C. C. & Potter, G. B. Thyroid hormone action in neural development. Cereb Cortex 10, 939–945 (2000).
Kim, H. Y. & Mohan, S. Role and Mechanisms of Actions of Thyroid Hormone on the Skeletal Development. Bone Res 1, 146–161 (2013).
Flood, D. E., Fernandino, J. I. & Langlois, V. S. Thyroid hormones in male reproductive development: evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen Comp Endocrinol 192, 2–14 (2013).
Sinha, R. & Yen, P. M. In Endotext (eds L. J. De Groot et al.) (2000).
Stoupa, A., Kariyawasam, D., Carre, A. & Polak, M. Update of Thyroid Developmental Genes. Endocrinol Metab Clin North Am 45, 243–254 (2016).
Shepard, T. H. Onset of function in the human fetal thyroid: biochemical and radioautographic studies from organ culture. J Clin Endocrinol Metab 27, 945–958 (1967).
Fagman, H. & Nilsson, M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol 323, 35–54 (2010).
De Felice, M. & Di Lauro, R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 25, 722–746 (2004).
Kimura, S. et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10, 60–69 (1996).
Mansouri, A., Chowdhury, K. & Gruss, P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19, 87–90 (1998).
De Felice, M. et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet 19, 395–398 (1998).
Martinez Barbera, J. P. et al. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127, 2433–2445 (2000).
Damante, G. & Di Lauro, R. Thyroid-specific gene expression. Biochim Biophys Acta 1218, 255–266 (1994).
Ohno, M., Zannini, M., Levy, O., Carrasco, N. & di Lauro, R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol 19, 2051–2060 (1999).
Damante, G., Tell, G. & Di Lauro, R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol 66, 307–356 (2001).
Nettore, I. C., Cacace, V., De Fusco, C., Colao, A. & Macchia, P. E. The molecular causes of thyroid dysgenesis: a systematic review. J Endocrinol Invest 36, 654–664 (2013).
Fernandez, L. P., Lopez-Marquez, A. & Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat Rev Endocrinol 11, 29–42 (2015).
Nettore, I. C. et al. Identification and functional characterization of a novel mutation in the NKX2-1 gene: comparison with the data in the literature. Thyroid 23, 675–682 (2013).
Krude, H. et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest 109, 475–480 (2002).
Carre, A. et al. Polymorphic length of FOXE1 alanine stretch: evidence for genetic susceptibility to thyroid dysgenesis. Hum Genet 122, 467–476 (2007).
Santarpia, L. et al. TTF-2/FOXE1 gene polymorphisms in Sicilian patients with permanent primary congenital hypothyroidism. J Endocrinol Invest 30, 13–19 (2007).
Castanet, M. & Polak, M. Spectrum of Human Foxe1/TTF2 Mutations. Horm Res Paediatr 73, 423–429 (2010).
Meeus, L. et al. Characterization of a novel loss of function mutation of PAX8 in a familial case of congenital hypothyroidism with in-place, normal-sized thyroid. J Clin Endocrinol Metab 89, 4285–4291 (2004).
Grasberger, H. et al. Autosomal dominant resistance to thyrotropin as a distinct entity in five multigenerational kindreds: clinical characterization and exclusion of candidate loci. J Clin Endocrinol Metab 90, 4025–4034 (2005).
Ngan, E. S. et al. A germline mutation (A339V) in thyroid transcription factor-1 (TITF-1/NKX2.1) in patients with multinodular goiter and papillary thyroid carcinoma. J Natl Cancer Inst 101, 162–175 (2009).
Tomaz, R. A. et al. FOXE1 polymorphisms are associated with familial and sporadic nonmedullary thyroid cancer susceptibility. Clin Endocrinol (Oxf) 77, 926–933 (2012).
Pereira, J. S. et al. Identification of a novel germline FOXE1 variant in patients with familial non-medullary thyroid carcinoma (FNMTC). Endocrine 49, 204–214 (2015).
Gregory Powell, J. et al. The PAX8/PPARgamma fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARgamma inhibition. Oncogene 23, 3634–3641 (2004).
Liu, C. et al. C14orf93 (RTFC) is identified as a novel susceptibility gene for familial nonmedullary thyroid cancer. Biochem Biophys Res Commun, 10.1016/j.bbrc.2016.1011.1078 (2016).
Cooper, C. D. et al. Identification and characterization of peripheral T-cell lymphoma-associated SEREX antigens. PLoS One 6, e23916 (2011).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Christofk, H. R., Wu, N., Cantley, L. C. & Asara, J. M. Proteomic screening method for phosphopeptide motif binding proteins using peptide libraries. J Proteome Res 10, 4158–4164 (2011).
Antonica, F. et al. Generation of functional thyroid from embryonic stem cells. Nature 491, 66–71 (2012).
Ruf, J. & Carayon, P. Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 445, 269–277 (2006).
Tunbridge, W. M. et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin. Endocrinol. (Oxf.) 7, 481–493 (1977).
Vanderpump, M. P. et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. (Oxf.) 43, 55–68 (1995).
Barbe, L. et al. Toward a confocal subcellular atlas of the human proteome. Molecular & cellular proteomics: MCP 7, 499–508 (2008).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos 81472580, 81272282, 81502322, 31271547 and 31470081), the Natural Science Foundation of Tianjin, China (Nos 13JCQNJC10200 and 14JCYBJC23600), the Program for New Century Excellent Talents (NCET-13-0293), and the 111 Project Grant (B08011).
Author information
Authors and Affiliations
Contributions
Y.Y., C.L., J.Z., M.Z., W.W., X.R., D.L., and S.Z. performed the experiments, and M.G. and L.C. conceived the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, Y., Liu, C., Zhang, J. et al. Rtfc (4931414P19Rik) Regulates in vitro Thyroid Differentiation and in vivo Thyroid Function. Sci Rep 7, 43396 (2017). https://doi.org/10.1038/srep43396
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43396
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.