Abstract
Inflammatory bowel diseases (IBD) are widespread inflammatory diseases that cause debilitating health problems including cancer. In this study, we show that ZnO nanoparticle (ZnONP) treatment has markedly dose-dependent effects on the remission of dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. We demonstrate the mechanism involves the antioxidant and anti-inflammatory abilities of ZnONPs to suppress ROS and malondialdehyde (MDA) production; increase GSH level; suppress proinflammatory cytokines IL-1β and TNF-α and myeloperoxidase (MPO). The ZnONP treatment is able to activate the Nrf2 pathway in the cellular antioxidant defense system. The novel finding is that ZnONP combined with mesalazine (5-ASA) can enhance the therapeutic efficacy of 5-ASA in the treatment of DSS-induced colitis. Lastly, we found that ZnONP treatment can restore the changes in special colonic bacteria of DSS-mice while the drug 5-ASA cannot. These results indicate that ZnONPs can act as a medical additive for the therapy of IBD.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is an idiopathic disease primarily includes two types of chronic intestinal disorders: ulcerative colitis (UC) and Crohn’s disease (CD)1 which affect approximately 1.4 million Americans with an accidence of 29.6 per 100,0001,2,3,4. The clinical manifestations of IBD are characterized by weight loss, colonic mucosal ulceration and diarrhea with blood or mucus5,6. So far, the exact etiology of IBD remains poorly understood. Oxidative stress is considered as a vital factor to initiate and propagate the development of IBD7,8. Recent studies have shown that the intestinal mucosa of UC, colon cancer and Helicobacter pylori infected tissues had 10- to 100-fold increased ROS and imbalance in antioxidant defense9. Ongoing efforts to widen the benefit-to-risk window of IBD therapy are urgently needed. The efforts are required on a number of complementary fronts. One potential therapeutic strategy is to effectively scavenge excessively generated ROS.
Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that plays an essential role in the promoter region of genes encoding antioxidant and/or detoxifying enzymes and stress-responsive proteins10,11. Activation of Nrf2 and antioxidant enzymes quinone oxidoreductase-1 (NQO-1) can attenuate inflammatory damage and neutralize ROS of cells/tissues from inflammatory injuries12,13. Recent studies have demonstrated that activation of Nrf2 can protect against dextran sulfate sodium (DSS)-induced colitis and inflammation-associated colorectal cancer by maintenance of intestinal integrity and regulation of proinflammatory cytokines3,11,14,15. Nrf2 gene mutation might enhances the susceptibility and progression in DSS-induced colitis mice3,15. Activation of Nrf2 signaling with small molecule may represent a novel, safe and effective therapy in IBD patients.
Zinc (Zn) is one of the essential trace elements for several aspects of normal human development and health16,17. Growing evidence suggests that Zn involves in redox-regulated signaling and contributes to maintain the cell redox balance. which is associate with IBD17,18,19,20,21,22. Zinc supplementary therapy has been confirmed to effectively reduce the duration of acute and persistent diarrhea19. Zinc oxide (ZnO) is considered as a safe metal oxide compound and is approved by US Food and Drug Administration (FDA) for food and drug additive23. ZnO nanoparticles (ZnONPs) are commonly used in various applications including as drug carriers and fillings in medical materials and nutritional supplements24,25. Thus far, the potential therapeutic effect of ZnONPs on DSS-induced colitis is unknown.
In this study, we used biomedical approaches to investigate the therapeutic efficacy of ZnONPs and their protective mechanism in attenuating DSS-induced colitis model mice. We estimated the anti-oxidative and anti-inflammatory effects of ZnONPs in colitis tissues. Our data show that ZnONPs present more efficient therapeutic option for DSS-induced colitis than ZnO microparticle (ZnOMP) due to the more persistent Zn2+ release from ZnONPs. Moreover, we designed a novel therapeutic strategy of combining ZnONPs with antiulcer drug, mesalazine (5-aminosalicylic acid, 5-ASA), one of a commonly used drug for treating IBD, to the treatment of DSS-induced colitis in mice. The results obtained will have great potential to provide a new type of adjuvant agent for therapy of IBD.
Results
Characterization of ZnONP and ZnOMP suspension solution
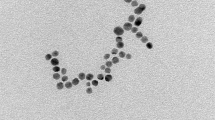
Representative TEM and SEM images show that the diameter of ZnONPs is 29.7 ± 4.0 nm (Fig. 1A and B). The ZnONPs were readily dispersed in 1% CMC solution, with a zeta potential of −59.4 ± 3.8 mV and a narrow size distribution of 69.4 ± 13.0 nm (Fig. 1C). The SEM images show the diameter of ZnOMPs is 701.3 ± 418.7 nm (Fig. 1D).
The releasing rates of Zn2+ from the particles in the suspension solution were evaluated. The data show that Zn2+ released from ZnONPs was very quick and was in a dose-dependent manner. Within 0.5~1 h post-dispersion, approximately 9.5, 13.8, and 23.2~26.5 μg/mL Zn2+ were detected in the supernatants of 1, 10 and 100 ZnONP suspensions, respectively; for 100 mg/mL ZnOMP suspension, 9.7 μg/mL Zn2+ were found in the supernatant, indicating that Zn2+ could quickly release from the surface of the particles and ZnONPs had much higher Zn2+ shedding rates than ZnOMPs (Supplementary Figure S1).
Safety observation of ZnONPs on the mouse intestinal tract
The disease activity index (DAI) was used to estimate some early histopathological changes in colon of mice after ZnONP treatment (Supplementary Figure S2A). As the results, no significant increase in DAI was observed at 0.5, 5 and 50 mg/kg doses of ZnONP treatment (Figures S2B and C). Meanwhile, no obvious histological lesion and colon length changes in ZnONP-treated mice were observed (Supplementary Figure S2D and E). The results indicate that ZnONP treatment did not induce any obvious colitis-like clinic changes in mice at the tested doses.
Effects of ZnONPs on DSS-induced colitis
To assess the efficiency of ZnONPs on colitis in mice, the DSS mice were orally administrated with ZnONPs for 7 days (Fig. 2A). The DSS-injured mice showed a significant increase in DAI, with visible blood in stool and marked colon shortening (Figs 2B,C and 3A,B). The obvious histological changes of epithelial ulceration, severe edema/inflammation and loss of crypts were found (Fig. 3C and D). As shown in Figs 2 and 3, ZnONP treatment can attenuate the DSS-induced colitis in mice with dose-dependent manner, including reduction DAI and histological lesion scores, and increase in colon length (p < 0.05, p < 0.01), indicating the potentially supportive therapy of ZnONPs on colitis.
(A) Experimental design of ZnONP treatment for DSS-injured mice. (B) Changes in DAI. Asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group (n = 5), respectively. (C) Representative photographs of viable blood observation in mice. C1: Control; C2: DSS; C3: ZnONPs 0.5 + DSS; C4: ZnONPs 5 + DSS; C5: ZnONPs 50 + DSS; C6: ZnOMPs 50 + DSS groups.
Representative images (A) and statistical analysis (B) of colon length. Representative H&E images (C) and histological scores (D) of the colon tissue in mice. Plus ++ denotes p < 0.01 compared with control group (n = 5); asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group (n = 5), respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with 50 mg/kg ZnOMPs + DSS group (n = 5), respectively. (C) Representative H&E-stained sections of colons, epithelial ulceration (black arrow), severe edema/inflammation (yellow asterisk), retention/regeneration of crypts (red arrow), and evidence of repair of the epithelium (box); scale bars, 100 μm.
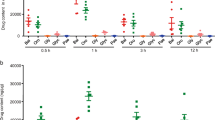
The changes of blood hematological and serum biochemical values were monitored to evaluate the potential effects of ZnONPs on DSS-induced colitis in mice (Supplementary method). The high white blood cell, lymphocytes and intermediate cell counts and low hemoglobin, albumin and triglyceride contents showed that DSS treatment could affect the immune system and cause severe inflammatory responses in the mouse colon (Fig. 4). Interestingly, administration of 50 mg/kg ZnONPs significantly induced reduction in the number of white blood cell, lymphocytes and intermediate cell counts and also induced the elevation of hemoglobin, albumin and triglyceride levels with a dose-dependent manner (Fig. 4A–F, p < 0.05, p < 0.01). Comparatively, the similar treatment by ZnOMPs (50 mg/kg) showed lower efficacious than by ZnONPs (Figs 2, 3 and 4).
After 7 days of treatment, blood hematologic (A–C) and biochemical (D–F) analysis were performed. Plus ++ denotes p < 0.01 compared with control group; asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group, respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with 50 mg/kg ZnOMPs + DSS group, respectively. n = 5.
In the meantime, the efficacy of Zn2+ (75 μg/kg bw, which based on the amount of Zn2+ released from 2 mg/mL ZnONP suspension solution) against DSS-induced colitis was evaluated. The data show that the Zn2+ treatment helped reduce the DAI scores and alleviate the histological damage, but its efficiency was not as significant as ZnONP treatment (Supplementary Figure S3). The results suggest that the released Zn2+ may contribute to the attenuation of colonic damage by ZnONP treatment.
Effects of combination therapy with ZnONPs and mesalazine on DSS-induced colitis
5-ASA is a bowel-specific antiulcer and is considered as an anti-inflammatory drug for clinical treatment of IBD26. The present results show that after 5-ASA therapy, the DAI and histological lesion scores had reduced and colon length increased in DSS-induced colitic mice (Fig. 5). Comparatively, the supportive therapy of 50 mg/kg ZnONPs (ZnONPs 50) with 5-ASA was more efficacious than 5-ASA or ZnONPs 50, respectively, with more significant reduction in DAI and increasing in colon length in DSS-induced colitis mice (Fig. 5B and C; p < 0.05, p < 0.01). The histopathological analysis of the colon tissues shows a more effective supportive effect of ZnONPs 50 for 5-ASA treatment compared with only 5-ASA or ZnONPs 50 treatment, the evidence includes less inflammatory infiltration, more regeneration of crypts and complete epithelial in the colon of DSS mice (Fig. 5D and E). Correspondingly, the levels of white blood cell, lymphocytes and intermediate cell counts significantly decreased by the combination therapy (Fig. 5F–H, p < 0.05, p < 0.01). The results demonstrate that ZnONPs can enhance the antiulcer activity of 5-ASA on DSS-induced colitis in mice.
(A) Experimental design. Changes in DAI (B) and colon length (C). Representative H&E images (D) and histological scores (E) of the colon tissues in mice. Mean and standard deviation of white blood cell count (F), lymphocytes count (G) and intermediate cell count (H) of mice by different treatments. Plus ++ denotes p < 0.01 compared with control group; asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group, respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with ZnONPs 50 + 5-ASA + DSS group, respectively. n = 5. (D) Representative H&E-stained sections of colons, D1: Control; D2: DSS; D3: ZnONPs 50 + DSS; D4: 5-ASA + DSS; D5: ZnONPs 50 + 5-ASA + DSS groups; epithelial ulceration (black arrow), severe edema/inflammation (yellow asterisk), retention/regeneration of crypts (red arrow), and evidence of repair of the epithelium (box); scale bars, 100 μm.
Treatment with ZnONPs reduces oxidative stress in the colon of DSS-induced colitis in mice
The levels of ROS and MDA significantly increased, and GSH significantly decreased in the colon of DSS-mice as compared to the control group, indicating that DSS treatment induced significant oxidative damage in the colon of mice (Fig. 6A–C, p < 0.01). The ZnONP treatments (0.5, 5, 50 mg/kg) significantly reduced the levels of ROS and MDA, and increased GSH in colon of DSS mice in a dose-dependent manner (Fig. 6A–C, p < 0.05). The results demonstrate that ZnONPs can exert antioxidative effects on DSS-induced colitis in mice. It is worth noting that the combination therapy of ZnONPs 50 + 5-ASA shows the highest antioxidative efficacy among the single 5-ASA, ZnONPs 50 and ZnOMPs 50 treatments (Fig. 6A–C).
Levels of ROS (A), GSH (B), MDA (C), MPO activity (D), IL-1β (E) and TNF-α (F) in colon of mice. Plus ++ denotes p < 0.01 compared with control group; asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group, respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with 50 mg/kg ZnONPs + DSS group, respectively; ampersand & and && denote p < 0.05 and p < 0.01, compared with 5-ASA + DSS group, respectively. n = 5.
In the study, the myeloperoxidase (MPO) activity in DSS-induced colitic mice elevated about four times as compared with the control mice (Fig. 6D), which is consistent with the previous studies27. The oral administration of single ZnONPs and ZnONPs + 5-ASA combination significantly reduced MPO activity in the DSS-mice (Fig. 6D, p < 0.01). To determine the effect of ZnONPs on pro-inflammatory cytokine expression, colonic interleukin (IL)-1β, and tumor necrosis factor (TNF-α), which are well-known markers of inflammation and play an important role in UC27, were determined. The data show that ZnONP treatment significantly decreased the expression of IL-1β and TNF-α in DSS-treated colon (Fig. 6E and F, p < 0.01). Moreover, ZnONPs can enhance the anti-inflammatory effects of 5-ASA against DSS-induced colitis in mice (Fig. 6D and E, p < 0.01; Fig. 6F, p < 0.05). Comparatively, the anti-inflammatory effect of ZnONPs is better than that of ZnOMPs (Fig. 6D and E, p < 0.01; Fig. 6F, p < 0.05).
These results demonstrate the potential anti-oxidant and anti-inflammatory effects of ZnONPs against DSS-induced colitis in mice, especially of their combination with 5-ASA.
Modulation of Nrf2 and NQO-1 expression by ZnONPs against DSS-induced colitis in mice
Nrf2 is an important cytoprotective transcription factor and can induce the expression of antioxidant enzymes, such as NQO-1, which can attenuate oxidative and inflammatory damage28,29. DSS treatment induced reduction of Nrf2 and NQO-1 expressions compared with the control group in colon tissues (Fig. 7). Interestingly, ZnONP treatment led to significantly dose-dependent up-regulation of Nrf2 and NQO-1 in colitic colon (Fig. 7B and C, p < 0.05). Comparatively, the combination treatment of ZnONPs 50 + 5-ASA induced the most obvious results. In contrast, the level of Nrf2 and NQO-1 expression in colon tissues in ZnOMP-treated mice was lower than that in ZnONP-treated ones (Fig. 7B and C, p < 0.01). These results indicated that ZnONPs protected against DSS-induced colitis by activating the Nrf2 signaling pathway.
(A) Western blotting analysis of the expression change of Nrf2 and NQO-1 proteins in the colon of mice, β-actin was used as a housekeeping control; Full-length gels are presented in Supplementary Figure S9. (B,C) The statistical analysis showed that ZnONP treatment led to a significant increase in the expression of Nrf2 and NQO-1 in the colon of DSS-induced colitis mice. All protein expression levels were normalized to housekeeping control. Plus ++ denotes p < 0.01 compared with control group; asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group, respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with 50 mg/kg ZnONPs + DSS group, respectively; ampersand & and && denote p < 0.05 and p < 0.01, compared with 5-ASA + DSS group, respectively. n = 5.
Effect of ZnONPs on colonic microflora in DSS-induced colitis mice
Several evidences suggest that the gut microbiota play a vital role in the pathogenesis of IBD30. The changes of gut bacteria may contribute to the development of DSS-induced colitis30,31. To investigate the alterations of gut bacteria composition in colitis mouse model, the relative abundance of five common gut bacterium species in mouse colon, including Enterobacterium, Enterococcus, Staphylococcus aureus, Lactobacillus and Bifidobacterium were analyzed (Supplementary Figures S3–S7). The data show that the number of Enterobacterium, Enterococcus and Staphylococcus aureus significantly increased, meanwhile, the number of probiotics of Lactobacillus and Bifidobacterium significantly decreased in colon of DSS-injured mice compared with the control group (Fig. 8, p < 0.01). The 5-SAS therapy effectively decreased the number of Enterobacterium, Enterococcus and S. aureus. Interestingly, the treatments of ZnONPs alone decreased the number of Enterobacterium, Enterococcus and S. aureus, meanwhile, increased the probiotics Lactobacillus and Bifidobacterium in a dose-dependent manner. The combination therapy of 50 mg/kg ZnONPs + 5-ASA induced the similar antibacterial effects. In contrast, ZnONPs show more effective antibacterial activity than the similar dose of ZnOMPs (Fig. 8, p < 0.01).
Statistical analysis of the number of Enterobacter (A), Enterococcus (B), Staphylococcus aureus (C), Lactobacillus (D) and Bifidobacterium (E) in the colon of mice. Plus ++ denotes p < 0.01 compared with control group; asterisks * and ** denote p < 0.05 and p < 0.01 compared with DSS group, respectively; crosshatch # and ## denote p < 0.05 and p < 0.01 compared with 50 mg/kg ZnONPs + DSS group, respectively.
Discussion
IBD (includes UC and CD) is a chronic intestinal disorder of the gastrointestinal tract that affects millions of patients worldwide1. It is reported that after 30 years of living with the diseases, UC and CD patients pose a risk of developing colitis-associated colon cancer, the third most common malignancy and one of the major causes of cancer related death in humans6. Up to now, the precise of the initiation and/or propagation of the inflammation for IBD is still unknown and is difficult to remove. Many efforts have been made to find effective therapeutic treatments for the disease. Mesalazine (5-ASA) is the first-line therapy for IBD, especially for maintenance of remission32. The pharmacodynamic studies show that 5-ASA inhibits inflammatory cell functions, plasma cell antibody production and TNF activity, decreases IL-1 production, and acts as a free oxygen radical scavenger33. More importantly, a large amount of molecular data support the notion that 5-ASA acts as chemopreventive compounds to interfere with colorectal cancer development, though the exact mechanism remains unknown26. However, although 5-ASA preparations are generally well tolerated by most patients, adverse reactions after high dosage and long term intake have been described, including nausea, vomiting, worsening colitis, and rarely nephrotoxicity, hepatotoxicity, pericarditis, pancreatitis, etc33. Therefore, a supportive therapy agent that can increase the curative effect is urgently required.
The DSS-induced colitis in mice is one of the commonly used murine models of IBD, which is very similar to human ulcerative colitis30,34. Our study shows that therapy by 5-ASA, as expected, obviously attenuated the severity of colitis in mice, including decrease DAI, increase colon length and alleviate the damage of colonic mucosal, furthermore, showed anti-inflammatory effects that suppressed proinflammatory mediators of MPO, IL-1β and TNF-α. The interesting result is that the ZnONP treatments showed markedly dose-dependent effects on the remission of the disease. In this study, we confirm that ZnONPs can antagonize oxidative stress and inflammatory responses, and importantly, restore the balance of intestinal flora in DSS-induced colitis mice. Activating Nrf2 pathway is probable to be the underlying molecular mechanism of ZnONP treatment in the cellular antioxidant defense system. The combination therapy ZnONPs with 5-ASA on DSS-induced colitis can enhance the antiulcer activity of 5-ASA, demonstrating a potentially supportive therapy of ZnONPs for the treatment of IBD.
Oxidative stress is hypothesized as a potential etiological and/or triggering factor for IBD, though the molecular pathways of colon cell damage are not completely understood7. Clinical data show that ROS increases in IBD patients, causing oxidative cellular damage and promoting carcinogenesis7. In the study, the DSS injury induced significant elevation of ROS production; decreased GSH and increased MDA levels in colon tissues, indicating the colitis in mice is correlated with oxidative stress. Meanwhile, significant high concentrations of proinflammatory cytokines IL-1β and TNF-α were generated, which are implicated in the progression of UC35. ZnONPs have gained a great advantage to apply in medical and nutritional applications due to their unique properties and biological safety23. Several actions of ZnONPs have been reported to the beneficial effects on human health, such as anticancer, antimicrobial and antioxidant activities23,36. In the study, the ZnONP treatments show significant dose-dependent efficacy for reduction of oxidative stress in colon of colitic mice, and meanwhile, suppress proinflammatory cytokines (IL-1β and TNF-α) and MPO, indicating ZnONPs possess anti-oxidant and anti-inflammatory activities. The 5-ASA treatments, as known, significantly reduced the oxidative stress levels, which is consistent with the previous reports26,32. The important finding is that the combination therapy of ZnONPs 50 + 5-ASA is more effective than monotherapy of ZnONPs and 5-ASA. Therefore, it is interesting to explore whether ZnONPs and 5-ASA involve in the similar antioxidant pathway in colitic mouse.
Nrf2 is a basic leucine zipper redox sensitive transcriptional factor that plays an important role in antioxidant response element-mediated induction of diverse antioxidant enzymes3,11,29. Khor et al. found that mice lacking Nrf2 were more susceptible to DSS-induced colitis3. The Nrf2−/− mice was found to be associated with more intense of proinflammatory cytokines, such as IL-1β and TNF-α, and more severe colonic colitis, including loss of colonic crypt, occurrence with massive infiltration by inflammatory cells than WT mice29. The similarly findings were found in the DSS-mice the colonic colitis increased in IL-1β and TNF-α, and reduced the expression of protein Nrf2 and phase II protein NQO-1, indicating DSS treatment decreased Nrf2 activation. Zinc is known as a major component of the cell antioxidant network that maintains cell redox homeostasis through various mechanisms including the regulation of antioxidant protective responses of Nrf218. The ZnONP treatments led to a dose-dependent increase in the expression of Nrf2 and anti-oxidant enzyme NQO-1 in the colon of colitic mice, demonstrating the activation of Nrf2 was involved in the response of ZnONPs acting as antioxidants in the colitic mice. The noteworthy result is that ZnONPs have more effective antioxidant activity than ZnOMPs when treated to the colitic mice. These results are in consistent with the previous findings that ZnONPs display extremely high scavenging capacity of free radicals due to their large surface area and high catalytic activity of the nanoparticles37. The antioxidative activity of 5-ASA show correlation with the responses of Nrf2 activation, but the levels of Nrf2 and NQO-1 expression are lower than ZnONPs-50 and higher than ZnOMPs-50 treatments. However, the combination therapy of 5-ASA with ZnONPs induced relatively the highest increased expression of Nrf2 and NQO-1, demonstrating that the combination therapy of 5-ASA with ZnONPs attenuates oxidative stress in colitis mice by modulating Nrf2 signaling pathways.
Recent studies have demonstrated that the resident intestinal bacteria play a role in initiating and maintaining IBD38. The disruption of the gut microbiota may drive the abnormal inflammatory response in the disease31,38. Antibiotics or antimicrobial peptide has shown to ameliorate or prevent inflammation in patients and in murine models of IBD, demonstrating the major impact of gut microbiota on disease pathogenesis30. For instant, a marked reduction in antimicrobial activity against B. vulgatus, E. coli, and E. faecalis was observed in extracts from Crohn patients39. The administration of a mixture of probiotics: Lactobacillus, Bifidobacterium and Streptococcussalivarious strains, has shown to have beneficial effects on colitis mouse models and IBD patients40. Our study shows that ZnONPs possess effects of supportive therapy for DSS-induced colitis in mice. However, the influence of ZnONPs on the colonic microflora remains unknown. The analysis of colonic microflora indicates that the DSS injury led to the changes in the intestinal microbiota of mice, i.e., the relative abundance of Enterobacter, Enterococcus and S. aureus increased and the probiotics, such as Lactobacillus and Bifidobacterium reduced. Interestingly, the ZnONP treatments alone obviously alter in the community of the gut bacteria in colon of the colitis mice, especially, in a dose-dependent manner, significantly increased the number of Lactobacillus and Bifidobacterium, of which are known as probiotics that have antagonistic effects against pathogenic bacteria in biological system, and in the meantime, decreased the numbers of Enterobacter, Enterococcus and S. aureus. In contrast, as an anti-inflammatory drug, 5-ASA treatment significantly decreased all the tested gut bacteria, including both the pathogens and probiotics, which is consistent with the previous reports26. Notably, the alteration of the gut microbacteria by the ZnONPs, 5-ASA and ZnONPs 50 + 5-ASA treatments accompany with the remission of the severity of colitis in mice. It is known that the invading bacteria, such as E. coli, contribute to inflammation by adhering to and invading epithelial cell lines and human ileal mucosa38,41; persist and replicate in macrophages, and secrete large quantities of TNF38. Our results indicate that the anti-inflammatory effect of ZnONPs associate with the reduction of translation and infection by commensal bacteria in the colonic mucosa. Since ZnONPs could also enhance the number of probiotics like Lactobacillus and Bifidobacterium in colon of colitis mice, ZnONP treatment can be applied in nutritional or supportive therapy in the treatment of IBD. The results suggest that the novel therapeutic strategy of ZnONPs conjunction with traditional drug (e.g., 5-ASA) could enhance the effectiveness of IBD therapy.
Additionally, the research demonstrates that ZnONPs are more efficacious than ZnOMPs in the treatment of colitis in mice. The results may due to several reasons: 1) ZnONPs have stronger antioxidant and anti-inflammatory activity than ZnOMPs; 2) restoration on the alteration of the abnormal intestinal microbiota by ZnONPs is more effective than by ZnOMPs; 3) ZnONPs are easier diffusion in the mucosa compared with larger-sized particles42, and more zinc ions may release from the nano-surface than micro-surface43, thus shows more obvious efficacy than that by ZnOMPs. The results indicate that ZnONPs is a promising zinc species with potential application in attenuation of IBD.
In summary, our study demonstrates that ZnONPs could attenuate DSS-induced colitis in mice, including reduction in DAI and histological lesion score, increase in colon length, which has the better therapeutic effects than that of ZnOMP treatment. The mechanism of the above effects may partially be due to the ability of ZnONPs to improve the colonic anti-oxidant and anti-inflammatory potential via suppressing ROS and MDA production; induction the antioxidant enzyme of GSH; meanwhile, suppression of proinflammatory cytokines (IL-1β and TNF-α) and MPO in the colitic mice. In addition, ZnONP treatment could induce upstream regulation of Nrf2 and NQO-1, which are the proteins representative antioxidant and cytoprotective factors involved in colitis and cancer chemoprevention12. Importantly, ZnONP treatment is first found beneficial to restore the unhealthy change of the intestinal microbiota in colitic mice, which has been demonstrated to be a vital role in initiating and maintaining IBD38. The combination therapy of ZnONPs with 5-ASA on DSS-induced colitis shows that ZnONPs can enhance the antiulcer activity of 5-ASA. Our results collectively suggest that ZnONPs acts as a supportive therapy for the treatment of IBD.
Methods
Preparation of ZnONP and ZnOMP suspension solution
ZnONPs and ZnOMPs were purchased from Hangzhou Wanjing New Material Co. Ltd (Hangzhou, China). Before animal experiments, ZnONPs were dispersed in 1% sodium carboxymethylcellulose (CMC) solution and the suspensions were sonicated at 12–15 °C for 0.5 h. Finally, the 10 mg/mL well-dispersed ZnONP and ZnOMP suspension solutions were obtained. For animal treatments, the suspension solution was further diluted with deionized water and sonicated for 15 min.
Animal
Male BABL/c mice (7-week-old, approximately 20 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The mice were housed two per cage at 24–26 °C with 60 ± 2% humidity and a 12-h light-dark cycle, having ad libitum access to water and food. All animal experiments were performed according to the guidelines for ethical conduct in the care and use of animals in research by Chinese Society of Toxicology, and were approved by the Office of Scientific Research Management of Institute (March 1, 2012 CCNU-IACUC-2012-011).
DSS-induced colitis in mice
The experimental acute colitis was induced in mice by feeding 3% (w/v) DSS (M.W. = 36,000–50,000 Da; MP Biomedicals) in their drinking water for 7 days.
Protective role of ZnONPs against colitis in mice
A total of 30 male mice were randomly divided into 6 groups (n = 5 per group) and treated as follows: (a) Control group (Control); (b) 3% DSS-induced colitis group (DSS); (c) 0.5 mg/kg bw ZnONPs + DSS group (ZnONPs 0.5 + DSS); (d) 5 mg/kg bw ZnONPs + DSS group (ZnONPs 5 + DSS); (e) 50 mg/kg bw ZnONPs + DSS group (ZnONPs 50 + DSS); and (f) 50 mg/kg bw ZnOMPs + DSS group (ZnOMPs 50 + DSS). The particle suspension was orally administrated to DSS mice once a day for 7 days.
As a comparison, the effects of Zn2+ that released from ZnONPs on DSS mice were estimated (Supplementary Data).
Therapeutic effect of combination ZnONPs with mesalazine against colitis in mice
The mice were randomly divided into 5 groups (n = 5): (a) Control group (Control); (b) 3% DSS-induced colitis group (DSS); (c) 50 mg/kg bw ZnONPs + DSS group (ZnONPs 50 + DSS); (d) 5-ASA + DSS group (5-ASA + DSS) and (e) 50 mg/kg ZnONPs + 5-ASA + DSS group (ZnONPs 50 + 5-ASA + DSS). The drug 5-ASA (200 mg/kg/day) and ZnONP suspensions were orally administered daily to the mice for 7 days.
DAI and colon length measurements
During 7 days of treatment, the changes in body weight, visible stool consistency and fecal bleeding were recorded daily. Disease activity index (DAI) is the summation of the stool consistency index (0–3); fecal bleeding index (0–3); and weight loss index (0–4)44. After 7 days of treatment, mice were anesthetized with diethyl ether and the entire colon (from cecum to rectum) was collected. Colon was gently washed with PBS (pH = 7.4) and the length was measured and recorded.
Colonic bacteria analysis
Freshly sterile colonic samples were collected and homogenized in ice-cold PBS (pH 7.4) in a glass homogenizer. 10-fold serial dilutions (10−1–10−7) were performed in medium. The intestinal bacteria: Enterobacter, Enterococcus and Staphylococcus aureus were cultured on Eosin-Methylene Blue agar, Enterococcus Agar and Mannitol Salt Agar (Qingdao Nissui Biological Technology Co. Ltd., Qingdao, China), respectively; Lactobacillus and Bifidobacterium were cultured on Lactobacillus Selective Agar and TPY Agar (Qingdao Nissui Biological Technology Co. Ltd., Qingdao, China) in an anaerobic jar containing an AnaeroGenTM bag (Oxoid), respectively. The colonies were incubated at 37 °C for 24–48 h and the counts were expressed as log CFU/g.
Statistical analysis
Data are presented as mean ± SD. Statistical graphs were generated using GraphPad Prism 5.0. One-way ANOVA combined with Fisher’s protected t-test were used to determine the statistical differences between groups. p < 0.05 and p < 0.01 were considered significant.
Additional Information
How to cite this article: Li, J. et al. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci. Rep. 7, 43126; doi: 10.1038/srep43126 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abraham, C. & Cho, J. H. Mechanisms of Disease. N Engl J Med 361, 2066–78 (2009).
Neumann, H. & Kiesslich, R. Endomicroscopy and endocytoscopy in IBD. Gastrointest Endosc Clin N Am 23, 695–705 (2013).
Khor, T. O. et al. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res 66, 11580–11584 (2006).
Dou, W. et al. Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int Immunopharmacol 23, 170–178 (2014).
Mishra, S. K. et al. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J Ethnopharmacol 143, 524–532 (2012).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–34 (2007).
Rezaie, A., Parker, R. D. & Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 52, 2015–21 (2007).
Takagi, T., Uchiyama, K. & Naito, Y. Oxidative Stress in Inflammatory Bowel Disease. In: Tsukahara, H., Kaneko, K., editors. Studies on Pediatric Disorders, New York, NY: Springer, New York, p. 301–314 (2014).
Wilson, D. S. et al. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 9, 923–928 (2010).
Kim, J., Cha, Y. N. & Surh, Y. J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690, 12–23 (2010).
Khor, T. O. et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 1, 187–91 (2008).
Lee, K. M., Kang, K., Lee, S. B. & Nho, C. W. Nuclear factor-E2 (Nrf2) is regulated through the differential activation of ERK1/2 and PKC alpha/betaII by Gymnasterkoreayne B. Cancer Lett 330, 225–32 (2013).
Chen, X.-L. & Kunsch, C. Induction of Cytoprotective Genes Through Nrf2/Antioxidant Response Element Pathway: A New Therapeutic Approach for the Treatment of Inflammatory Diseases. Curr Pharm Des 10, 879–891 (2004).
Trivedi, P., Jena, G. & Kumar, V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis‐associated colon carcinogenesis. Mol Carcinog 55, 255–267 (2015).
Osburn, W. O. et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer 121, 1883–1891 (2007).
Uriu-Adams, J. Y. & Keen, C. L. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res Part B: Dev Reprod Toxicol 89, 313–325 (2010).
Hambidge, M. Human Zinc Deficiency. The Journal of Nutrition 130, 1344S–1349S (2000).
Oteiza, P. I. Zinc and the modulation of redox homeostasis. Free Radic Biol Med 53, 1748–59 (2012).
Bhutta, Z. et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr 135, 689–697 (1999).
Luk, H. H., Ko, J. K. S., Fung, H. S. & Cho, C. H. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol 443, 197–204 (2002).
Massironi, S. et al. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin Nutr 32, 904–910 (2013).
Eiden, K. A. Nutritional Considerations in Inflammatory Bowel Disease: In: Parrish, C. R. Series Editor. Nutrition issues in gastroenterology, Series #5, Nutrition Support Specialist, Barnes-Jewish Hospital, St. Louis, MO. p. 33–54 (2003).
Vimala, K. et al. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem 49, 160–172 (2014).
Martirosyan, A. & Schneider, Y. J. Engineered nanomaterials in food: implications for food safety and consumer health. Int Environ Res Public Health 11, 5720–50 (2014).
Jones, N., Ray, B., Ranjit, K. T. & Manna, A. C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279, 71–76 (2008).
Lyakhovich, A. & Gasche, C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther 31, 202–9 (2010).
Ramasamy, S. et al. Intestinal Alkaline Phosphatase Has Beneficial Effects in Mouse Models of Chronic Colitis. Inflammatory bowel diseases 17, 532–542 (2011).
Pandurangan, A. K., Saadatdoust, Z., Hamzah, H. & Ismail, A. Dietary cocoa protects against colitis‐associated cancer by activating the Nrf2/Keap1 pathway. Biofactors 41, 1–14 (2015).
Li, W. et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76, 1485–9 (2008).
Nagalingam, N. A., Kao, J. Y. & Young, V. B. Microbial ecology of the murine gut associated with the development of DSS-colitis. Inflamm Bowel Dis 17, 917–926 (2011).
Gkouskou, K. K., Deligianni, C., Tsatsanis, C. & Eliopoulos, A. G. The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol 4, 28 (2014).
Iacucci, M., de Silva, S. & Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can J Gastroenterol 24, 127–133 (2010).
Schroeder, K. W. Is Mesalamine Safe? Gastroenterology & Hepatology 3, 878–879 (2007).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Ferguson, L. R. Chronic inflammation and mutagenesis. Mutat Res 690, 3–11 (2010).
Prasad, A. S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 43, 370–377 (2008).
Singh, B. N. et al. Biosynthesis of Stable Antioxidant ZnO Nanoparticles by Pseudomonas aeruginosa Rhamnolipids. PLoS ONE 9, e106937 (2014).
Sartor, R. B. & Mazmanian, S. K. Intestinal Microbes in Inflammatory Bowel Diseases. Am J Gastroenterol Suppl 1, 15–21 (2012).
Nuding, S., Fellermann, K., Wehkamp, J. & Stange, E. F. Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut 56, 1240–7 (2007).
Isaacs, K. & Herfarth, H. Role of probiotic therapy in IBD. Inflamm Bowel Dis. 14, 1597–1605 (2008).
Chen, H. et al. Coculture with Low-Dose SWCNT Attenuates Bacterial Invasion and Inflammation in Human Enterocyte-like Caco-2 Cells. Small 11, 4366–4378 (2015).
Lamprecht, A., Schäfer, U. & Lehr, C.-M. Size-Dependent Bioadhesion of Micro- and Nanoparticulate Carriers to the Inflamed Colonic Mucosa. Pharm Res 18, 788–793 (2001).
Wang, B. et al. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res 10, 263–276 (2008).
Cooper, H. S., Murthy, S., Shah, R. & Sedergran, D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69, 238–249 (1993).
Acknowledgements
This work was supported by the National Basic Research Program (2016YFA0201603), and the National Natural Science Foundation of China (11505193, 11475195, 91543118 and U1432245).
Author information
Authors and Affiliations
Contributions
Jinquan Li, Hanqing Chen, Xu Yang, Zhifang Chai, Weiyue Feng conceived and designed the experiments. Jinquan Li, Hanqing Chen, Bing Wang, Chengxu Cai performed the experiments. Jinquan Li, Hanqing Chen, Weiyue Feng analyzed the data. Jinquan Li, Hanqing Chen, Bing Wang contributed reagents, materials and analysis tools. Jinquan Li, Hanqing Chen, Xu Yang, Weiyue Feng wrote the manuscript. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, J., Chen, H., Wang, B. et al. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep 7, 43126 (2017). https://doi.org/10.1038/srep43126
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43126
This article is cited by
-
The progression of inorganic nanoparticles and natural products for inflammatory bowel disease
Journal of Nanobiotechnology (2024)
-
Toxicity and Hepatoprotective Effects of ZnO Nanoparticles on Normal and High-Fat Diet-Fed Rat Livers: Mechanism of Action
Biological Trace Element Research (2024)
-
Dietary Zinc Ameliorates TNBS-Induced Colitis in Mice Associated with Regulation of Th1/Th2/Th17 Balance and NF-κB/NLRP3 Signaling Pathway
Biological Trace Element Research (2024)
-
Anthelmintic and Hepatoprotective Activities of the Green-Synthesized Zinc Oxide Nanoparticles Against Parascaris equorum Infection in Rats
Acta Parasitologica (2024)
-
Role of Combination Treatment of Aspirin and Zinc in DMH-DSS-induced Colon Inflammation, Oxidative Stress and Tumour Progression in Male BALB/c Mice
Biological Trace Element Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.