Abstract
Tuberculosis (TB) poses a serious public health problem in Angola. No surveillance data on drug resistance is available and nothing is known regarding the genetic diversity and population structure of circulating Mycobacterium tuberculosis strains. Here, we have genotyped and evaluated drug susceptibility of 89 Mycobacterium tuberculosis clinical isolates from Luanda. Thirty-three different spoligotype profiles corresponding to 24 different Shared International Types (SIT) and 9 orphan profiles were detected. SIT 20 (LAM1) was the most prevalent (n = 16, 18.2%) followed by SIT 42 (LAM9; n = 15, 17.1%). Overall, the M. tuberculosis population structure in this sample was dominated by LAM (64.8%) and T (33.0%) strains. Twenty-four-loci MIRU-VNTR analysis revealed that a total of 13 isolates were grouped in 5 distinct clusters. Drug susceptibility data showed that 22 (24.7%) of the 89 clinical isolates were resistant to one or more antibacillary drugs of which 4 (4.5%) were multidrug resistant. In conclusion, this study demonstrates a high predominance of LAM strains circulating in the Luanda setting and the presence of recent transmission events. The rate and the emergence dynamics of drug resistant TB found in this sample are significant and highlight the need of further studies specifically focused on MDR-TB transmission.
Similar content being viewed by others

Introduction
Despite the importance that the African region plays in a global TB epidemiological context, many countries still lack data on the prevalence of specific M. tuberculosis strains and drug resistance1. This is the case for Angola, which presently lacks any data concerning drug resistance rates and prevalence of specific Mycobacterium tuberculosis genotypes and respective population structure. The latest World Health Organization (WHO) estimates for Angola suggest the occurrence of 90 000 new cases and an incidence rate of 370 cases per 100 000 habitants in 2014 combined with an increasing trend over the last two decades1. Furthermore, these estimates point towards the occurrence of 1500 cases of multidrug resistant (MDR) TB that were not yet bacteriologically confirmed1.
The situation is particularly concerning in Luanda, the capital city of Angola, and its province, which accounts for approximately one-third of the country’s TB cases (21 281 cases notified in 2013)2,3.
Understanding how TB transmission takes place is a key component to strategically manage TB from a public health perspective4,5,6. Furthermore, given the unexpected degree of genetic diversity more recently unveiled by whole genome sequencing and since different strains have the ability to elicit distinct immunopathological events, tracking specific strains plays an even more important role7,8,9,10. Several typing methods have been developed over the past decades to investigate the clonality, population structure and transmission of M. tuberculosis5,11,12,13,14. These, have gradually led to the development of online databases and have enabled the identification of epidemiologically links between patients and risk factors that otherwise would be nearly impossible to identify with the use of traditional epidemiological investigation and contact tracing6,15,16.
Currently, there is no data concerning M. tuberculosis diversity, population structure and drug resistance in Angola. Herein, we have characterized the genetic diversity and drug susceptibility profiles of circulating M. tuberculosis strains recovered from patients followed at a central hospital in Luanda.
Methods
Patients and Clinical Isolates
A total of 106 sputum samples, positive for sputum smear acid-fast bacilli, were collected from patients clinically diagnosed with TB in Hospital da Divina Providência (HDP) in Luanda district. HDP is located in the Kilamba-Kiaxi municipality and serves an estimated population of 990 892 inhabitants. Regarding TB treatment, HDP has a unit dedicated to the diagnosis of respiratory diseases (approximately 600 new TB cases/year) and directly observed treatment. Sample collection was performed between March to June 2014, where all sputum smear positive samples, comprising approximately 18% of the hospitals yearly diagnosed cases, were collected to avoid unbiased selection criteria. Patients’ demographical and clinical data were collected from clinical records. The study was approved by the Angolan Ministry of Health’s Ethics Committee, and all methods were performed in accordance with the relevant guidelines and regulations, including informed consent from all patients enrolled in the study.
Sputum sample decontamination was carried out using the NaOH/N-acetyl-L-Cysteine method and cultured on Lowenstein-Jensen slants and Middlebrook 7H9 medium supplemented with oleic acid, albumin, dextrose and catalase (OADC) and an antibiotic cocktail comprised by carbenicillin (final concentration: 5 μg/ml), trimethoprim (15 μg/ml), amphotericin B (1 μg/ml) and polymyxin B (20 U/ml).
High molecular weight genomic DNA was extracted from mycobacterial grown on Lowenstein-Jensen media by the cetyltrimethylammonium bromide (CTAB) method17.
All isolates were identified as belonging to the M. tuberculosis complex by positive PCR amplification of an internal IS6110 fragment17.
Drug Susceptibility Testing (DST)
All M. tuberculosis complex isolates were tested for first-line DST to all first-line antibacillary drugs (isoniazid (INH), rifampicin (RIF), ethambutol (EMB), streptomycin (STP) and pyrazinamide (PZA)) through the BACTEC™ MGIT™ 960 system (Becton Dickinson Diagnostic Systems, Sparks, MD, USA) using the standardized procedure according to the manufacturer’s instructions18.
Spoligotyping and MIRU-VNTR typing
Spoligotyping was performed as described previously by Kamerbeek et al.12. Detection of the hybridization patterns was carried out using the ECL® Chemiluminescence Detection System (GE Healthcare®, Cleveland, OH, USA).
All isolates were typed by 24-loci MIRU-VNTR using the multiplex amplification procedure described by Supply et al.11.
Spoligotyping and MIRU-VNTR profiles were assigned to lineage, clade, shared international type (SIT) and MIRU International Type (MIT) using the SITVIT WEB international database (http://www.pasteur-guadeloupe.fr:8081/SITVIT_ONLINE/index.jsp) and/or the SPOTCLUST tool (http://tbinsight.cs.rpi.edu/run_spotclust.html)16,19.
A dendrogram was constructed based on the MIRU-VNTR and spoligotyping data, as appropriated, using the MIRU-VNTRplus international database15. A cluster was defined as group of two or more strains with identical profile. The Hunter-Gaston index of diversity was computed as described previously20.
A minimum spanning tree was also constructed using the MIRU-VNTRplus database to investigate phylogenetic relationships within the sample and identify clonal complexes. A clonal complex was defined as groups of isolates that are within dual-locus variants of each other.
All statistical analyses were conducted using the IBM© SPSS© Statistics v.21 (IBM Corporation, Armonk, NY, USA).
Results
Patients and Clinical Isolates
In the present study we have collected 106 sputum samples, positive for acid-fast bacilli, in HDP in 2014. After culture and identification, five samples were excluded due to contamination; seven samples were duplicated from the same patient; and, five samples showed no growth after a 3 month incubation period. The remaining samples yielded 89 M. tuberculosis complex clinical isolates, each corresponding to a different patient (Fig. 1).
From the 106 sputum samples positive for acid-fast bacilli, 7 were excluded since they were duplicates from the same patient, 5 were contaminated and another 5 showed no growth, leaving a total of 89 M. tuberculosis clinical isolates. Drug susceptibility testing revealed that 22 isolates were resistant to one or more antibacillary drugs, of which four corresponded to MDR-TB isolates. Genotyping analysis allowed the identification of 13 clustered isolates across five different MIRU-VNTR clusters with one isolate excluded due to mixed strain infection. Spoligotyping analysis revealed a population structure dominated mostly by LAM and T strains.
It was only possible to collect partial demographical and clinical data for 60 (67.4%) of the 89 studied patients (see Supplementary Table 1). The majority of the patients resided in the Kilamba-Kiaxi municipality (n = 34/58, 58.6%), where HDP is located, followed by a significant proportion of patients living in Viana (n = 19/58, 32.8%) (Supplementary Table 1).
The majority of the patients comprising this sample were registered as new patients (n = 53/60, 88.3%), approximately half presented with cavitary lung disease (n = 29/50, 58.0%) and eight out of 55 patients (14.5%) were co-infected with HIV. Fifty-one (87.9%) out of 58 patients started the 2HRZE/4HR treatment regimen while the remaining seven started the 2HRZES/4HR treatment regimen. The majority of the patients (n = 39/60, 65.0%) achieved both bacteriological and clinical cure after completing the treatment regimen (Supplementary Table 1).
Drug Resistance
We have detected eight different drug-resistance profiles and overall, 22 (24.7%) isolates showed resistance to one or more antibacillary drugs (Table 1). Four (4.5%) isolates exhibited a multidrug resistant (MDR) profile, i.e. resistance to at least isoniazid (INH) and rifampicin (RIF), whereas 13 of the 22 drug resistant isolates were resistant to INH or RIF and can thus be considered pre-MDR-TB isolates.
Population structure and Molecular Epidemiology
To gain insight on the sample’s population structure all strains were genotyped by spoligotyping and 24-loci MIRU-VNTR. One isolate (HDP7743) showed double alleles in 5 of the 24 MIRU-VNTR loci and was therefore excluded from the genotypic analysis. Spoligotyping analysis of the remaining 88 M. tuberculosis isolates enabled the detection of 33 different spoligotype profiles corresponding to 24 different Shared International Types (SIT) and 9 orphan profiles (Table 2, Fig. 2). SIT 20 (LAM1) was the most prevalent SIT found (n = 16, 18.2%) followed by SITs 42 (LAM9; n = 15, 17. 1%) and 53 (T1; n = 12, 13.6%).
Comparatively, the 24-loci MIRU-VNTR analysis showed, as expected, a superior discriminatory power in which only 13 isolates were clustered across 5 clusters (Fig. 2, Table 2). Analysis of the genotypic data using the 12, 15 and 24-loci MIRU-VNTR sets showed that the 15- and 24-loci sets had comparable discriminatory ability as illustrated by the comparable values of the Hunter-Gaston Index of diversity (D) (Table 3)20,21. Both spoligotyping and 12-loci MIRU-VNTR, owing to a lesser capability of genotypic discrimination, yielded a high degree of clustering, which does not necessarily illustrate recent transmission events but rather be associated with an earlier common origin followed by diversification at the genotypic level, in particular at loci with a faster molecular clock11.
Drug resistant isolates were found across distinct clades and MIRU-VNTR clusters although the largest MIRU-VNTR cluster detected (n = 5) harboured 3 drug resistant isolates, including one MDR isolate (Fig. 1). A two-isolate cluster composed by pre-MDR strains (mono-INH resistance) was also detected.
To better understand transmission, a minimum spanning tree was constructed using double-locus variants from 24-loci MIRU-VNTR data as a maximum distance to link and define clonal complexes (Fig. 3). We have identified 12 clonal complexes which ranged in size from two to 17 isolates. The largest clonal complex, CC1, was comprised by 17 LAM isolates, mostly belonging to the LAM1 clade and MIT10 (Supplementary Table 2). A second large LAM9 clonal complex, CC2, was also detected comprised by six isolates. T strains were mostly detected on three clonal complexes, CC3–6, comprised by 6, 5 and 4 isolates, respectively. Furthermore, 32 singleton profiles were identified (Table 4).
The tree was constructed based on 24-loci MIRU-VNTR genotypic data and clonal complexes defined as MIRU-VNTR profiles within double-locus variants of each other. Clonal complexes have been highlighted and annotated on the tree (CC1-12) along with each node SIT, whenever each node is composed by strains of the same SIT and the spoligotype profile is not orphan. Numbers on the lines connecting each node indicate the loci difference between each node.
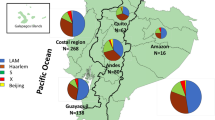
Next, we have combined the genotypic data with the geographical data, i.e. patient residency area. Geographical case dispersion analysis was restricted to the Kilamba-Kiaxi and Viana since all but 5 patients resided in these municipalities. This is expected since HDP is located in the eastern zone of Kilamba-Kiaxi, near the Viana municipality and primarily serves these two municipalities. Although not meeting statistical significance, genotypic dispersion through these municipalities appears to exhibit substantial differences for some of the clades or clonal complexes assessed by spoligotyping or MIRU-VNTR, respectively (Table 5). Generally, LAM strains were more prevalent in Kilamba-Kiaxi in comparison to the Viana municipality (LAM: 70.59% vs 42.11%, respectively), which consequently holds a higher percentage of T strains (52.63%). Stratification by clonal complex, clade and SIT allowed picturing the distribution of these genetic groups at a finer resolution: in Kilamba-Kiaxi CC1 stands out as the most prevalent MIRU-VNTR clonal complex, which correlates with a high SIT20/LAM1 prevalence in this municipality (Table 5). CC3 also appears to play an important role in Kilamba-Kiaxi’s epidemiological context, consistent with the prevalence of T1 strains. Although no clonal complex appears to be more prevalent in Viana, SIT53/T1 and SIT42/LAM9 strains exhibited a higher prevalence in this setting than in Kilamba-Kiaxi (Table 5).
Discussion
In the present study we made the first characterization of the genetic diversity and drug resistance of M. tuberculosis complex strains circulating in Luanda, Angola’s most important setting concerning TB epidemiology2.
Drug susceptibility testing revealed a worrying situation concerning resistance rates. Approximately one-quarter of the studied isolates were resistant to one or more antibacillary drugs. This situation is particularly notorious for INH resistance which was detected on 18% of the clinical isolates. Pre-MDR-TB strains (i.e. monoresistant to INH or RIF) were found to represent 14.6% of the studied samples highlighting an increased potential for MDR-TB development during the course of treatment by resistance amplification due to standardized treatment regimens and particularly, in cases of poor adherence22,23. The potential risk for resistance amplification is well patent in the Lisboa3 and Q1 strains in Portugal, or the KZN strains in South Africa that have gradually acquired resistance to an increasing number of drugs over the years in a stepwise manner8,24,25. Also, the MDR-TB rate appears to be moderate (4.5%) but may in fact represent a serious problem from a public health perspective. Assuming that the rate found in this study is the same across the entire province setting, new MDR-TB cases can be estimated to be at least around 958 cases based on the official province data estimate for 2013, of 21 281 new cases/year2. This situation clearly contrasts with the data reported in a study conducted in the Ndola district, in Zambia, a neighbouring country, in which from 193 clinical isolates only 8.8% and 0.5% showed resistance to any antibacillary drug or MDR, respectively26. A clear example of the consequences of not having an adequate laboratory support on the follow up of TB patients under treatment, a recurrent situation in many countries with unreported high levels of acquired MDR-TB27,28,29.
Besides drug resistance we also investigated the M. tuberculosis genetic diversity, population structure and transmission dynamics. Genotypic analysis through spoligotyping and 24-loci MIRU-VNTR revealed a population structure dominated by LAM and the ill-defined T strains. Comparing our data with the recently published global framework for the LAM lineage proposed by Mokrousov et al.30 and the SNP barcode system for phylogenetic classification proposed by Coll et al.10, we find that: (i) the single strain belonging to SIT33 exhibits the VNTR signature characterized by loci 2401_2 and 3171_1 alleles in agreement to what has been proposed previously; (ii) 11 (12.5%) isolates bearing LAM or possibly LAM-derived spoligotype profiles formed a monophyletic clade bearing the locus 154_1 allele, characteristic of the RD115-sublineage/sublineage 4.3.3; 44 (50.0%) isolates bearing LAM profiles were found to exhibit the VNTR locus 802_1 allele proposed to be a marker for the RD174-sublineage/sublineage 4.3.410,30. The data herein, presented therefore contributes to the VNTR data available for sub-Saharan Africa and is consistent with an increasing LAM prevalence when moving southwards on the African continent30.
It is also important to consider when structuring M. tuberculosis samples by spoligotyping clades that spoligotyping profiles show a tendency to converge and that a given clade does not necessarily share a more recent common ancestor. Such is particularly true, e.g., among the ill-defined T lineage and LAM9 clade10. From a public health standpoint such polyphyletic clades may therefore enclose a higher degree of genetic diversity and underpin distinct transmission networks.
Again, when comparing with the neighbouring countries, only Zambia and Namibia have sufficient data on SITVIT WEB database to allow such comparison, as the M. tuberculosis population structure in the Democratic Republic of the Congo is presently unknown16. While Zambia exhibits a more diverse population structure dominated by the LAM11-ZWE (not found in the present study) but including other LAM, CAS, MANU and EAI strains, Namibia exhibits a more restricted diversity mainly dominated by SIT 20 (LAM1) strains that account to 78.5% of the strains deposited in SITVIT WEB for this country16. Although we cannot rule out epidemiological influence from the Democratic Republic of the Congo in the Angolan population structure, for Zambia there is no data supporting any epidemiological influence in Angola since no LAM11-ZWE strains were found in our sample. On the other hand, Namibia’s M. tuberculosis population structure might be or have been of importance in shaping the current Angolan epidemiological context concerning genetic diversity as the most prevalent SIT in Namibia (SIT 20) was also the most prevalent SIT in our study albeit with a significant difference in prevalence (78.5% vs 18.2%, respectively). The relations that both countries have maintained during the Angolan civil war might have been of particular importance in strain circulation between both countries as a peak of 30 881 Angolan citizens took refuge in Namibia in 2001, most of which have later returned to the country of origin31. Similarly, the Democratic Republic of the Congo, for which no data on the M. tuberculosis genetic diversity presently exists, hosted up to 186 879 Angolan refugees in the same period, which is likely to have influenced the M. tuberculosis population structure on both countries. Strain spreading from the Democratic Republic of the Congo, and eventually from Republic of Congo, is plausible given the documented spread of HIV-1 variants from these countries to Angola32.
However, another factor shaping the population structure found herein might be located further away, pertaining the Community of Portuguese Speaking Countries. In fact a very similar M. tuberculosis population structure is found in Portugal when it comes to the prevalence of LAM strains16. All SIT20/LAM1 isolates (n = 16, 18.2%), and derived SITs, bear the VNTR locus 2461_2 allele while Lisboa3 clade strains in Portugal harbour the 2461_1 allele, which on the one hand hampers the establishment of a more direct link but, on the other hand, makes it possible to hypothesize that Lisboa3 strains might have derived from such SIT20/LAM1 strains bearing the 2461_2 allele during its evolutionary trajectory towards XDR-TB8,30. Such hypothesis warrants, however, further studies as the SIT20/LAM1 strains found in this study might have originated in Latin America as well16,30. Furthermore, 13 (18.8%) isolates were also found to harbour the VNTR locus 1644_1_allele that according to Mokrousov et al.30 is only observed in few isolates across the RD174-sublineage with the exception of Q1 (SIT1106/LAM4) clade strains. This latter finding also raises the hypothesis of a common evolutionary origin between these strains, and countries, that merits further investigation8,30. Historical ties connect the Community of the Portuguese Speaking Countries, which likely favour, at a macroepidemiological level, an enhanced strain circulation between these countries.
There is some paucity of data concerning clustering rates in African countries. The clustering rates obtained in this study, particularly with the 15 or 24-loci MIRU-VNTR sets, are comparable with the ones reported by Homolka et al.33 in a study involving strains from Sierra Leone but considerably lower than the clustering rates obtained by Mulenga et al.34 in a study from Ndola district in Zambia33,34. It is also likely that the clustering rate in our study might be underestimated due the restricted sampling timeframe34. Overall, the population structure provided by MIRU-VNTR typing was consistent with the spoligotyping population structure. Nevertheless, as reported previously, MIRU-VNTR allows a deeper discrimination between isolates and is therefore more adequate for epidemiological surveillance11. Still in this regard, no significant difference exists between the discriminatory ability of 24 vs the 15 MIRU-VNTR loci sets as measured by the Hunter and Gaston Index of Diversity (0.996 vs 0.993, respectively) for this setting. For routine epidemiological surveillance the 15-loci set is therefore recommended with the 24-loci set being more adequate for M. tuberculosis phylogenetic studies, as proposed by Supply et al.11.
Besides demonstrating the presence of recent transmission events, complex transmission chains with a common origin may be underlying local strain diversification. In particular, the genotypic diversification exhibited by CC1, in comparison with the other clonal complexes, and its association with SIT20/LAM1 clades suggest an earlier introduction of LAM1 strains in this setting when compared with other clade-associated clonal complexes of restricted genotypic diversification. Concerning its global importance, SIT20/LAM1/MIT10 strains have already been previously described in Portugal as associated with drug resistance, highlighting, again, the putative epidemiological links between Portuguese-speaking countries8.
Additionally, given the high number of singleton profiles and non-clustered isolates, another important aspect to consider is that TB reactivation may be playing an important role in this setting’s TB epidemiology. Tackling TB by preventive treatment on specific sub-populations associated with known risk factors for disease reactivation may contribute to slowing down the country’s increasing TB incidence35.
In conclusion, this first cross-sectional study conducted in Luanda, Angola, provides a framework for future studies and programmatic management of TB in Angola. We provide sufficient evidence for cluster-based transmission with differential geographic dispersion. Furthermore, the moderate rate of MDR-TB found in this sample has major public health implications and highlights the need for further studies specifically focused on MDR-TB transmission.
Additional Information
How to cite this article: Perdigão, J. et al. Genetic diversity, transmission dynamics and drug resistance of Mycobacterium tuberculosis in Angola. Sci. Rep. 7, 42814; doi: 10.1038/srep42814 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
World Health Organization. Global Tuberculosis Report 2015. (World Health Organization, Geneva, 2015).
Direcção Provincial de Saúde. Relatório Anual de 2013 - Província de Luanda. (Direcção Provincial de Saúde, Governo Provincial de Luanda, República de Angola, Luanda, 2013).
World Health Organization. Global Tuberculosis Report 2014. (World Health Organization, Geneva, 2014).
Alland, D. et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med 330, 1710–1716 (1994).
Genewein, A. et al. Molecular approach to identifying route of transmission of tuberculosis in the community. Lancet 342, 841–844 (1993).
Small, P. M. et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med 330, 1703–1709 (1994).
Reed, M. B. et al. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431, 84–87 (2004).
Perdigao, J. et al. Unraveling Mycobacterium tuberculosis genomic diversity and evolution in Lisbon, Portugal, a highly drug resistant setting. BMC Genomics 15, 991 (2014).
Caws, M. et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 4, e1000034 (2008).
Coll, F. et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun 5, 4812 (2014).
Supply, P. et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 44, 4498–4510 (2006).
Kamerbeek, J. et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35, 907–914 (1997).
Supply, P. et al. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol 36, 762–771 (2000).
Thierry, D. et al. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol 28, 2668–2673 (1990).
Weniger, T., Krawczyk, J., Supply, P., Niemann, S. & Harmsen, D. MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res 38, W326–331 (2010).
Demay, C. et al. SITVITWEB–a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol 12, 755–766 (2012).
van Soolingen, D., de Haas, P. E. W. & Kremer, K. Restriction fragment length polymorphism (RFLP) typing of mycobacteria. Bilthoven, The Netherlands: National Intitute of Public Health and The Environment. 52 (2002).
Siddiqi, S. & Rüsch-Gerdes, S. MGIT960 Procedure Manual for BACTECTM MGIT 960TM TB System (Also applicable for Manual MGIT). Mycobacteria growth indicator tube (MGIT) culture and drug susceptibility demonstration projects. (Foundation for innovative new diagnostics Ed., 2006).
Vitol, I., Driscoll, J., Kreiswirth, B., Kurepina, N. & Bennett, K. P. Identifying Mycobacterium tuberculosis complex strain families using spoligotypes. Infect Genet Evol 6, 491–504 (2006).
Hunter, P. R. & Gaston, M. A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol 26, 2465–2466 (1988).
Torkaman, M. R. et al. Estimation of Recent Transmission of Mycobacterium Tuberculosis Strains among Iranian and Afghan Immigrants: A Cluster-Based Study. J Clin Diagn Res 8, DC05–08 (2014).
Munang, M. L., Kariuki, M. & Dedicoat, M. Isoniazid-resistant tuberculosis in Birmingham, United Kingdom, 1999–2010. QJM 108, 19–25 (2015).
Cox, H. et al. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med 3, e384 (2006).
Pillay, M. & Sturm, A. W. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin Infect Dis 45, 1409–1414 (2007).
Cohen, K. A. et al. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med 12, e1001880 (2015).
Mulenga, C. et al. Low Occurrence of Tuberculosis Drug Resistance among Pulmonary Tuberculosis Patients from an Urban Setting, with a Long-Running DOTS Program in Zambia. Tuberc Res Treat 2010, 938178 (2010).
He, G. X. et al. Follow-up of patients with multidrug resistant tuberculosis four years after standardized first-line drug treatment. PLoS ONE 5, e10799 (2010).
Lew, W., Pai, M., Oxlade, O., Martin, D. & Menzies, D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med 149, 123–134 (2008).
Rabna, P. et al. Direct Detection by the Xpert MTB/RIF Assay and Characterization of Multi and Poly Drug-Resistant Tuberculosis in Guinea-Bissau, West Africa. PLoS ONE 10, e0127536 (2015).
Mokrousov, I. et al. Latin-American-Mediterranean lineage of Mycobacterium tuberculosis: Human traces across pathogen’s phylogeography. Mol Phylogenet Evol 99, 133–143 (2016).
United Nations High Commissioner for Refugees. 2004 UNHCR Statistical Yearbook. (United Nations High Commissioner for Refugees, Geneva, 2006).
Bartolo, I. et al. Highly divergent subtypes and new recombinant forms prevail in the HIV/AIDS epidemic in Angola: new insights into the origins of the AIDS pandemic. Infect Genet Evol 9, 672–682 (2009).
Homolka, S. et al. High genetic diversity among Mycobacterium tuberculosis complex strains from Sierra Leone. BMC Microbiol 8, 103 (2008).
Mulenga, C. et al. Diversity of Mycobacterium tuberculosis genotypes circulating in Ndola, Zambia. BMC Infect Dis 10, 177 (2010).
Huynh, G. H. et al. Tuberculosis control strategies to reach the 2035 global targets in China: the role of changing demographics and reactivation disease. BMC Med 13, 88 (2015).
Acknowledgements
The authors wish to thank the laboratory, clinical, and nursing staff of HDP, whose high quality of service and cooperation have made this study possible. Financial support was provided by the Fundação para a Ciência e a Tecnologia (FCT) Portugal [PTDC/SAU-EPI/122400/2010], part of the EDCTP2 program supported by the European Union. JP was supported by a post doc fellowship from project [PTDC/SAU-EPI/122400/2010] and by fellowship [SFRH/BPD/95406/2013] from FCT. JP is thankful to the European Society of Clinical Microbiology and Infectious Diseases for a Research Grant, and would like to acknowledge the Study Group for Mycobacterial Infections. DM was supported by FCT fellowship [SFRH/BPD/100688/2014] and DM, IC MV are thankful to [GHTM UID/Multi/04413/20139] from FCT. CS was supported by FCT [SFRH/BD/73579/2010].
Author information
Authors and Affiliations
Contributions
J.P., S.C., N.T. and I.P. designed the study. S.C. and P.M. performed the collection of sputum samples and clinical/demographical data. J.R., D.M., I.C. and M.V. have performed the drug susceptibility assays. J.P. and C.S. have isolated, identified and genotyped the clinical isolates. J.P. analysed the data. J.P., N.T., M.V. and I.P. wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Perdigão, J., Clemente, S., Ramos, J. et al. Genetic diversity, transmission dynamics and drug resistance of Mycobacterium tuberculosis in Angola. Sci Rep 7, 42814 (2017). https://doi.org/10.1038/srep42814
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42814
This article is cited by
-
Spread of Mycobacterium tuberculosis in Southern Brazilian persons deprived of liberty: a molecular epidemiology study
European Journal of Clinical Microbiology & Infectious Diseases (2023)
-
Molecular epidemiology of drug resistant Mycobacterium tuberculosis in Africa: a systematic review
BMC Infectious Diseases (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.