Abstract
Myocardial ischemia reperfusion injury is a negative pathophysiological event that may result in cardiac cell apoptosis and is a result of coronary revascularization and cardiac intervention procedures. The resulting loss of cardiomyocyte cells and the formation of scar tissue, leads to impaired heart function, a major prognostic determinant of long-term cardiac outcomes. Photobiomodulation is a novel cardiac intervention that has displayed therapeutic effects in reducing myocardial ischemia reperfusion related myocardial injury in animal models. A growing body of evidence supporting the use of photobiomodulation in myocardial infarct models has implicated multiple molecular interactions. A systematic review was conducted to identify the strength of the evidence for the therapeutic effect of photobiomodulation and to summarise the current evidence as to its mechanisms. Photobiomodulation in animal models showed consistently positive effects over a range of wavelengths and application parameters, with reductions in total infarct size (up to 76%), decreases in inflammation and scarring, and increases in tissue repair. Multiple molecular pathways were identified, including modulation of inflammatory cytokines, signalling molecules, transcription factors, enzymes and antioxidants. Current evidence regarding the use of photobiomodulation in acute and planned cardiac intervention is at an early stage but is sufficient to inform on clinical trials.
Similar content being viewed by others
Introduction
Heart failure is an increasing health burden worldwide, with myocardial infarct (MI) size suggested as the major determinant of adverse outcomes1. Initial cardiomyocyte death due to ischemic conditions is followed by subsequent apoptosis and myocardial dysfunction, instigated by the reperfusion of areas devoid of blood flow. Post infarction remodelling along with the incurred cardiomyocyte death, results in reduced contractility, excessive left ventricular chamber dilation, infarct related wall thinning, compensatory hypertrophy of non-infarcted regions and increased deposition of fibrillar collagen1,2. Although interventions targeted at myocardial ischemic-reperfusion (MIR) insult have become less invasive and more effective in reducing mortality, ongoing management of morbidity among survivors is a substantial challenge1.
When the myocardium no longer receives oxygenated blood from the compromised coronary vessel the “area at risk” is rendered ischemic and subject to distinct metabolic processes that result in necrosis and cell death1,3. Under ischemic conditions, oxidative phosphorylation ceases, which reduces mitochondrial membrane potential and the availability of cellular ATP. This causes the engagement of anaerobic glycolysis, which in turn induces an influx of Na+ through the Na+/H+ exchanger. Mitochondrial Na+ levels are further exacerbated by the reduction of Na+/K+ ATPase, which requires ATP for activation. In an attempt to restore cellular pH, Na+ is removed by the 2Na+-Ca2+ ion exchanger, causing a substantial influx of Ca2+ 3,4. Accumulated mitochondrial Ca2+ remains within the mitochondria while mitochondrial permeability transition pores (MPTP), which are dependent on intracellular pH levels, remain closed5.
When blood flow is restored, reperfusion causes additional injury, thought to be related to the surge of oxygen return. Yellon and Hausenloy6 have suggested that reperfusion alone can contribute up to 30% to 40% of total infarct size following coronary artery occlusion. Microvascular obstruction has been suggested to cause damage during coronary events, however its contribution to infarct size, if any, remains unclear4. Under conditions of rapid re-oxygenation, the shift in ionic flux results in restoration of cellular pH and the rapid alteration of cellular pH, rather than the return of oxygen, may be the stimulus that activates processes leading to cell death5. A speed dependant relationship of pH restoration has been identified, where the intensity at which oxygen returns determines the amount of reactive oxygen species (ROS) released and the opening of the MPTP, allowing accumulated Ca2+ into the cytoplasm. Disordered intracellular Ca2+/ROS balance ultimately leads to dysregulation of the MPTP and rupture of the sarcolemma5,7,8. A more severe form of apoptosis is oncosis, where cell death is characterised by cell swelling and karyolysis during MIR injury. Factors that mitigate against oncosis include the presence of melatonin9.
Secondary damage as a result of the innate immune response has been suggested as an immediate and delayed process that also contributes to infarct size. Persistent inflammation has been identified as harmful, preventing infarct repair2. However, the exact involvement of inflammation has yet to be fully elucidated4. Neutrophils, monocytes, and macrophages, which are responsible for remodelling and removal of dead or dying tissue, depend on specific spatiotemporal and quantitative signalling for their activation10. Modulation of these signalling processes, especially in the acute vulnerable period following MIR injury, presents another pathway for reducing infarct size.
MIR injury is also associated with potentially serious systemic effects, such as increased morbidity, increased mortality (9%) at one year11 and neurological impairment, where it is estimated that only 10% of resuscitated patients are neurologically intact when discharged from hospital7. MIR injury as a sequela of cardiac surgery, may also lead to a greater rate of post-operative cognitive dysfunction (POCD) than other types of surgery12. Thus recovery from MI is the gold standard for effectiveness of treatments that precondition against MIR injury.
While there are many therapies to reduce the effect of ischemia, there has been less success in treating reperfusion injury, although a number of novel potential therapies have been proposed1,11,13. These include ischemic preconditioning, where brief episodes of ischemia followed by reperfusion, are introduced before a sustained ischemia. Ischemic preconditioning has been shown to reduce infarct size substantially (30–80%) and can last for 2 to 3 hours after the preconditioning event, as well as having a second window of protection that occurs 24 hours after preconditioning and can last for about 48 hours14. Ischemic post-conditioning, where the rate of reperfusion is slowed by short episodes of myocardial ischemia (using, for example, an angioplasty balloon) may also be effective in reducing MIR injury14. Remote ischemic conditioning, where brief non-lethal episodes of ischemia and reperfusion are applied to an area remote from the heart, such as an arm or leg, has also been shown to have some effect on MI size6. While ischemic conditioning has been demonstrated in animal models, there has been less success in the translation of this therapy to clinical trials15,16, although balloon angioplasty post-conditioning and hypothermia have shown some positive effects17. Ischemic preconditioning is also limited to conditions where the potential for MIR insult can be predicted, such as coronary artery bypass grafts (CABG) surgery, and ischemic post-conditioning is limited by the very short window (5 to 10 minutes) during which the treatment can be effective14. In addition to ischemic conditioning, there are a number of experimental pharmaceutical products with the potential to reduce MI size. Alpha-melanocyte-stimulating hormone (α-MSH) has shown some promise18, the use of volatile anaesthetics for cardioprotection during open heart surgery can produce modest effects19 and the use of cyclosporin A20 and exenatide21 have been shown to produce reductions in MI size. The disadvantage of drug and anaesthetic intervention is the potential to introduce serious side effects, complicated by the proteostasis of the patient, as well as disease (e.g., diabetes) and interactions with other drugs22. These side effects can potentially be as severe as Parkinson’s disease and Huntington’s disease-like symptoms7.
In short, MIR injury involves a complex redox stress response, for which there is so far no effective treatment, although a number of novel treatments have been proposed. A goal for such treatment would be a mechanism to switch between the deleterious redox stress reactions, towards protective redox conditions. The complexity, however, of the switching mechanism has hindered an effective therapeutic regime23. A potential target for treatment intervention is the modification of the mitochondrial response to oxidative stress.
Photobiomodulation (PBM) is the low power (1–500 mW) non-thermal delivery of photons in the visible or near infrared spectrum (405–1000 nm) that elicits a beneficial biological response in cells and tissues24. PBM can include light emitting diodes (LED) and low-level laser therapy (LLLT). Phototherapy has a long history of application in medicine. The earliest scientific report was the use of red light in the treatment of smallpox scars by Neil Finsen in 190325 and his use of ultraviolet light for the treatment of lupus vulgaris, which resulted in a Nobel Prize in 1903. Laser light was used in radiation ulcer attenuation after the Chernobyl nuclear accident26 and light has been used as a treatment for bilirubin dysfunction in neonates for many decades27. Interestingly, when light is used for premature neonate jaundice treatment, there is a concomitant effect in patent ductus arteriosus, where the systemic effect of the light application can cause cardiac vasodilation effects and prevents the closure of the patent ductus, which can be avoided by the use of light impermeable chest shielding28. Recently 670 nm light has been proposed as a treatment for oxygen-induced retinal disease in neonates29. PBM has been increasingly used for treatment of ulcers30, wounds31, neuro-inflammation32,33, pain34, lympoedema35, macular degeneration36 and tendon healing37. The use of LLLT in various clinical applications has been reviewed by Chow et al.34 in the area of chronic neck pain, by Khan and Arany38 for wound healing, by Geneva et al.39 for retinal disease, by Agrawal et al.40 as a preconditioning treatment for various diseases and by Carlos et al.41 for cardiac remodeling after myocardial infarction. Animal models have also suggested a role for the PBM treatment of neurodegenerative diseases42, such as Alzheimer’s disease43, Parkinson’s disease44, multiple sclerosis45 and as a preconditioning treatment against post-operative cognitive dysfunction46. Natural light sources have also been shown to be important in recovery from spinal surgery47 and management of cardiac risk patients48. An emerging body of experimental evidence as well as some clinical trials support the application of PBM in conjunction with routine cardiac interventions, which warrants a systematic review of PBM application and MIR injury.
The aim of this contribution is to report the results of a systematic review into the experimental evidence in tissue studies, animal studies and clinical trials for the use of PBM in the treatment, intervention and management of myocardial reperfusion injury, and to summarize the underlying mechanisms and metabolic signalling pathways found to underpin this effect. The results of the review can be used to inform scientists and clinicians on the potential scope of further experimental studies and clinical trials, the creation of best practice guidelines and the use of PBM in other areas of cardiovascular intervention.
Results and Discussion
Quality and Limitations of Studies
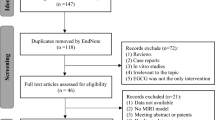
The review of articles was conducted according to PRISMA guidelines (http://www.prisma-statement.org) (Fig. 1). Since most of the articles reviewed pertain to animal or tissue experiments, with only one clinical trial, the extent to which results can be extended to human effects is necessarily limited. In the single clinical trial, assessment using the JADAD method49 resulted in a score of 4, indicating high quality. The use of the SYRCLE risk of bias tool50 to assess quality of animal studies indicated a high or unknown risk of bias for most studies in the majority of categories (Table 1 and Fig. 2). Only two categories were assessed as having a low risk of bias for the majority of studies. While reporting bias (item 9, Table 1) was assessed as low, the possibility of publication bias, in that results have been selectively reported, cannot be discounted, especially as no adverse effects or negative findings of the effect of PBM were described. It should also be kept in mind that it is difficult to translate results of animal studies to humans, due in part to the variations in physiology and anatomy between the species (for example, rats have fewer collateral coronary arteries than dogs and humans).
Risk of bias score for each risk item in animal studies, as assessed using the SYRCLE tool50.
Characteristics of Studies
Experimental designs in the studies reviewed ranged from in vitro cardiomyocyte cultures to a single human clinical trial. Of the 23 studies included, 17 (74%) were conducted using animal models (13 on rats, 2 on mice, 1 on rabbits and 1 on dogs and rats), 2 were conducted on isolated rat hearts, 3 on tissues or cell cultures and 1 was a clinical trial (Table 2). The application site for PBM in animal studies varied with experimental design, with 6 studies using trans-thoracic application of PBM (with skin shaved or removed), 12 irradiating the myocardium directly and 3 studies irradiating sites distal to the heart (leg muscle or tibia). In the clinical trial there was a transthoracic administration of PBM. Studies on isolated tissue and cell samples involved irradiation without direct contact. PBM was administered as preconditioning treatment, as post-conditioning treatment, as treatment during surgery or reperfusion, or as a combination of treatments. Total intervention dose ranged from 0.6 J to 36 J, with a mean of 6.26 J. All trials used wavelengths between 630 nm and 830 nm, with the human trial using infrared light (810 nm) and the majority of animal trials used red light (at around 660 nm). All animal studies reported physical or histological outcomes after PBM intervention and 18 reported molecular changes.
Infarct Size Reduction, Histological Profile and Long-Term Effects
The most significant finding from this review was the positive effect of PBM on modulating infarct size and the improvement of cardiac remodelling51,52,53,54,55,56,57,58,59,60,61,62,63. These results were achieved using a range of experimental designs, wavelengths and dosages. Three studies59,60,61 reported a reduction in total infarct size of greater than 60% with one60 reporting MI size reduction of 76% when irradiation was applied to the tibia of the rat (remote preconditioning), compared to a 31% reduction when applied locally to myocardium. Yaakobi et al.58 reported consistently reduced infarct size compared to controls throughout a 45-day follow-up period. The results agreed with other studies that have shown PBM can significantly decrease the narrowing of coronary arteries, decrease rates of restenosis after stenting procedures64,65,66 and is a therapeutic option for severe medically refractory angina67 and healing of sternotomy incisions68.
Histological analysis in a number of studies revealed improvements in cardiomyocyte arrangement51,53,58,69, including reductions in dense collagen, reduced ventricular wall thinning and increased mesenchymal and cardiac stem cells. Gavish et al.70 also reported that PBM modulated collagen synthesis in a porcine aorta model, along with changes to the levels of matrix metalloproteinase-2 (MMP-2), a regulator of collagen synthesis.
PBM Dose, Timing and Treatment Regime
All studies showed the safety of PBM in animal and human subjects, including direct myocardial irradiation and the clinical trial did not report any side effects or adverse reactions in patient populations as a result of PBM treatment. Both LLLT and LED were shown to be effective as a source of PBM and all wavelengths tested showed positive results, including red and infrared wavelengths (Table 2). As yet there have been no studies on super-pulsed laser infrared (904–980 nm) wavelengths. The reported effects of PBM on MIR injury were dose dependant and mirrored PBM effects that have been reported in the literature for treatment of conditions such as chronic pain, neurodegenerative disease, lymphoedema and macular degeneration34,35,36,42.
There appears to be wide windows for the dose and timing of PBM treatment. In a number of studies, PBM was shown to be effective both as a preconditioning treatment and if applied during reperfusion or in the immediate post (4 hour) surgical window. There was also good evidence60,71 that PBM administered to a distal area (tibia or leg muscle) produced a positive effect on infarct size. This is consistent with the abscopal effect seen in application of PBM72 with, for example, neuroprotection where targeting remote tissues with PBM can decrease the symptoms of Parkinson’s disease73. This abscopal or systemic effect has been attributed to the downregulation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines73,74 as well as to the proliferation of mesenchymal stem cells59,60,75, both of which PBM is known to influence76. It may also possibly be due to a systemic mitokine response (see below).
One study found that a higher PBM dose was more effective in the initial phase of MIR to reduce injury58, 804 nm LLLT at 12 mW/cm2 having more effective cardioprotection than 6 mW/cm2. This is in contrast to the most effective dose given 1.5 hour after MIR being a combination of 12 mW/cm2 and 5 mW/cm2, which was found to be superior to two doses of 12 mW/cm2, leading to the conclusion that LLLT may be less beneficial when given too frequently. The power density required to achieve a reduction in MI size was similar for rats, dogs and humans. For infrared 803–810 nm the dose window indicated is 5 to 15 mW/cm2 (with 25 mW/cm2 less effective)53,77, which equates to 1 to 6 J/cm2 for rats58 and 6 J/cm2 for the human trial78. This is consistent with other studies of treatment with PBM that have demonstrated a biphasic dose response, where a therapeutic effect is achieved with an optimal dose within a wide dose window, outside of which there is no effect79. Oron et al.53 stressed that the complex sequential physiological processes after MIR injury required different doses of PBM at different stages of reperfusion. PBM administered after the reperfusion event (post-conditioning) was found to be effective in a number of studies, even after a considerable delay. In fact, when a delayed post-conditioning treatment was omitted from the treatment regime, the effect on MI size was reduced58. Evidence for treatment protocols from a range of animal studies and the clinical trial would seem to suggest that a combination of preconditioning, immediate and post-application (with red or infrared wavelengths of LED or Laser) produce the most positive effects. All of these dose and timing factors would be important for administration of PBM in any planned clinical trial.
The achievement of a therapeutic dose in small experimental animals is not in question using near infrared wavelengths80, however there is the possibility that transthoracic PBM may not reach a therapeutic dose in cardiac tissue in humans. While the transmission of light to the heart does not appear in the literature per se, it can be inferred from other studies. For example, the use of PBM to treat traumatic brain injury has included studies that have demonstrated that transcranial LLLT can reach therapeutic doses 40 to 50 cm deep with infrared irradiation (800–830 nm), using head simulations81, formalin fixed82 and unfixed human cadaver heads83. The average parasternal, skin-to-heart distance has been reported as 32.1 ± 7.9 mm84. In addition, in the clinical trial reviewed here, the transthoracic application of PBM resulted in changes to cardiac and inflammation markers78.
Mitochondrial Effects
The overall trend from the studies was a positive effect on mitochondrial respiration pathways, including an increase in the availability of ATP and nitric oxide (NO). Zhu et al.85 noted 15% higher ATP levels in isolated hearts during induced cardioplegia when treated with PBM and Oron et al.53 showed a 21% reduction in mitochondrial damage in an animal model, which, it was suggested, may account for sustained ATP production.
One of the major photon receptors is generally believed to be the cytochrome C oxidase (COX) enzyme in the electron transport chain and stimulation of this enzyme sets in motion multiple cascading signal transduction pathways86. Heart tissue, however, responds differently from other tissues to mitochondrial COX signalling pathways, due to the tissue specific expression of signalling molecules7, which might make heart tissue more susceptible to ischemic and reperfusion injury than other tissues. The response of the mitochondria to reperfusion is a critical factor in the health or otherwise of myocardial cells and tissues. Protection of the mitochondria, particularly the MPTP, has been called “the Holy Grail” of cardioprotection13 and phosphorylation events that regulate COX are ideal targets for therapeutic interventions7. The effect of PBM on COX is generally accepted2 and is believed to be a major reason for the effect PBM on mitochondrial health (Fig. 3), due to the increased production of ATP and the activation of ATP dependent ion pumps86,87. Recently however, it has been demonstrated that near IR has no effect on isolated COX protein88, which may suggest a more complex interaction between light and the mitochondria, possibly an indirect effect. This was also implied in a study of retinopathy in diabetic mice89 and in diabetic rats90, where there was no evidence found for a PBM effect on mitochondria. Never-the-less, an effect of PBM on the mitochondria was suggested by a number of the studies reviewed here (Fig. 3), with a positive effect of PBM on both mitochondrial respiration and mitochondrial retrograde signalling and major impacts on the regulation of Ca2+ ion channel flux, NO production through COX and increased in ATP levels. These factors ensure the maintenance of mitochondrial retrograde signalling, which has been identified as a regulator of many cellular activities in both normal and pathological states91, including mitochondrial retrograde signalling in MIR92 and the ischemic preconditioning effect of cardioprotection40. Mitochondrial homeostasis is important in the cell stress response93, which can be regulated by the level of cellular melatonin in the MIR injury response, where elevated melatonin is neuroprotective against myocardial cell oncosis9. Modulation of the mitochondrial retrograde signalling response would also have an effect on systemic metabolism through the mitochondrial unfolded protein response (UPRmt)94,95. Since proteomic stress can induce changes in redox stress across tissues, this may be important in the limitation of cardiomyocyte death and scar formation96 as well as in organism wide neuroprotection. This may involve a mitokine response95, which could also have implications in preconditioning against ischemic reperfusion injury. The modulation of the UPRmt would restore homeostasis through mitochondrial hormesis (mitohormesis) (see Fig. 3).
Non-Mitochondrial Signalling by PBM
Non-mitochondrial effects of PBM involve signalling pathways that originate from photon absorption and protein conformational modulation at the cell membrane (Fig. 3). Photons can be absorbed by ion channels (including TRPV1, K+ and Ca2+ channels), membrane receptors including tyrosine kinases and a variety of photoactive molecules described as opsins38. Downstream signal transduction is known to affect inflammatory cytokines97, heat shock proteins98, endothelial and axonal cytoskeleton morphology99,100, antioxidant IL10101, growth factors vascular epithelial growth factor (VEGF) and MMP-2102, inducible nitrite oxide synthase (iNOS)103, and superoxide dismutase (SOD)104 as well as modifying ROS and NO. These have a variety of effects, such as improving tissue responses, including cardiac tissue, endothelial tissue and arterial lumen diameter.
An example of a signalling pathway modified by PBM in MIR is the suppression of the ASK1/p38/NF-κB signalling pathway71, which is important in cardiomyocyte necrosis following myocardial infarction2 and which has also been shown, in an in vitro model of wound healing, to enhance tissue regeneration and angiogenesis105. A pathway that was not monitored in any study reviewed here is the Akt/GSK-3β pathway106, involved in neuronal survival in traumatic brain injury. This pathway has been demonstrated to be modulated by PBM and to be neuroprotective against apoptosis induced by amyloid β peptide107. This pathway might be predicted to also be involved in protection against MIR injury by PBM.
PBM was shown in a number of the studies reviewed here to influence a variety of non-mitochondrial signal transduction pathways, which are linked to a decreased cell adverse stress response, improved tissue responses (decreased MI size, reduced restenosis, etc.) and a restoration of homeostasis (see Fig. 3). While these modulations of cell signalling can also be achieved using drug and anaesthetic strategies5, these treatments have the potential for serious side effects, as previously noted.
Redox State and Antioxidants
In addition to the respiratory chain, NO is also produced in a number of reactions outside of the mitochondria. A number of studies reviewed here demonstrated an increase in NO following PBM treatment, as well as increased levels of iNOS and endothelial nitric oxide synthase (eNOS). Tuby et al.77 reported a significant elevation in iNOS, which increased with increasing PBM dose. The increase in iNOS levels was recorded after 2.5 hours and peaked at 2 days, which indicated an ongoing role for NO after the acute insult. Interestingly, enhanced levels of iNOS were also observed in non-infarcted but irradiated myocardium, which raises questions concerning the modulating effect of PBM on this enzyme in non-traumatic pathological states. Manchini et al.63 however reported reductions in iNOS in the intervention group, 3 days post injury, although plasma nitrite and nitrate concentrations (NOx) were markedly higher, indicating a transient effect of PBM on NO levels.
In one study62, NO was shown to be released from nitrosyl heme proteins in the irradiation group and two other studies54,55 reported an increase in NO from MbNO and HbNO, independent of nitric oxide synthase.
NO is important in the PBM myocardial protection during ischemic reperfusion injury, both in the cell stress response and also in regulating Ca2+ homeostasis108 and thus limiting the effect of mitochondrial Ca2+ overload from MPTP. NO signalling may also lead to mitochondrial S-nitrosation, which slows reactivation of mitochondrial metabolism at the onset of re-oxygenation7. This slowing of reactivation in post-conditioning may be particularly important to mitigate against further MIR injury. Non-NOS sources of NO have been argued to be a major mechanism behind PBM protection against MIR injury55.
Increased levels of a number of anti-oxidants and ROS were reported after PBM intervention. These included creatine phosphokinase (CPK)78, lactate dehydrogenase (LDH)78, dichlorofluorescein (DCFH)109, glutathione (GSH)98, SOD110,111,112 and interleukin 10 (IL-10)57,71,113,114.
While two studies reported that levels of SOD increased within 1 hour post irradiation110,111, others109,115 reported a reduction in SOD activity in PBM treated groups, when irradiation occurred 4 and 3 weeks after rats were exposed to ischemic insult. These results suggest that PBM induces an acute transient increase in SOD activity immediately after MIR injury. This was confirmed in an experiment where SOD activity was shown to double when isolated rat hearts were irradiated immediately after MIR injury112. Interestingly Malinovaskaya et al. found that while non-coherent light resulted in an increase in SOD, laser brought about a decrease in SOD activity111.
Yaakobi et al.58 found a 2.2-fold increase of heat shock protein inducible factor (Hsp70i), 5 hours post irradiation using 810 nm. Zhang et al.110 reported increased levels of gastrin-releasing peptide (GRP) 78 (a chaperone to Hsp70) at 1 hour, 1 day and 1 week, indicating a sustained up-regulation of this protein in the later stages of the tissue recovery cycle. Heat shock proteins have a role in mitochondrial membrane homeostasis and have an important role in protein folding, unfolding and the cell stress response116. Hsp70 is also an important factor in the amelioration of acute lung injury induced by gut ischemia in the rat model, where PBM up-regulates peroxisome proliferator-activated receptor-γ (PPARγ), which in turn results in an increase in Hsp70 production and a decrease in inflammation and lung injury85.
Inflammatory Cytokines
Inflammatory cytokines regulate the cellular stress response and the inflammatory cascade associated with the ischemic event that causes injury23. The balance between pro and anti-inflammatory cytokines is important in determining prognosis after MIR injury, especially the balance in redox signalling molecules, which appears to act as a switch between protective oxidative signalling and damaging oxidative signalling23. While this switching mechanism is not yet fully understood, it appears that PBM is able to affect the switching in a positive way, resulting in cardioprotection. This was consistent across all studies and parallels the PBM effect on cytokines in non-cardiac studies, including macular degeneration36, wound healing38, muscle pre-conditioning40, gut ischemia and reperfusion98 and neuroprotection against Alzheimer’s and Parkinson’s diseases42 and, potentially, POCD46.
Six studies reported changes in levels of the inflammatory markers IL-1α, 1β, 2, 4, 6 and 8 after PBM57,63,71,114. In a human clinical trial with patients undergoing angioplasty and cardiac stenting (excluded from this review), Derkacz et al.114 also found that IL-1β, and IL6 were reduced when irradiated with 808 nm with a total treatment dose of 9 J/cm2. In contrast to these findings, Manchini et al.63 found strong increases in IL6 in both control and PBM groups (660 nm and 22.5 J/cm2) three days post intervention. In a comparable study, Hentschke et al.71 showed that a four-week delay in PBM application resulted in reduced IL6 levels in the group treated with 660 nm 21 J/cm2 but significantly higher IL6 levels in the group treated with 3 J/cm2. Together these results suggest that there was a dose window and a dose dependant relationship between PBM and levels of IL6.
In the clinical trial reviewed here78, repeated doses of 808 nm PBM resulted in reduced white blood cell, lymphocyte and neutrophil activity 5 days post cardiac artery bypass graft (CABG) surgery. In a rat reperfusion injury model98 it was reported that an acute increase in myeloperoxidase (MPO) occurred after irradiation with 660 nm laser, indicating an increase in neutrophil granulocyte activity. Manchini et al.63 found that kinin B2 receptor mRNA expression and Mas receptor protein expression was increased after MI and PBM, whereas PBM significantly decreased the kinin B1 and significantly reduced angiotensin-converting enzyme (ACE) mRNA expression, all of which affect vasodilation.
Growth Factor Modulation
Three studies showed significant increases in expression of VEGF after PBM. Tuby et al.77 demonstrated significant increases in expression as early as 2.5 hours after treatment, which continued to 24 and 48 hours, reverting back to pre-treatment levels by 72 hours. There was also a dose dependent relationship, with power densities 5, 12, and 17 mW/cm2 increasing expression rates by 2, 2.3, and 1.3 fold respectively. Zhang et al.110 also found significant increases in VEGF 1 hour and 1 day post intervention, with no significant difference in levels after 1 week. This is consistent with other studies investigating the mechanisms of PBM action102. The increase in VEGF promotes the proliferation of endothelial cells and angiogenesis, important in the cell stress response under hypoxic conditions117 or under high concentrations of ROS94. VEGF is also important in the homeostatic control of the unfolded protein response in the endoplasmic reticulum and in the mitochondria94. In addition, PBM has an indirect action on mesenchymal stem cell proliferation60,69 and the interaction between mesenchymal stem cells and VEGF mRNA modulates cell adhesion and proliferation in a nutritionally deprived model118.
Cardiac Markers
There was a reduction in cardiac enzyme marker troponin in the rat model52 and creatine phosphokinase (CPK) in the rat52 and the human study78. These are clinically important markers for cardiac damage, which may be important in the design of PBM trials for the evaluation of the success of dose and timing of PBM protocols.
Cytoskeleton Modulation by PBM
In a study on nutrient stressed rabbit aortic endothelial cells99, it was shown that irradiation with laser at 685 nm at 8 J/cm2 for 7 days caused reorganisation of actin filament stress fibres and filament proliferation, such that the cell regained its structure to a pre-stressed state. This may be a similar mechanism to the reorganization of the cytoskeleton seen after PBM application to dorsal root ganglion nerves in pain blockade46,100; a process strikingly similar to the cytoskeleton remodulation in the neuroprotective mechanism of rapid ischemic tolerance defence against NMDA excitotoxicity. These mechanisms have been reviewed by Liebert et al.46. This rapid ischemic tolerance mechanism may be relevant to the PBM effect in the animal model for infarct size reduction in MIR. It is often observed that rapid ischemic tolerance induce by PBM (such as in pain blockade) requires a higher dose in the initial stages, which is also the case in cardioprotection77, suggesting a similar mechanism.
Conclusion
Cardiac protection, both in clinical use and preconditioning applications, will become progressively more important with increasing numbers of cardiac procedures in the future and the further burden of cardiac disease, evident in the incidence of morbidity and potential mortality and the escalating cost of treatment. The evidence outlined in this review from a range of in vitro and in vivo animal studies, as well as one clinical trial, suggests that PBM may have a role as a cardioprotective agent against MIR injury and could protect against the initial cardiac ischemic event and the ongoing damage caused by reperfusion. PBM has been shown to affect a variety of signal transduction pathways that are critical to switching from the deleterious redox stress reactions that occur as a result of reperfusion, towards the more protective redox conditions that can limit injury and promote repair. This could ultimately lead to improved tissue responses, including reduced infarct size and lower rates of restenosis. PBM is non-invasive, simple to administer, inexpensive and has no known side effects, unlike other interventions that appear to have limited evidence of efficacy and potentially deleterious side effects. PBM could therefore be considered as a potential alternative to drug and anaesthetic pre-treatments for MIR injury. It also appears to be an effective post-conditioning therapeutic protocol, thus extending the available time for intervention to treat MIR injury.
The number of well-designed clinical trials is limited (one in this review), but the available evidence from animal and tissue studies suggest that further clinical trials are warranted. The timing and dosage of PBM appears to be complex, with different doses required at different stages of reperfusion, in order to achieve optimal treatment outcomes; something that must be considered when planning clinical trials. The cardiac markers of troponin, CPK and other novel biochemical indices such as osteopontin119 could be used as markers of MIR injury in these future trials. Evidence would suggest that a combination of preconditioning, immediate and post application treatment would be appropriate, with a potential role for remote application of PBM to produce a cardioprotective preconditioning abscopal effect.
Methods
Search Strategy
This review was conducted according to PRISMA guidelines and the overall search strategy is shown in Fig. 1. The search of published articles was conducted on the 28th of August 2015. PubMed, Ovid (OvidMedline), Scopus, and Web of Science, journal databases were searched with restrictions set to 1995-current, and English publications only (see Supplementary Table 1). Keywords used for the search included “laser, low level” and was combined with “myocardial”. Initial searches were expanded by adding derivatives: “ischemia”; “infarct”; “rupture”; “arrest”; and “failure”. Preliminary search results indicated that certain keywords such as “cardiovascular” and “disease” returned an excessive number of results when searching databases. These keywords were not used in order to limit the number of irrelevant papers captured during the initial search, which may have meant that studies that included these keywords were overlooked. Duplicate articles from the database search results were removed. Titles and abstracts of each paper were screened, and irrelevant articles removed. The reference lists of relevant papers were searched for additional articles, which were then added to the list of articles.
Eligibility Criteria
Once all full-text copies were obtained, each article was submitted to the eligibility criteria set out by authors (Supplementary Table 2). Articles investigating therapeutic, procedural or methodological applications of PBM were accepted, including application of PBM as an adjunct to surgery, or with standard medical management in a clinical population. The application of the irradiation could be to any part of the body. Due to the small number of clinical trials available, research conducted using animal models, isolated tissues and cell cultures were also accepted. Experimental design had to be representative of either ischemic and/or reperfusion injury. The primary outcome of the review was changes to mortality, cardiac tissues or cells or a change to molecular markers of cardiac function. Secondary outcomes were changes to other molecular markers, such as signalling molecules, redox markers or cytokines. All studies had to report a minimum of wavelength, power output and dose intervention parameters, or the missing parameter had to be calculable using alternate parameters, such as fluency and power density. Studies using combination therapies were omitted to ensure clarity when collating treatment effects. Populations with diseases or co-morbidities, with the exception of those directly involving MIR pathophysiological processes, were excluded to ensure conformity of examined population. Likewise, investigations involving organisms or cell structures with atypical genetics, or those containing disease-causing microorganisms were excluded to avoid false negative results.
Language and Time Restrictions
Language was restricted to English. Preliminary searches returned 21 articles not available in English. Titles of these papers were screened and a further 12 papers identified as potentially relevant. A sample of these were selected and translated into English. Upon review by AL, it was deemed that these articles were not suitable for inclusion. Examination of the publication trend indicated an increase in article number after 2004, with 50% of included articles published since 2010. Articles were therefore limited to those published after 1995. While the decision to only review articles after 1995 limited the number of articles captured, it is highly likely that key insights and findings from before 1995 would have been re-examined in later articles.
Qualitative assessment
Twelve articles did not meet the inclusion criteria. These articles are shown, along with reasons for exclusion, in Supplementary Table 3. The remaining studies were then divided between AK, AL and NG for data extraction. The experimental design, including population studied, number, outcome measures, treatment protocol, and laser dose parameters were recorded. All recorded data was collated into results tables and synthesized (BB and AK) to identify analogous results. Laser dose parameters were standardized by conversion into total dose administered, using the formula from Bjordal et al.120. Qualitative analysis of the clinical trial was conducted using the method of Jadad & McQuay49. Risk of bias for animal studies was assessed using the SYRCLE tool of Hooijmans et al.50. Interpretation of the articles in this review is necessarily descriptive, due to the limited number but wide range of studies that have investigated the effect of PBM in MIR injury.
Additional Information
How to cite this article: Liebert, A. et al. A Role for Photobiomodulation in the Prevention of Myocardial Ischemic Reperfusion Injury: A Systematic Review and Potential Molecular Mechanisms. Sci. Rep. 7, 42386; doi: 10.1038/srep42386 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ibanez, B., Heusch, G., Ovize, M. & Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Amer. Coll. Cardiol. 65, 1454–1471 (2015).
Hu, Y. et al. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res. Cardiol. 106, 1311–1328 (2011).
Turer, A. T. & Hill, J. A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 106, 360–368 (2010).
Hausenloy, D. J. & Yellon, D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 (2013).
Burwell, L. S., Nadtochiy, S. M. & Brookes, P. S. Cardioprotection by metabolic shut-down and gradual wake-up. J. Mol. Cell. Cardiol. 46, 804–810 (2009).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Eng. J Med. 357, 1121–1135 (2007).
Hüttemann, M. et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. BBA Bioenergetics 1817, 598–609 (2012).
Garcia-Dorado, D., Ruiz-Meana, M., Inserte, J., Rodriguez-Sinovas, A. & Piper, H. M. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 94, 168–180 (2012).
Liu, L. F. et al. Effect of melatonin on oncosis of myocardial cells in the myocardial ischemia/reperfusion injury rat and the role of the mitochondrial permeability transition pore. Genet. Mol. Res. 14, 7481–7489 (2015).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Bulluck, H., Yellon, D. M. & Hausenloy, D. J. Reducing myocardial infarct size: challenges and future opportunities. Heart 102, 341–348 (2015).
Meybohm, P. et al. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One 8, e64743 (2013).
Heusch, G. Molecular basis of cardioprotection signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 116, 674–699 (2015).
Penna, C., Mancardi, D., Rastaldo, R. & Pagliaro, P. Cardioprotection: A radical view: Free radicals in pre and postconditioning. BBA Bioenergetics 1787, 781–793 (2009).
Pilcher, J. M. et al. A systematic review and meta-analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. J. Roy. Soc. Med. 105, 436–445 (2012).
Wever, K. E. et al. Determinants of the efficacy of cardiac ischemic preconditioning: a systematic review and meta-analysis of animal studies. PloS One 10, e0142021 (2015).
Hausenloy, D. J. et al. Translating cardioprotection for patient benefit: position paper from the working group of cellular biology of the heart of the European society of cardiology. Cardiovasc. Res. 98, 7–27 (2013).
Vecsernyes, M. et al. The administration of α-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur. J. Pharacol. 470, 177–183 (2003).
Suleiman, M. S., Zacharowski, K. & Angelini, G. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Bri. J. Pharmacol. 153, 21–33 (2008).
Piot, C. et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Eng. J Med. 359, 473–481 (2008).
Lønborg, J. et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 33, 1491–1499 (2012).
Nwamba, C. & Ibrahim, K. The role of protein conformational switches in pharmacology: its implications in metabolic reprogramming and protein evolution. Cell Biochem. Biophys. 68, 455–462 (2014).
Pagliaro, P. & Penna, C. Redox signalling and cardioprotection: translatability and mechanism. Br. J. Pharmacol. 172, 1974–1995 (2015).
Mandel, A. & Hamblin, M. R. A renaissance in low-level laser (light) therapy–LLLT. Photonics. Lasers. Med. 1, 231–234 (2012).
Finsen, N. The red-light treatmet of small-pox. Lancet June 6 1903, 1297–1298 (1903).
Peter, R. U. et al. Chronic cutaneous damage after accidental exposure to ionizing radiation: The Chernobyl experience. J. Am. Acad. Dermatol. 30, 719–723 (1994).
Ennwver, J. Blue light, green light, white light, more light: treatment of neonatal jaundice. Clin. Perinatol. 17, 467–481 (1990).
Bhola, K., Foster, J. P. & Osborn, D. A. Chest shielding for prevention of a haemodynamically significant patent ductus arteriosus in preterm infants receiving phototherapy. Cochrane DB Syst. Rev. CD009816, doi: 10.1002/14651858.CD009816.pub2 (2015).
Natoli, R. et al. 670 nm photobiomodulation as a novel protection against retinopathy of prematurity: evidence from oxygen induced retinopathy models. PLoS One 8, e72135 (2013).
Ahmed, A. To evaluate the safety and efficiency of low level laser therapy (LLLT) in treating decubitus ulcers: a review. Proc. SPIE. 9309F, doi: 10.1117/12.2077991 (2015).
Avci, P. et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin. Cutan. Med. Surg. 32, 41–52 (2013).
Song, S., Zhou, F. & Chen, W. Low-level laser therapy regulates microglial function through src-mediated signalling pathways: implications for neurogenerative diseases. J. Neuroinflamm. 9, 219–225 (2012).
Zhang, Q., Zhou, C., Hamblin, M. R. & Wu, M. X. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J. Cereb. Blood Flow Metab. 34, 1391–1401 (2014).
Chow, R. T., Johnson, M. I., Lopes-Martins, B., R. A. & Bjordal, J. M. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 374, 1897–1908 (2009).
Smoot, B., Chiavola-Larson, L., Lee, J., Manibusan, H. & Allen, D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J. Cancer Surviv. 9, 287–304 (2015).
Rutar, M., Natoli, R., Albarracin, R., Valter, K. & Provis, J. 670-nm light treatment reduces complement propagation following retinal degeneration. J. Neuroinflamm. 9, doi: 10.1186/1742-2094-9-257 (2012).
Neves, M. A. I. et al. Different power settings of LLLT on the repair of the calcaneal tendon. Photomed. Laser Surg. 29, 663–668 (2011).
Khan, I. & Arany, P. Biophysical approaches for oral wound healing: emphasis on photobiomodulation. Adv. Wound Care 4, 724–737 (2015).
Geneva, I. I. Photobiomodulation for the treatment of retinal diseases: a review. Int. J. Ophthal. 9, 145–152 (2016).
Agrawal, T., Gupta, G. K., Rai, V., Carroll, J. D. & Hamblin, M. R. Pre-conditioning with low-level laser (light) therapy: light before the storm. Dose-Response 12, 619–649 (2014).
Carlos, F. P. et al. Role of low-level laser therapy on the cardiac remodeling after myocardial infarction: A systematic review of experimental studies. Life Sci. 151, 109–114 (2016).
Johnstone, D. M., Moro, C., Stone, J., Benabid, A. L. & Mitrofanis, J. Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front. Neurosci. 9, doi: 10.3389/fnins.2015.00500 (2016).
Purushothuman, S., Johnstone, D., Nandasena, C., Mitrofanis, J. & Stone, J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex - evidence from two transgenic mouse models. Alzheimers Res. Ther. 6, doi: 10.1186/alzrt232 (2014).
Johnstone, D. et al. The potential of light therapy in Parkinson’s disease. Chronophysiol. Ther. 4, 1–14 (2014).
Gonçalves, E. D. et al. Low-level laser therapy ameliorates disease progression in a mouse model of multiple sclerosis. Autoimmunity 49, 1–11 (2015).
Liebert, A. D., Chow, R. T., Bicknell, B. T. & Varigos, E. Neuroprotective effects against POCD by photobiomodulation: Evidence from assembly/disassembly of the cytoskeleton. J. Exp. Neurosci. 10, 1–19 (2016).
Walch, J. M. et al. The effect of sunlight on postoperative analgesic medication use: a prospective study of patients undergoing spinal surgery. Psychosom. Med. 67, 156–163 (2005).
Beauchemin, K. M. & Hays, P. Dying in the dark: sunshine, gender and outcomes in myocardial infarction. J. Roy. Soc. Med. 91, 352–354 (1998).
Jadad, A. R. & McQuay, H. J. Meta-analyses to evaluate analgesic interventions: A systematic qualitative review of their methodology. J. Clin. Epidemiol. 49, 235–243 (1996).
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, doi: 10.1186/1471-2288-14-43 (2014).
Yang, J. et al. Effect of low-level laser irradiation on oxygen free radicals and ventricular remodeling in the infarcted rat heart. Photomed. Laser Surg. 31, 447–452 (2013).
Quirk, B. J., Sonowal, P., Jazayeri, M.-A., Baker, J. E. & Whelan, H. T. Cardioprotection from ischemia-reperfusion injury by near-infrared light in rats. Photomed. Laser Surg. 32, 505–511 (2014).
Oron, U. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation 103, 296–301 (2001).
Keszler, A., Baumgardt, S., Hwe, C. & Bienengraeber, M. Far red/near infrared light-induced cardioprotection under normal and diabetic conditions. Proc SPIE. 9309, doi: 10.1117/12.2079880 (2015).
Keszler, A. et al. Far red/near infrared light-induced protection against cardiac ischemia and reperfusion injury remains intact under diabetic conditions and is independent of nitric oxide synthase. Front. Physiol. 5, doi: 10.3389/fphys.2014.00305 (2014).
Gatsura, S., Gladkikh, S. & Titov, M. Effect of low-energy laser irradiation on the area of experimental myocardial infarction, lipid peroxidation, and hemoglobin affinity for oxygen. B. Exp. Biol. Med. 137, 355–357 (2004).
Yang, Z. et al. Low-level laser irradiation alters cardiac cytokine expression following acute myocardial infarction: a potential mechanism for laser therapy. Photomed. Laser Surg. 29, 391–398 (2011).
Yaakobi, T. et al. Long-term effect of low energy laser irradiation on infarction and reperfusion injury in the rat heart. J. Appl. Physiol. 90, 2411–2419 (2001).
Tuby, H., Maltz, L. & Oron, U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Laser. Surg. Med. 38, 682–688 (2006).
Tuby, H., Maltz, L. & Oron, U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Laser. Surg. Med. 43, 401–409 (2011).
Ad, N. & Oron, U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int. J. Cardiol. 80, 109–116 (2001).
Lohr, N. L. et al. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J. Mol. Cell. Cardiol. 47, 256–263 (2009).
Manchini, M. T. et al. Amelioration of cardiac function and activation of anti-inflammatory vasoactive peptides expression in the rat myocardium by low level laser therapy. PloS One 9, e101270 (2014).
Derkacz, A., Protasiewicz, M., Poreba, R., Szuba, A. & Andrzejak, R. Usefulness of intravascular low-power laser illumination in preventing restenosis after percutaneous coronary intervention. Am. J. Cardiol. 106, 1113–1117 (2010).
De Scheerder, I. et al. Long-term follow-up after coronary stenting and intravascular red laser therapy. Am. J. Cardiol. 86, 927–930 (2000).
De Scheerder, I. K. et al. Intravascular low-power laser irradiation after coronary stenting: Long-term follow-up. Laser. Surg. Med. 28, 212–215 (2001).
Salem, M., Rotevatn, S. & Nordrehaug, J. E. Long-term results following percutaneous myocardial laser therapy. Coron. Artery Dis. 17, 385–390 (2006).
Lima, A. C. G. et al. Photobiomodulation (laser and LED) on sternotomy healing in hyperglycemic and normoglycemic patients who underwent coronary bypass surgery with internal mammary artery grafts: A randomized, double-blind study with follow-up. Photomed. Laser Surg., doi: 10.1089/pho.2016.4143 (2016).
Tuby, H., Maltz, L. & Oron, U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Laser. Surg. Med. 39, 373–378 (2007).
Gavish, L., Perez, L. & Gertz, S. D. Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Laser. Surg. Med. 38, 779–786 (2006).
Hentschke, V. S. et al. Low-level laser therapy improves the inflammatory profile of rats with heart failure. Laser. Med. Sci. 28, 1007–1016 (2013).
Liebert, A., Bicknell, B. & Adams, R. Protein conformational modulation by photons: A mechanism for laser treatment effects. Med. Hypoth. 82, 275–281 (2014).
Johnstone, D. et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism - An abscopal neuoroprotective effect. Neuroscience 274, 93–101 (2014).
Zhevago, N. A. & Samoilova, K. A. Pro-and anti-inflammatory cytokine content in human peripheral blood after its transcutaneous (in vivo) and direct (in vitro) irradiation with polychromatic visible and infrared light. Photomed. Laser. Surg. 24, 129–139 (2006).
Oron, A. & Oron, U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: A minireview. Photomed. Laser Surg. 34, 627–630 (2016).
Fekrazad, R., Asefi, S., Allahdadi, M. & Kalhori, K. A. Effect of photobiomodulation on mesenchymal stem cells. Photomed. Laser Surg. 34, 533–542 (2016).
Tuby, H., Maltz, L. & Oron, U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med 38, 682–688 (2006).
Khoo, N. K., Babazadeh, K., Lajevardi, M., Dabaghian, F. H. & Mostafavi, E. Application of low-level laser therapy following coronary artery bypass grafting (CABG) surgery. J. Lasers Med. Sci. 5, 86–91 (2014).
Huang, Y.-Y., Chen, A.-H., Carroll, J. & Hamblin, M. Biphasic dose response in low level light therapy. Dose-Response 7, 358–383 (2009).
Anders, J. J. & Wu, X. Comparison of light penetration of continuous wave 810 nm and superpulsed 904 nm wavelength light in anesthetized rats. Photomed. Laser Surg. 34, 418–424 (2016).
Strangman, G. E., Zhang, Q. & Li, Z. Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. NeuroImage 85, Part 1, 136–149 (2014).
Jagdeo, J. R., Adams, L. E., Brody, N. I. & Siegel, D. M. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One 7, e47460 (2012).
Tedford, C. E., DeLapp, S., Jacques, S. & Anders, J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Laser. Surg. Med. 47, 312–322 (2015).
Rahko, P. S. Evaluation of the skin-to-heart distance in the standing adult by two-dimensional echocardiography. J. Am. Soc. Echocardiog. 21, 761–764 (2008).
Zhu, Q. et al. Photo-irradiation improved functional preservation of the isolated rat heart. Laser. Surg. Med. 20, 332–339 (1997).
Karu, T. Mitochondrial signaling in mammalian cells activated by red and near-ir radiation. Photochem. Photobiol. 28, 1091–1099 (2008).
Gao, X. & Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 16, doi: 10.1186/1423-0127-16-4 (2009).
Quirk, B. J. & Whelan, H. T. Effect of red-to-near infrared light on the reaction of isolated Cytochrome c oxidase with Cytochrome c. Photomed. Laser Surg. 34, 631–637 (2016).
Saliba, A. et al. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: evidence from intervention studies in pigmented mice. PloS One 10, e0139003 (2015).
Tang, J. et al. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro . Invest. Ophthalmol. Vis. Sci. 54, 3681–3690 (2013).
Butow, R. A. & Avadhani, N. G. Mitochondrial signaling: The retrograde response. Mol. Cell. 14, 1–15 (2004).
Stetler, R. A. et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog. Neurobiol. 114, 58–83 (2014).
Calabrese, V. et al. Nitric oxide in cell survival: a janus molecule. Antioxid. Redox Sign. 11, 2717–2739 (2009).
Pellegrino, M. W., Nargund, A. M. & Haynes, C. M. Signaling the mitochondrial unfolded protein response. BBA Mol. Cell Res. 1833, 410–416 (2013).
Lee, M. S. Effect of mitochondrial stress on systemic metabolism. Ann. NY Acad. Sci. 1350, 61–65 (2015).
Kirstein, J. et al. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. EMBO J. 34, 2334–2349 (2015).
Wu, S. & Xing, D. Intracellular signaling cascades following light irradiation. Laser Photonics Rev. 8, 115–130 (2014).
de Lima, F. et al. low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem. Photobiol. 89, 179–188 (2013).
Ricci, R., Pazos, M., Borges, R. E. & Pacheco-Soares, C. Biomodulation with low-level laser radiation induces changes in endothelial cell actin filaments and cytoskeletal organization. J. Photoch. Photobio. B 95, 6–8 (2009).
Chow, R., David, M. & Armati, P. 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root dorsal root ganglion: implications for the analgesic effects of 830 nm laser. J. Peripher. Nerv. Syst. 12, 28–39 (2007).
Aimbire, F. et al. Low-level laser therapy induces dose-dependent reduction of TNFα levels in acute inflammation. Photomed. Laser Surg. 24, 33–37 (2006).
Hsieh, Y. L. et al. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: Possible involvements in hypoxia-inducible factor 1α (HIF-1α). J. Comp. Neurol. 520, 2903–2916 (2012).
Rizzi, C. F. et al. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-κB signaling pathway in traumatized muscle. Laser. Surg. Med. 38, 704–713 (2006).
Assis, L. et al. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Laser. Surg. Med. 44, 726–735 (2012).
Cheng, Y. J., Ou, H. C. & Lee, C. H. Low level laser therapy prevent endothelial cells from TNF-alpha/cycloheximide-induced apoptosis by modulating P38 MAPK/NF-kB pathway. Physioth. 101, Supplement 1, e236, doi: 10.1016/j.physio.2015.03.411 (2015).
Song, J.-Q., Teng, X., Cai, Y., Tang, C.-S. & Qi, Y.-F. Activation of Akt/GSK-3β signaling pathway is involved in intermedin1–53 protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis 14, 1299–1307 (2009).
Liang, J., Liu, L. & Xing, D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radical Biol. Med. 53, 1459–1467 (2012).
Duchen, M. R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 529, 57–68 (2000).
Biasibetti, M. et al. The influence of low-level laser therapy on parameters of oxidative stress and DNA damage on muscle and plasma in rats with heart failure. Laser. Med. Sci. 29, 1895–1906 (2014).
Zhang, H., Xing, D., Wu, S. & Sun, X. Protein kinase C δ promotes cell apoptosis induced by high fluence low-power laser irradiation. Proc. SPIE. 7519, doi: 10.1117/12.841566 (2009).
Malinovskaya, S., Monich, V. & Artifeksova, A. Effect of low-intensity laser irradiation and wideband red light on experimentally ischemized myocardium. Bull. Exp. Biol. Med. 145, 573–575 (2008).
Monich, V. A., Drugova, O. V., Lazukin, V. F. & Bavrina, A. B. Low-power light and isolated rat hearts after ischemia of myocardium. Proc. SPIE. 7552, doi: 10.1117/12.840841 (2010).
Cook, J. R. et al. Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome. J. Clin. Invest. 124, 1329–1339 (2014).
Derkacz, A., Protasiewicz, M., Poreba, R., Doroszko, A. & Andrzejak, R. Effect of the intravascular low energy laser illumination during percutaneous coronary intervention on the inflammatory process in vascular wall. Laser. Med. Sci. 28, 763–768 (2013).
Zhang, X. et al. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. J. Neurosci. 33, 17737–17748 (2013).
Morimoto, R. I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22, 1427–1438 (2008).
Pereira, E. R., Frudd, K., Awad, W. & Hendershot, L. M. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J. Biol. Chem. 289, 3352–3364 (2014).
de Oliveira, T. S. et al. Effects of low level laser therapy on attachment, proliferation, and gene expression of VEGF and VEGF receptor 2 of adipocyte-derived mesenchymal stem cells cultivated under nutritional deficiency. Laser. Med. Sci. 30, 217–223 (2015).
Yang, Z. et al. Low-level laser irradiation alters cardiac cytokine expression following acute myocardial infarction: a potential mechanism for laser therapy. Photomed. Laser Surg. 29, 391–398 (2011).
Bjordal, J. M., Couppé, C., Chow, R. T., Tunér, J. & Ljunggren, E. A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 49, 107–116 (2003).
Zhang, H. et al. Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. J. Cell. Mol. Med. 14, 1975–1987 (2010).
Plass, C. A., Wieselthaler, G. M., Podesser, B. K. & Prusa, A. M. Low-level-laser irradiation induces photorelaxation in coronary arteries and overcomes vasospasm of internal thoracic arteries. Laser. Surg. Med. 44, 705–711 (2012).
Author information
Authors and Affiliations
Contributions
A.L. and H.K. conceived the paper: A.K. and N.G. reviewed and categorized the reviewed articles; A.L., B.B. and H.K. interpreted data; A.L. and B.B. wrote the manuscript, with contributions from N.G.; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
B.B. is an agent for a company that manufactures medical laser products. The other authors have no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liebert, A., Krause, A., Goonetilleke, N. et al. A Role for Photobiomodulation in the Prevention of Myocardial Ischemic Reperfusion Injury: A Systematic Review and Potential Molecular Mechanisms. Sci Rep 7, 42386 (2017). https://doi.org/10.1038/srep42386
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42386
This article is cited by
-
Influence of Intravascular Laser Irradiation of Blood (ILIB) on inflammatory cytokines and nitric oxide in vivo: a systematic review
Lasers in Medical Science (2024)
-
Far-infrared-emitting fabric improves neuromuscular performance of knee extensor
Lasers in Medical Science (2022)
-
Light-emitting diode therapy protects against ventricular arrhythmias by neuro-immune modulation in myocardial ischemia and reperfusion rat model
Journal of Neuroinflammation (2019)
-
Effects of early- and mid-life stress on DNA methylation of genes associated with subclinical cardiovascular disease and cognitive impairment: a systematic review
BMC Medical Genetics (2019)
-
Photobiomodulation of the microbiome: implications for metabolic and inflammatory diseases
Lasers in Medical Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.