Abstract
Activating mutations leading to ligand-independent signaling of the stem cell factor receptor KIT are associated with several hematopoietic malignancies. One of the most common alterations is the D816V mutation. In this study, we characterized mice, which conditionally express the humanized KITD816V receptor in the adult hematopoietic system to determine the pathological consequences of unrestrained KIT signaling during blood cell development. We found that KITD816V mutant animals acquired a myeloproliferative neoplasm similar to polycythemia vera, marked by a massive increase in red blood cells and severe splenomegaly caused by excessive extramedullary erythropoiesis. Moreover, we found mobilization of stem cells from bone marrow to the spleen. Splenectomy prior to KITD816V induction prevented expansion of red blood cells, but rapidly lead to a state of aplastic anemia and bone marrow fibrosis, reminiscent of post polycythemic myeloid metaplasia, the spent phase of polycythemia vera. Our results show that the extramedullary hematopoietic niche microenvironment significantly influences disease outcome in KITD816V mutant mice, turning this model a valuable tool for studying the interplay between functionally abnormal hematopoietic cells and their microenvironment during development of polycythemia vera-like disease and myelofibrosis.
Similar content being viewed by others
Introduction
The blood forming system is characterized by a remarkable regenerative capacity, required for the continuous replacement of mature blood cells. During that process, the balance between cell proliferation and differentiation has to be tightly controlled to prevent hematopoietic malignancies. The KIT receptor, expressed by hematopoietic stem cells (HSCs) and progenitor cells (HPCs) and several lineage-committed precursors, is involved in cell maintenance, proliferation, survival and terminal differentiation1,2,3,4,5,6. Uncontrolled KIT signaling is associated with several myeloproliferative disorders7,8,9.
KIT belongs to the type III subfamily of tyrosine kinases and gets activated by its ligand stem cell factor (SCF), which is expressed as a membrane bound or soluble form10,11,12. SCF is produced by stromal cells in the hematopoietic bone marrow (BM) niche. For HSCs, interaction of KIT with membrane bound SCF was shown to be important for positioning to the niche13,14. Furthermore, KIT was described to be important for the maintenance of long-term HSCs5. In most lineages, KIT is downregulated during differentiation2, while in mast cells high KIT expression is maintained15. KIT deficient mice die in utero due to defects in fetal liver erythropoiesis, demonstrating its important function in red blood cells16. In erythroid progenitors, KIT regulates proliferation and maintenance of the undifferentiated state17.
Several KIT mutations have been described that cause constitutive receptor activation without ligand binding. The D816V substitution is one of the most commonly described mutations associated with hematopoietic neoplasia8,9,18. We previously described the generation of a humanized transgenic mouse model for conditional KITD816V expression and analyzed effects of KITD816V signaling on fetal liver erythropoiesis19. Here, we used R26-LSL-KITD816V mice to investigate sustained KITD816V signaling in the adult hematopoietic system and found development of a myeloproliferative neoplasm (MPN) reminiscent of polycythemia vera (PV), which was transplantable and characterized by massively increased red cell mass and splenomegaly. Furthermore, stem cells were mobilized from BM to the spleen. Splenectomy of KITD816V mutants prevented the increase in red cell mass but promoted BM failure and myelofibrosis, clinical features observed upon transformation of PV to post polycythemic myeloid metaplasia. The fact that course of disease in KITD816V mutants is influenced by splenectomy demonstrates the relevance of the niche and provides a unique model to study the interdependency of hematopoietic cells and the microenvironment.
Results
KITD816V induces a polycythemia vera-like disease
We previously described the generation of the R26-LSL-KITD816V mouse line, harboring a conditional knock in of a humanized KITD816V receptor linked to a green fluorescent protein (GFP) in the ROSA26 genomic locus. The D816V mutation has been implicated in the pathology of acute myeloid leukemia, mastocytosis and other oncogenic malignancies7,8,9,18,20. To extend the knowledge on how KIT regulates hematopoiesis and contributes to myeloproliferative disorders, we studied the effects of ectopic KITD816V expression in the adult hematopoietic system. We mated R26-LSL-KITD816V with HSC-SCL-Cre-ERT mice, which express a tamoxifen-inducible Cre recombinase under control of the stem cell enhancer of the Scl gene locus21. HSC-SCL-Cre-ERT-mediated recombination has been demonstrated in HSCs/HPCs and endothelial cells. Double transgenic HSC-SCL-Cre-ERT:R26-LSL-KITD816V animals (hereafter called HSC-SCL:KITD816V) were viable and developed normally. For induction of KITD816V expression (Fig. 1a), we treated adult HSC-SCL:KITD816V mice with a daily dose of 1.5 mg tamoxifen (TX) for 5 consecutive days. TX-treated wildtype and single transgenic littermates were used as controls. Quantitative real-time PCR validated KITD816V expression in hematopoietic compartments of HSC-SCL:KITD816V animals after induction, with transcript levels comparable to endogenous Kit expression in controls. To validate GFP co-expression, GFP-positive and -negative fractions were also analyzed for KITD816V expression (Supplementary Fig. S1).
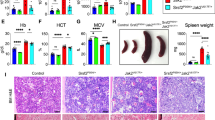
(a) Schematic illustration of tamoxifen (TX)-dependent KITD816V transgene induction in HSC-SCL:KITD816V mice. A loxP-flanked transcriptional STOP cassette precedes the the KITD816V cDNA that is fused to a GFP (pontellina plumata) via a 2A-peptide. The Cre-ERT protein is expressed in hematopoietic cells and translocates to the nucleus upon TX binding. Thereafter, GFP-KITD816V expression is driven by the endogenous ROSA26 promoter, mediating moderate transgene expression64,65. 1: ROSA26 Exon1. pA: polyadenylation sequence. SA: splice acceptor. (b) Indicated pB parameters of HSC-SCL:KITD816V and control mice were analyzed 4, 8, 10 and 18 weeks after TX injection with a hematology analyzer. 4 W - Control: N = 20. HSC-SCL:KITD816V: N = 14. 8 W - Control: N = 17. HSC-SCL:KITD816V: N = 9. 10 W - Control: N = 7. HSC-SCL:KITD816V: N = 6. 18 W - Control: N = 4. HSC-SCL:KITD816V: N = 2. (c) 8 weeks after TX injection, pB from HSC-SCL:KITD816V and control mice was subjected to erythrocyte lysis and nucleated cells were analyzed for expression of CD45 and markers for lineage committed blood cells via flow cytometry. The WBC was used to calculate the absolute number of cells per unit of blood volume. Control: N = 12. HSC-SCL:KITD816V: N = 7. EryB: Erythroblasts. Gran: Granulocytes. Mono: Monocytes. (d) Blood smears of HSC-SCL:KITD816V and control mice were prepared 8 weeks after TX injection and subjected to May-Grünwald-Giemsa staining. (e) Left: Number of splenic CFU-E colonies obtained at indicated concentrations of supplemented erythropoietin. As MethoCult methylcellulose medium includes fetal bovine serum as a possible source of erythropoietin, data demonstrate hyper-responsiveness of HSC-SCL:KITD816V CFU-Es to erythropoietin but cannot prove full erythropoietin-independency. Analysis carried out 10 weeks after induction. Control: N = 4. HSC-SCL:KITD816V: N = 4. Right: Serum erythropoietin levels were determined 8 weeks after induction using ELISA. Control: N = 6. HSC-SCL:KITD816V: N = 6. Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
In total, 44 HSC-SCL:KITD816V and 45 control mice were monitored for 4–10 weeks after treatment. Only a limited group of 2 HSC-SCL:KITD816V and 4 control animals was monitored up to 18 weeks, as we observed a high rate of spontaneous mortality for HSC-SCL:KITD816V animals (29.55%; 13/44 mice) within the first 10 weeks after induction. Aside from moderate enlargement of the abdomen in some cases, HSC-SCL:KITD816V mice showed no signs of morbidity before death. From the control group, all animals survived the observation period.
Cell counts in peripheral blood (pB) were analyzed 4, 8, 10 and 18 weeks after induction. The red blood cell count (RBC), hemoglobin (Hb), mean platelet volume (MPV) and white blood cell count (WBC) were significantly elevated in HSC-SCL:KITD816V mice compared to controls (Fig. 1b, Supplementary Fig. S2). The hematocrit (Hct) was initially elevated to high levels and slightly decreased thereafter. The mean corpuscular volume (MCV) and the platelet count (PLT) were decreased in HSC-SCL:KITD816V mice.
We further investigated alterations in pB cell lineages via flow cytometry. Analyses demonstrated elevated amounts of CD45-positive cells in pB of HSC-SCL:KITD816V mice, caused by increased monocyte and B-cell populations and a mild increase in granulocytes. Staining for erythroid markers CD71 and Ter-11922 revealed the presence of CD45-negative erythroblasts, which are normally not released into circulation (Fig. 1c, Supplementary Fig. S2). In addition, the reticulocyte frequency was increased (Supplementary Fig. S2). Figure 1d shows pB smears of HSC-SCL:KITD816V and control mice 8 weeks after induction. Accumulation of erythrocytes and mature myeloid cells is a hallmark of PV23. PV furthermore often includes thrombocytosis, but similar to murine PV models harboring the JAK2V617F mutation24,25,26 we found no elevation of platelets in HSC-SCL:KITD816V mice. As PV is additionally marked by endogenous erythroid colony formation and decreased serum erythropoietin23, we further analyzed these parameters. Indeed, for HSC-SCL:KITD816V mice markedly decreased serum erythropoietin levels and high numbers of splenic colony forming unit-erythroids (CFU-Es) in assays with low erythropoietin concentration were found, indicating erythropoietin hyper-responsiveness (Fig. 1d). Thrombopoietin serum levels were also reduced in HSC-SCL:KITD816V mice (Supplementary Fig. S2).
HSC-SCL:KITD816V mice develop splenomegaly with massive extramedullary erythropoiesis
BM and spleen were analyzed 10 weeks after KITD816V induction or when enlargement of the abdomen was observed. Hematoxylin and eosin (HE) staining revealed no apparent differences in BM histology of HSC-SCL:KITD816V and control animals. However, siderophages (mononuclear phagocytes containing hemosiderin, a product of hemoglobin catabolism) were scattered throughout control BM, whereas HSC-SCL:KITD816V mutants showed almost no siderophages, indicating reduced iron storage (Fig. 2a). Quantification further demonstrated a slight reduction in BM megakaryocytes. Immunohistochemical staining for active Caspase3 revealed a slight elevation of apoptosis in HSC-SCL:KITD816V BM, while Ki67 staining showed no differences in proliferation (Supplementary Fig. S3). Compared to controls, total BM cellularity of HSC-SCL:KITD816V mice was moderately increased (Fig. 2b). GFP-fluorescence was examined to estimate efficiency of KITD816V induction in HSC-SCL:KITD816V animals, demonstrating transgene expression in 57.40 ± 6.41% of total BM cells (Fig. 2b, dashed bar). While the frequency of CD45-positive cells was decreased in HSC-SCL:KITD816V BM, the overall frequency of CD45-negative erythroblasts was elevated in comparison to controls. Flow cytometric quantification of discrete developmental stages19,27 demonstrated a trend towards increased early and late erythroblasts, whereas reticulocytes were reduced, indicating a mild shift to more immature cells (Fig. 2b, Supplementary Fig. S3). To also investigate early BM erythropoiesis, we performed assays for CFU-E progenitors, showing no differences between HSC-SCL:KITD816V and control mice (Supplementary Fig. S3).
Hematopoietic organs of HSC-SCL:KITD816V and control mice were analyzed 7–10 weeks after induction of KITD816V expression with TX. (a,b) Analysis of BM. (a) Paraffin sections were subjected to HE staining and Prussian Blue staining for hemosiderin. (b) Graphs show quantification of indicated cell populations. For HSC-SCL:KITD816V mice, the frequency of GFP-positive cells within each population is depicted. Right bar graph in Log10 scale. BM Cellularity - Control: N = 7. HSC-SCL:KITD816V: N = 5. CD45-positive cells - Control: N = 9. HSC-SCL:KITD816V: N = 7. Erythroid populations - Control: N = 11. HSC-SCL:KITD816V: N = 8. (c,d) Analysis of the spleen. (c) Left: Macroscopic view. Scale bars: 1 cm. Middle: Scatter plot shows spleen weight in g. Control: N = 15. HSC-SCL:KITD816V: N = 11. Right: HE stainings of paraffin sections. (d) Quantification of indicated cell populations in the spleen. Right graph in Log10 scale. CD45-positive cells - Control: N = 9. HSC-SCL:KITD816V: N = 7. Erythroid populations - Control: N = 10. HSC-SCL:KITD816V: N = 8. EryB: Erythroblasts; ProE: Proerythroblasts; BasoE: Basophilic EryB; PolyE: Polychromatic EryB; OrthoE: Orthochromatic EryB; Retic: Reticulocytes. Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
Gross examination of the mice revealed massive splenomegaly in HSC-SCL:KITD816V animals, another diagnostic criterion for PV23. KITD816V mutants showed a 19.81 ± 10.09-fold increase in spleen weight and altered spleen histology (Fig. 2c). Lymphoid nodules, normally marked by clusters of CD3-positive cells, were virtually absent in the spleen of mutant mice (Supplementary Fig. S3).
Flow cytometric examination demonstrated CD45 expression in almost 80% of control spleen cells. In HSC-SCL:KITD816V mice, this frequency was reduced to less than 20% (Fig. 2d). This reduction was due to a massive increase in splenic erythropoiesis, as CFU-Es and erythroblast frequency were significantly elevated in HSC-SCL:KITD816V mice (Figs 1c and 2d, Supplementary Fig. S3). The high ratio of GFP-positive erythroblasts substantiates that this expansion was KITD816V-dependent.
In summary, these data demonstrate that chronic KITD816V signaling causes a PV-like disease based on moderately increased BM erythropoiesis and a massive induction of splenic erythropoiesis. As PV is often associated with high incidence of thrombosis due to elevated Hct23, we assume that abrupt death of HSC-SCL:KITD816V animals is a consequence of thrombotic events.
KITD816V signaling causes stem cell mobilization from BM and a shift of hematopoiesis to extramedullary sites
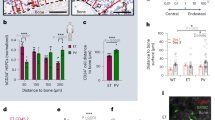
We next examined effects of KITD816V signaling on stem cells by analyzing the frequencies of LK (KITposSca-1negLinneg), LSK (KITposSca-1posLinneg) and HSC (LSK-CD48negCD150pos) populations among CD45-positive cells. BM HSC and LSK populations were increased in HSC-SCL:KITD816V animals compared to controls (Fig. 3a, Supplementary Fig. S4). To investigate the reasons for this expansion, we sorted LinnegKITpos BM cells and analyzed expression of important transcriptional regulators. Increased Gata2 transcript was found in the stem cell-enriched compartment of KITD816V mutants (Fig. 3b, Supplementary Fig. S4). Consistently, Gata2 has been shown to mediate proliferation and survival in hematopoietic stem cell compartments and in vitro differentiation of Gata2-deficient embryonic stem cells demonstrated impaired SCF-responsiveness28,29.
Indicated populations bearing different stem cell potential were analyzed by flow cytometry 7–10 weeks after KITD816V induction in HSC-SCL:KITD816V and control mice. (a) Graphs illustrate the fold change in frequency of CD45-positive cells for HSC-SCL:KITD816V mice in relation to controls; Log10 scale. BM - Control: N = 11. HSC-SCL:KITD816V: N = 8. Spleen - Control: N = 9. HSC-SCL:KITD816V: N = 7. (b) The LinnegKitpos cell population was purified from HSC-SCL:KITD816V and control BM 4 to 6 weeks after TX injection by flow cytometric cell sorting and analyzed for expression of indicated genes by quantitative real-time PCR. Data are presented as relative expression normalized to Gusb and Sdha. Control: N = 3. HSC-SCL:KITD816V: N = 3. (c) Combined Ki67/DAPI staining was used to analyze the cell cycle status of indicated BM stem cell populations via flow cytometry. Control: N = 3. HSC-SCL:KITD816V: N = 3. (d) The frequency of LT-HSCs, ST-HSCs, MPPs or defined progenitor cells within the CD45-positive cell population was analyzed for indicated hematopoietic compartments. Control: N = 3. HSC-SCL:KITD816V: N = 3. CDP: Common dendritic progenitor. CLP: Common lymphoid progenitor. CMP: Common myeloid progenitor. GMP: Granulocyte-macrophage progenitor. HSC: Hematopoietic stem cell. LT-HSC: Long-term HSC. MDP: Macrophage-dendritic progenitor. MPP: Multipotent progenitor. MEP: Megakaryocyte-erythroid progenitor. MkP: Megakaryocyte progenitor. ST-HSC: Short-term HSC. Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
To examine if KITD816V signaling confers a proliferative advantage to stem cells, we analyzed Ki67 expression in BM stem cell populations (Fig. 3c, Supplementary Fig. S4). Interestingly, we found that the effects varied between the different populations. While actively cycling cells were increased in the HSC population of HSC-SCL:KITD816V animals, they were decreased in the LK population, suggesting that KIT stimulates proliferation in HSCs but supports cell cycle exit in the LK population. To see if we could find alterations in downstream signaling of KITD816V expressing stem cell populations, we stimulated BM cells with SCF or thrombopoietin and used phospho-flow cytometry to analyze phosphorylation of Erk1/2 and Akt in LK and LSK populations. However, for the analyzed pathways we could find no differences on the level of the examined cell populations (Supplementary Fig. S4). So, studies of additional pathways and more precisely defined cell populations will be necessary to find out more about signaling changes in KITD816V stem cells.
In the spleen, HSCs showed a mild but not significant elevation, while the LSK and LK populations bearing lower stem cell potential were significantly increased (Fig. 3a, Supplementary Fig. S4).
We further examined distribution of long-term (LT)-HSCs, short-term (ST)-HSCs and multipotent progenitors (MPPs) between BM, pB and spleen of HSC-SCL:KITD816V and control mice. We found BM LT-HSCs slightly increased in HSC-SCL:KITD816V mice, while the other populations in BM were not significantly altered. In contrast, ST-HSC and MPP frequencies were considerably elevated in pB and spleen (although changes were not significant for pB), indicating increased HSC activation and mobilization from BM to circulation. Consistently, progenitor cell populations were slightly reduced in BM but increased in spleen (Fig. 3d, Supplementary Fig. S4).
HSC analysis includes Kit as a cell surface marker. It is thus important to note that the KITD816V mutant receptor localizes to intracellular compartments30. In line with this, we found the fraction of cells positive for Kit surface expression similar in GFP-positive and GFP-negative BM populations in HSC-SCL:KITD816V mice, suggesting that only the endogenous receptor reaches the cell surface and ectopic KITD816V expression does not affect HSC analysis (Supplementary Fig. S5).
Unexpectedly, the frequency of GFP-positive cells was quite low in KITD816V HSCs, while it increased with progressive differentiation. To examine potential loss of KITD816V-positive HSCs or high Cre-recombination frequencies in differentiated cells, we analyzed kinetics of reporter gene expression in different hematopoietic populations 2, 4 and 6 weeks after induction. The GFP-frequency was stable in the LT-HSC compartment and gradually increased in ST-HSC and MPP populations, indicating no negative influence of KITD816V signaling on stem cell survival (Supplementary Fig. S5). Initially, the GFP-frequency in most progenitor and mature populations was lower than in stem cells, demonstrating Cre-recombination primarily in HSCs, albeit at low frequency. However, analyses suggested also low recombination in granulocytes and B-cells. As the GFP-positive fraction was especially high in the erythroid compartment, we analyzed erythroblasts directly after TX-induction, further revealing recombination in proerythroblasts to a comparatively high degree (Supplementary Fig. S5).
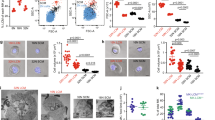
As we found alterations in stem cell distribution, we also analyzed distribution of mature blood cells. In BM, we found no alterations in granulocyte, B-cell and monocyte populations, while in the spleen we found a reduction in lymphocyte frequency for HSC-SCL:KITD816V animals (Fig. 4a, Supplementary Fig. S6). Analysis of dendritic cells (DCs) revealed a decrease in BM and an increase in the spleen of HSC-SCL:KITD816V mice. As KITD816V is strongly associated with mastocytosis8,9, we checked for mast cell infiltrations. Surprisingly, flow cytometry revealed a reduced number of peritoneal and skin mast cells in mutants, although changes were not significant (Fig. 4b, Supplementary Fig. S6).
(a) BM and spleen cells from HSC-SCL:KITD816V and control mice were analyzed 7–9 weeks after TX-treatment for indicated populations via flow cytometry. BM - Control: N = 3. HSC-SCL:KITD816V: N = 3. Spleen - Control: N = 5. HSC-SCL:KITD816V: N = 5. (b) Dendritic cells and mast cell infiltrations were analyzed in indicated organs 7 weeks after TX-treatment via flow cytometry. Control: N = 3. HSC-SCL:KITD816V: N = 3. DC: Dendritic cell. cDC: Conventional DC. pDC: Plasmacytoid DC. (c) Graph summarizes the alterations in analyzed HSC/HPC- and differentiated blood cell populations for BM and spleen of HSC-SCL:KITD816V mice. Data presented as fold change in frequency within the CD45-positive cell population for HSC-SCL:KITD816V mice in relation to controls; Log10 scale. CLP: Common lymphoid progenitor. CMP: Common myeloid progenitor. GMP: Granulocyte-macrophage progenitor. MDP: Macrophage-dendritic progenitor. CDP: Common dendritic progenitor. MEP: Megakaryocyte-erythroid progenitor. MkP: Megakaryocyte progenitor. Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
Taking together, our analyses demonstrate that stem cell and differentiated blood cell populations tend to be reduced in BM but increased in spleen (with the exception of lymphocytes), indicating a shift in hematopoiesis to extramedullary sites (Fig. 4c).
The KITD816V-mediated PV-like phenotype is transplantable
In addition to the hematopoietic compartment, HSC-SCL-Cre-ERT-mediated recombination also occurs in endothelial cells21. Hence, KITD816V signaling might also be activated in cells of the perivascular niche. Thus, we investigated whether the PV-like phenotype was a secondary event due to changes in the hematopoietic microenvironment. We treated HSC-SCL:KITD816V and control animals with TX and performed transplantation of unfractionated BM 6 weeks later. Recipients were analyzed 3 and 6 weeks after transplantation. In HSC-SCL:KITD816V transplanted mice we observed a gradual increase in RBC, Hb, Hct and WBC (Fig. 5a, Supplementary Fig. S7). Moreover, HSC-SCL:KITD816V recipients developed splenomegaly and progressively elevated erythroblast numbers (Fig. 5b, Supplementary Fig. S7). While splenic stem cell frequencies were generally elevated post-transplantation, absolute numbers were markedly higher for HSC-SCL:KITD816V recipients compared to controls (Fig. 5c, Supplementary Fig. S7). In summary, these data demonstrate that early PV-like disease develops autonomously from the medullary hematopoietic compartment.
Whole BM cells from HSC-SCL:KITD816V and control mice were used for BM transplantation 6 weeks after KITD816V induction. Recipients were analyzed 3 and 6 weeks post-transplantation. Control donors: N = 1. HSC-SCL:KITD816V donors: N = 3. 3 W - Control recipients: N = 1. HSC-SCL:KITD816V recipients: N = 3. 6 W - Control recipients: N = 2 for (a); N = 1 for (b,c). HSC-SCL:KITD816V recipients: N = 5. (a) Indicated pB parameters were analyzed with a hematology analyzer. (b) Representative macroscopic picture of the spleens of control and HSC-SCL:KITD816V recipients 6 weeks after transplantation. Graphs illustrate quantification of splenic erythroblasts (EryB) as analyzed by flow cytometry. (c) Quantification of splenic HSC/HPC populations shown as frequency of CD45-positive cells. For recipients of BM transplants, the frequency was calculated for the CD45.2-positive donor cell population. Data are presented as mean ± standard deviation.
Splenectomy protects HSC-SCL:KITD816V mice from increased red blood cell mass but promotes rapid hematopoietic failure
We assumed that the high mortality rate of HSC-SCL:KITD816V animals was a consequence of thrombotic events caused by the elevated Hct due to massive splenic erythropoiesis. We therefore hypothesized that splenectomy (SplE) might have a protective effect and subjected HSC-SCL:KITD816V and control animals to SplE prior to TX-treatment (afterwards termed SplE HSC-SCL:KITD816V and SplE control mice). Blood parameters determined 4 and 10 weeks after KITD816V induction demonstrated that SplE HSC-SCL:KITD816V mice indeed were protected from excessive red blood cell production, as RBC, Hb, Hct, MCV and reticulocyte frequency were similar to SplE controls (Fig. 6a, Supplementary Fig. S8). Moreover, although pB monocytes were significantly elevated after 18 weeks, the WBC and the overall number of CD45-positive cells were unaffected in SplE HSC-SCL:KITD816V animals (Supplementary Fig. S8). Surprisingly, however, 18 weeks after induction SplE HSC-SCL:KITD816V animals became anemic, with RBC, Hb, Hct and PLT values falling significantly below control values (Fig. 6a, Supplementary Fig. S8). Despite the decrease in erythrocytes, a considerable number of erythroblasts was found in pB of SplE HSC-SCL:KITD816V mice (Supplementary Fig. S8).
HSC-SCL:KITD816V and control mice were subjected to SplE, followed by TX-treatment. (a) Indicated pB parameters were analyzed 4, 10 and 18 weeks after KITD816V induction with a hematology analyzer. SplE Control: N = 4. SplE HSC-SCL:KITD816V: N = 3. (b,c) Analyses performed 18 weeks after KITD816V induction. SplE Control: N = 4. SplE HSC-SCL:KITD816V: N = 3. (b) Pictures show BM paraffin sections that were subjected to HE staining. The upper graph depicts quantification of the absolute cell number in BM. For the lower graphs, nucleated pB cells were analyzed via flow cytometry for CD45 and erythroid marker proteins. EryB: Erythroblasts. For HSC-SCL:KITD816V mice, the frequency of GFP-positive cells within each population is indicated. (c) CFC assays performed with BM cells. Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
Histologically, BM of SplE HSC-SCL:KITD816V mice appeared hypocellular compared to SplE controls. Consistently, the total cell number per femur and BM megakaryocyte counts were dramatically decreased (Fig. 6b, Supplementary Fig. S8). In SplE controls BM siderophages were abnormally abundant, indicating partial take-over of red blood cell destruction after spleen removal. In contrast, siderophages were absent in KITD816V mutants after SplE, again demonstrating affected iron storage (Supplementary Fig. S8).
After 18 weeks we found the frequency of CD45-positive cells in SplE HSC-SCL:KITD816V BM considerably reduced in favor of a significant increase in erythroblast frequency (Fig. 6b). However, an elevated ratio of early and late erythroblasts to reticulocytes indicated ineffective cell maturation. Moreover, given the overall cell loss in BM, total cell numbers were reduced for all erythroid populations (Supplementary Fig. S8). In line with the increase in erythroblast frequency, CFC-assays revealed significantly more CFU-E colonies for SplE HSC-SCL:KITD816V animals compared to SplE controls. In contrast, the number of non-erythroid colonies was reduced for SplE HSC-SCL:KITD816V mice (Fig. 6c). These data indicate, that KITD816V preferentially promotes erythropoiesis.
As extramedullary hematopoiesis can also occur in liver, SplE animals were examined for liver erythropoiesis (Supplementary Fig. S8). Indeed, a mild induction of erythropoiesis was found in livers of SplE HSC-SCL:KITD816V mice, but the overall extent was low and no hepatomegaly was observed.
Splenectomy is accompanied by stem cell loss and BM fibrosis in KITD816V mice
Analyzing stem cell populations of SplE HSC-SCL:KITD816V animals, we found a strong progressive reduction in BM HSCs/HPCs compared to SplE controls (Fig. 7a, Supplementary Fig. S9). This was in contrast to KITD816V mutants not subjected to SplE, which showed no indication of stem cell depletion after 10 weeks (Fig. 3a). We also examined Erk1/2 and Akt phosphorylation in BM LK and LSK populations of SplE animals, but again found no constitutive activation or altered reaction to SCF or thrombopoietin stimulation in KITD816V cells (Supplementary Fig. S9).
HSC-SCL:KITD816V and control mice were subjected to SplE, followed by TX-treatment. (a) Indicated BM cell populations were analyzed 10 and 18 weeks after induction. Graphs show fold change in frequency of CD45-positive cells for SplE HSC-SCL:KITD816V mice in relation to controls; Log10 scale. Representative flow cytometric panels from 18 weeks analysis are depicted. 10 weeks - SplE Control: N = 4. SplE HSC-SCL:KITD816V: N = 3. 18 weeks - SplE Control: N = 4. SplE HSC-SCL:KITD816V: N = 3. (b) 18 weeks after induction BM paraffin sections were analyzed by reticulin staining and immunohistological staining against active Caspase3. Reticulin staining demonstrated BM fibrosis in all analyzed SplE HSC-SCL:KITD816V mice (10 weeks N = 3/18 weeks N = 3). In 18 weeks samples a loose network of reticulin fibers was found (MF-1–MF-2 according to the European Consensus fibrosis grading). Data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test.
Our results demonstrate, that although SplE protects HSC-SCL:KITD816V mice from excessive red blood cell accumulation, it promotes rapid BM failure. Clinically, PV can progress to its spent phase polycythemic myeloid metaplasia (PPMM)31. There, the initially hypercellular BM becomes hypocellular and secondary myelofibrosis develops. Red blood cell production becomes ineffective and pB cell counts decrease, resembling aplastic anemia. In this study, HSC-SCL:KITD816V mice without SplE developed a malignancy reminiscent of early PV, whereas the phenotype observed in SplE HSC-SCL:KITD816V mice resembled PPMM. To further validate this, we performed staining for reticulin fibers to check for BM fibrosis. Indeed, fibrotic changes were found in SplE HSC-SCL:KITD816V BM 10 and 18 weeks after KITD816V induction32. In contrast, HSC-SCL:KITD816V mice not subjected to SplE neither displayed any signs of fibrosis in BM after 10 or 18 weeks nor increased reticulin deposition in spleen (Fig. 7b, Supplementary Fig. S9). In SplE HSC-SCL:KITD816V BM we also observed considerably elevated apoptosis, analyzed by immunostaining for active Caspase3 (Fig. 7b). Ki67 staining showed no apparent differences (Supplementary Fig. S9).
Our results indicate that removal of the splenic hematopoietic niche in KITD816V mutants dramatically influences the clinical picture of the PV-like disease.
Discussion
In this study, we analyzed the consequences of oncogenic KITD816V expression in the adult hematopoietic system and found MPN development reminiscent of early and advanced forms of PV, depending on pre-treatment of animals with SplE.
Upon induction of KITD816V expression, we observed massively increased red blood cell production. Under normal conditions, the erythrocyte pool is maintained by BM steady-state erythropoiesis, whereas extramedullary stress erythropoiesis mediates its rapid expansion upon acute anemia33. Different studies have reported a role of KIT in stress erythropoiesis34,35. Accordingly, we found the increase in red blood cells to depend on splenic erythropoiesis. Similar observations have been reported for KITV558∆;T669I/+ mice36. While this clearly demonstrates involvement of KIT in stress erythropoiesis, effects on BM steady-state erythropoiesis are difficult to evaluate in our model, since analysis is impeded by splenic erythropoiesis or hematopoietic failure. However, the erythroid expansion in BM of SplE KITD816V mutants indicates that KIT signaling regulates proliferative expansion during both, steady-state and stress erythropoiesis.
HSC-SCL:KITD816V mice had significantly increased pB monocytes, which is likely based on increased proliferation rather than mobilization, as the monocyte frequency in HSC-SCL:KITD816V BM or spleen37 was not reduced and pB monocytes were also elevated after SplE. Detailed investigation will be necessary to elucidate effects of KITD816V on the monocytic lineage. Untypical for PV, pB B-cells were also elevated in HSC-SCL:KITD816V mice. We suppose that this was primarily caused by displacement from the spleen, which showed markedly reduced B-cell frequency.
In HSC-SCL:KITD816V animals we observed mobilization of HSCs to the spleen. Several studies have indicated a role of KIT in mobilization of stem cells from the quiescent niche38,39. Mobilization of HSCs/HPCs from the BM niche was shown to depend on the release of soluble SCF mediated by matrix metalloproteinase-9 (MMP9)38. Moreover, KIT signaling is involved in cell mobilization triggered by functionally blocking cytoadhesion molecules VLA4/VCAM-1, suggesting an integrin/cytokine crosstalk39. We also observed an increased frequency of cycling HSCs and increased ST-HSCs in KITD816V mutants. Differences in the KIT expression level within the LSK-CD150posCD48neg HSC pool have been reported, with lower expression in quiescent cells40. These data indicate that oncogenic KITD816V contributes to activation and mobilization of dormant HSCs. Interestingly, mobilization of HSCs/HPCs has also been associated with primary myelofibrosis and PPMM41,42,43.
HSC-SCL:KITD816V animals pre-treated by SplE showed stem cell loss and myelofibrosis already 10 weeks after KITD816V induction. Interestingly, no fibrosis was detected in HSC-SCL:KITD816V mice without SplE, suggesting that disease pathogenesis depends not only on time but also on the interplay between KITD816V-positive cells and the medullary and extramedullary hematopoietic niches. This is in line with the “bad seeds in bad soil” concept proposed for primary myelofibrosis, presuming that an abnormal hematopoietic cell clone alters its microenvironment, resulting in niche dysfunctions42. One may hypothesize, that abnormal KITD816V-positive cells cycle between hematopoietic niches and upon splenectomy remain in or repopulate the BM and produce high levels of fibrogenic cytokines, stimulating stromal reticulin production. Consequently, displacement from the niche caused by KITD816V-induced stem cell mobilization and myelofibrosis leads to hematopoietic failure. Studies which have shown that the spleen serves as a reservoir of aberrant stem cells in primary myelofibrosis patients support this assumption44,45. Further, a study by Migliaccio et al. demonstrated that the spleen microenvironment is capable of supporting maturation of Gata1low mutant stem cells that fail to mature in the BM, suggesting the possibility that the BM niche may likewise not sustain maturation of KITD816V mutant cells46.
Future experiments with SplE HSC-SCL:KITD816V mice should investigate the disease-promoting cell population(s) and cytokine production to further substantiate this hypothesis.
As SplE can manipulate the course of disease, HSC-SCL:KITD816V mice provide an excellent model to study the interplay between hematopoietic cells and microenvironment in PV-like disease.
Noteworthy, while Philadelphia-negative MPN are highly associated with the JAK2V617F mutation found in hematopoietic and endothelial-like cells47,48, there is only one study reporting an association of KIT mutations with PV49. However, other sequencing studies did not confirm this association, raising the question for the relevance of activating KIT mutations in human MPN. It might be possible that alternative mechanisms lead to altered KIT signaling in human MPN. For instance, it was shown that cultured pB cells from primary myelofibrosis patients produce elevated levels of activated MMP950. As mentioned before, MMP9 can mediate release of soluble KIT ligand38. Furthermore, phospho-proteomic analysis of erythroblasts from PV patients revealed reduced total KIT and phospho-KIT-Y719 protein content, indicating altered KIT signaling51. It remains unclear if this results from hyper- or hypoactivation of the pathway, as the relative KIT-Y719 phosphorylation in PV and control groups was not compared. However, the reduction in total KIT on protein but not transcript level points to increased internalization upon activation in PV cells.
KITD816V represents a frequent mutation in mastocytosis in man8,9 and development of cutaneous mastocytosis with variable speed and degree has been demonstrated in a BAC transgenic KitD814V mouse model52. Thus, it was surprising that peritoneal and skin mast cells were reduced in HSC-SCL:KITD816V mice and no mastocytosis was observed. This discrepancy might be due to differential expression levels, as the the Kit promoter exhibits strong physiological activity in the mast cell lineage15,53 compared to moderate ROSA26-mediated expression. Moreover, mastocytosis in man is associated with additional mutations, such as Tet2, Srsf2, Asxl1, Cbl and Runx1, which are not present in HSC-SCL:KITD816V mice54,55. A recent study by Jawhar et al. has shown that mutations in TET2, SRSF2 or ASXL1 precede the KITD816V mutation in mastocytosis56. Thus, in the absence of such seed mutations the KITD816V mediated disease phenotype might well be different from the phenotype in KITD816V mastocytosis patients. In addition to mastocytosis, the KITD816V mutation is also frequently identified in the core binding factor leukemias, involving the AML1-ETO or CBFB/MYH11 genes (t[8;21] or inv[16]/t[16;16], respectively)7,57. A study by Wang et al. found strong evidence that in t(8;21) AML, AML1-ETO is the first genetic hit which is responsible for disease initiation, while KITD816V is a secondary mutation58.
Features reminiscent of early PV have also been observed in KITV558∆;T669I/+ mice36,59. However, the effects were rather mild, probably due the endogenous Kit promoter driving the mutated KIT, mediating no expression at later developmental stages. We show that KITD816V expression driven by the ROSA26 promoter causes a more severe and complex phenotype (PPMM/myelofibrosis). We speculate that our KITD816V allele, which is not downregulated upon differentiation might better mirror pathology of some MPN entities, where deregulated gene expression patterns are found60,61,62. Taking together, R26-LSL-KITD816V mice provide a valuable model to investigate interactions of aberrant KITD816V cells with the hematopoietic microenvironment leading to PV-like disease and myelofibrosis.
Materials and Methods
Animal studies
All experiments were compliant with the German law of animal protection and the European Directive 86/609/EEC and were approved by the local institutional animal care committees (Landesamt für Natur, Umwelt und Verbraucherschutz, North Rhine-Westphalia; approval-ID: #84-02.04.2013.A491/#84-02.04.2012.A256).
R26-LSL-KITD816V mice19 were maintained on 129 Sv/S2, HSC-SCL-Cre-ERT mice21 on C57BL/6 genetic background. For transgene induction animals received intraperitoneal injection of 1.5 mg TX/day for 5 consecutive days at a minimum age of 8 weeks. Peripheral blood samples were collected from the tail vein. Blood and BM cell counts were determined using an Hemavet 950 hematology analyzer (Drew Scientific, Miami Lakes, FL, USA). Studies were powered to detect a pre-specified RBC difference of 1 × 106 cells/μl between HSC-SCL:KITD816V and control animals (effect size = 1.48, two-sided α-value = 0.05/ß-value = 0.2). For splenectomy, animals were anesthetized and an incision was made at the left abdominal wall. Ligatures were set around splenic vessels before spleen removal. The incision was closed with wound clips. Following surgery, animals obtained carprofen for pain relief and were allowed to recover for one week before TX-treatment. For transplantation assays, R26-LSL-KITD816V mice were backcrossed to C57BL/6. CD45.1 recipient mice were subjected to lethal irradiation (2 × 5.5 Gray in 4 h interval) and 2 × 106 unfractionated BM cells from HSC-SCL:KITD816V CD45.2 donor mice were transplanted via tail vein injection. Reconstitution of CD45.2 donor cells was analyzed by flow cytometry. Splenectomy/transplantation studies were powered to detect a RBC difference of 7 × 106 cells/μl, based on earlier results (effect size = 4.69).
Colony forming cell assays and erythropoietin levels
Colony forming cell (CFC) assays were performed in technical triplicates using MethoCult methylcellulose medium (StemCell Technologies, Vancouver, Canada) according to manufacturers instructions with minor modifications. Per replicate, 5 × 104 nucleated cells were plated for BM and 2.5 × 105 for spleen. CFU-E colonies were scored after 2–4 days. If not stated otherwise, erythropoietin concentration was 3 U/ml. For GEMM and GM colonies, the medium was supplemented with 50 ng/ml SCF, 10 ng/ml IL3 and 10 ng/ml IL6 and the total colony number scored after 9–10 days. Serum erythropoietin and thrombopoietin levels were determined by ELISA (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
For flow cytometry, cells were stained with fluorochrome-conjugated antibodies listed in Supplementary Table S1 or with 1 μg/ml Hoechst H33342 for staining of nucleated cells. For reticulocyte staining, whole EDTA blood was stained using anti-Ter119-antibody and 1 ng/ml thiazol orange. Flow cytometry was performed on a BD FACSCanto™ or a BD FACSCanto™ II Flow Cytometer (Becton Dickinson, Heidelberg, Germany). Data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA). For marker combinations defining cell populations see Figure descriptions and Supplementary Table S2.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using RNeasy Mini or RNeasy Micro Kits (Qiagen, Hilden, Germany). cDNA synthesis was performed using RevertAid Premium reverse transcriptase (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). For amplification of target sequences Maxima SYBR Green/ROX (Fermentas) was used. Reference genes Gusb (glucuronidase) and Sdha (succinate dehydrogenase) were used for normalization. Primer sequences for qRT-PCR are given in Supplementary Table S3.
Histology and Immunohistochemistry
For histology, samples were fixed in 4% paraformaldehyde, dehydrated and paraffin-embedded. Immunohistochemistry was performed using the BrightVision Ultimate Plus Kit (Immunologic, Duiven, Netherlands) with antibodies listed in Supplementary Table 1. For Prussian Blue/hemosiderin staining, sections were deparaffinized, hydrated, stained in 1:2 potassium ferrocyanide (1–2%)/hydrochloric acid (1–2%) and counterstained with nuclear fast red. Reticulin fibers were stained using Gordon and Sweet’s silver staining protocol for reticulin63.
Statistical analysis
All data are presented as mean ± standard deviation. P-values were determined using two-tailed, unpaired Student’s t-test. Studies were neither randomized nor blinded, as all animal experiments were performed with homogeneous age, strain and similar variance.
Additional Information
How to cite this article: Pelusi, N. et al. The spleen microenvironment influences disease transformation in a mouse model of KITD816V-dependent myeloproliferative neoplasm. Sci. Rep. 7, 41427; doi: 10.1038/srep41427 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ikuta, K. & Weissman, I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. USA 89, 1502–6 (1992).
Ogawa, M. et al. Expression and function of c-kit in hemopoietic progenitor cells. J. Exp. Med. 174, 63–71 (1991).
Munugalavadla, V. et al. Repression of c-kit and its downstream substrates by GATA-1 inhibits cell proliferation during erythroid maturation. Mol Cell Biol 25, 6747–6759 (2005).
Lyman, S. D. & Jacobsen, S. E. W. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91, 1101–34 (1998).
Thorén, L. a. et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J. Immunol. 180, 2045–53 (2008).
Waskow, C., Paul, S., Haller, C., Gassmann, M. & Rodewald, H.-R. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity 17, 277–88 (2002).
Kim, H.-J. et al. KIT D816 mutation associates with adverse outcomes in core binding factor acute myeloid leukemia, especially in the subgroup with RUNX1/RUNX1T1 rearrangement. Ann. Hematol. 92, 163–71 (2013).
Bodemer, C. et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J. Invest. Dermatol. 130, 804–15 (2010).
Longley, B. J. et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc. Natl. Acad. Sci. USA 96, 1609–14 (1999).
Anderson, D. M. et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 63, 235–43 (1990).
Zsebo, K. M. et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63, 213–24 (1990).
Copeland, N. G. et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell 63, 175–83 (1990).
Kimura, Y. et al. c-Kit-mediated functional positioning of stem cells to their niches is essential for maintenance and regeneration of adult hematopoiesis. PLoS One 6, e26918 (2011).
Driessen, R. L., Johnston, H. M. & Nilsson, S. K. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp. Hematol. 31, 1284–91 (2003).
Maeda, K., Nishiyama, C., Ogawa, H. & Okumura, K. GATA2 and Sp1 positively regulate the c-kit promoter in mast cells. J. Immunol. 185, 4252–60 (2010).
de Aberle, S. B. A study of the hereditary anaemia of mice. Am. J. Anat. 40, 219–249 (1927).
Muta, K., Krantz, S. B., Bondurant, M. C. & Dai, C. H. Stem cell factor retards differentiation of normal human erythroid progenitor cells while stimulating proliferation. Blood 86, 572–80 (1995).
Malaise, M., Steinbach, D. & Corbacioglu, S. Clinical implications of c-Kit mutations in acute myelogenous leukemia. Curr Hematol Malig Rep 4, 77–82 (2009).
Haas, N. et al. Kit transduced signals counteract erythroid maturation by MAPK-dependent modulation of erythropoietin signaling and apoptosis induction in mouse fetal liver. Cell Death Differ. 22, 790–800 (2015).
Sakuma, Y., Sakurai, S., Oguni, S., Hironaka, M. & Saito, K. Alterations of the c-kit gene in testicular germ cell tumors. Cancer Sci. 94, 486–91 (2003).
Göthert, J. R. et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105, 2724–32 (2005).
Zhang, J., Socolovsky, M., Gross, A. W. & Lodish, H. F. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 102, 3938–46 (2003).
Spivak, J. L. Polycythemia vera: myths, mechanisms, and management. Blood 100, 4272–90 (2002).
Wernig, G. et al. EXEL-8232, a small-molecule JAK2 inhibitor, effectively treats thrombocytosis and extramedullary hematopoiesis in a murine model of myeloproliferative neoplasm induced by MPLW515L. Leukemia 26, 720–7 (2012).
Wernig, G. et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107, 4274–81 (2006).
Zaleskas, V. M. et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One 1, e18 (2006).
Sprüssel, A. et al. Lysine-specific demethylase 1 restricts hematopoietic progenitor proliferation and is essential for terminal differentiation. Leukemia 26, 2039–51 (2012).
Rodrigues, N. P. et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 106, 477–485 (2005).
Tsai, F. Y. et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–6 (1994).
Xiang, Z., Kreisel, F., Cain, J., Colson, A. & Tomasson, M. H. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol Cell Biol 27, 267–282 (2007).
Mesa, R. A., Elliott, M. A. & Tefferi, A. Splenectomy in chronic myeloid leukemia and myelofibrosis with myeloid metaplasia. Blood Rev. 14, 121–9 (2000).
Gianelli, U. et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod. Pathol. 25, 1193–1202 (2012).
Paulson, R. F., Shi, L. & Wu, D.-C. Stress erythropoiesis: new signals and new stress progenitor cells. Curr. Opin. Hematol. 18, 139–45 (2011).
Agosti, V., Karur, V., Sathyanarayana, P., Besmer, P. & Wojchowski, D. M. A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis. Exp. Hematol. 37, 159–171 (2009).
Perry, J. M., Harandi, O. F. & Paulson, R. F. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood 109, 4494–502 (2007).
Deshpande, S. et al. Kit receptor gain-of-function in hematopoiesis enhances stem cell self-renewal and promotes progenitor cell expansion. Stem Cells 31, 1683–1695 (2013).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–6 (2009).
Heissig, B. et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–37 (2002).
Papayannopoulou, T., Priestley, G. V. & Nakamoto, B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 91, 2231–9 (1998).
Matsuoka, Y. et al. Low level of c-kit expression marks deeply quiescent murine hematopoietic stem cells. Stem Cells 29, 1783–91 (2011).
Passamonti, F. et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+cells into peripheral blood in myeloproliferative disorders. Blood 107, 3676–82 (2006).
Le Bousse-Kerdilès, M.-C. Primary myelofibrosis and the ‘bad seeds in bad soil’ concept. Fibrogenesis Tissue Repair 5, S20 (2012).
Lataillade, J.-J. et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood 112, 3026–35 (2008).
Wang, X. et al. Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J. Clin. Invest. 122, 3888–99 (2012).
O’Malley, D. P., Orazi, A., Wang, M. & Cheng, L. Analysis of loss of heterozygosity and X chromosome inactivation in spleens with myeloproliferative disorders and acute myeloid leukemia. Mod. Pathol. 18, 1562–8 (2005).
Migliaccio, A. R. et al. Gata1 expression driven by the alternative HS2 enhancer in the spleen rescues the hematopoietic failure induced by the hypomorphic Gata1 low mutation. Blood 114, 2107–2121 (2009).
Levine, R. L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–97 (2005).
Sozer, S. et al. Human CD34+cells are capable of generating normal and JAK2V617F positive endothelial like cells in vivo . Blood Cells. Mol. Dis. 43, 304–12 (2009).
Fontalba, A. et al. Identification of c-Kit gene mutations in patients with polycythemia vera. Leuk. Res. 30, 1325–6 (2006).
Ciurea, S. O. et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 110, 986–993 (2007).
Hricik, T. et al. Transcriptomic and phospho-proteomic analyzes of erythroblasts expanded in vitro from normal donors and from patients with polycythemia vera. Am. J. Hematol. 88, 723–729 (2013).
Gerbaulet, A. et al. Mast cell hyperplasia, B-cell malignancy, and intestinal inflammation in mice with conditional expression of a constitutively active kit. Blood 117, 2012–21 (2011).
Orfao, A. et al. Flow Cytometric Analysis of Mast Cells from Normal and Pathological Human Bone Marrow Samples. Am. J. Pathol. 149, 1493–1499 (1996).
Schwaab, J. et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 122, 2460–2466 (2013).
Jawhar, M. et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia 30, 136–43 (2016).
Jawhar, M. et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia 29, 1115–1122 (2015).
Riera, L. et al. Core binding factor acute myeloid leukaemia and c-KIT mutations. Oncol. Rep., doi: 10.3892/or.2013.2328 (2013).
Wang, Y. et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia : Implication in stepwise leukemogenesis and response to Gleevec. Proc. Natl. Acad. Sci. USA 102, 1104–1109 (2005).
Bosbach, B. et al. Imatinib resistance and microcytic erythrocytosis in a KitV558Δ;T669I/+gatekeeper-mutant mouse model of gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA 109, E2276–83 (2012).
Hasselbalch, H. C. et al. High expression of carcinoembryonic antigen-related cell adhesion molecule (CEACAM) 6 and 8 in primary myelofibrosis. Leuk. Res. 35, 1330–1334 (2011).
Hasselbalch, H. C. et al. Transcriptional Profiling of Whole Blood Identifies a Unique 5-Gene Signature for Myelofibrosis and Imminent Myelofibrosis Transformation. PLoS One 9, e85567 (2014).
Pérez, C. et al. Aberrant DNA methylation profile of chronic and transformed classic Philadelphia-negative myeloproliferative neoplasms. Haematologica 98, 1414–1420 (2013).
Gordon, H. & Sweets, H. H. A Simple Method for the Silver Impregnation of Reticulum. Am. J. Pathol. 12, 545–552.1 (1936).
Nyabi, O. et al. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 37, e55 (2009).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–1 (1999).
Acknowledgements
NP is supported by the Deutsche José Carreras Leukämie-Stiftung. Experiments were supported by a collaborative grant from the Ministry of Innovation, Science and Research of the Federal State of North Rhine-Westphalia and the Interdisciplinary Clinical Research Center IZKF Aachen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pelusi, N., Kosanke, M., Riedt, T. et al. The spleen microenvironment influences disease transformation in a mouse model of KITD816V-dependent myeloproliferative neoplasm. Sci Rep 7, 41427 (2017). https://doi.org/10.1038/srep41427
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41427
This article is cited by
-
Niche-induced extramedullary hematopoiesis in the spleen is regulated by the transcription factor Tlx1
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.