Abstract
This study was to explore the association between thyroid dysfunction and albuminuria. 581 cases with chronic kidney disease (CKD) were included in this study. The clinical characteristics consisted of sex, age, serum creatinine, urinary albumin-to-creatinine ratio (ACR), thyroid function were recorded. Estimated glomerular filtration rate (eGFR) was calculated by CKD-EPI four-level race equation. Prevalence of different thyroid diseases was calculated by chi-square test. Levels of thyroid hormone were compared among different albuminuria groups by Kruskal-Wallis test. Spearman’s correlation was used to assess the association between albuminuria and thyroid hormone. Our study showed that total T4 and free T4 were significantly different among ACR < 30 mg/g, 30–300 mg/g and >300 mg/g (P < 0.001 and =0.007, respectively). Positive correlation between T4 (total T4 and free T4) and albuminuria was evaluated by correlation analysis (P = 0.001 and <0.001, respectively). Albuminuria was an independent influence factor of T4 after adjustment for age, sex, serum creatinine, albumin, hs-CRP, smoking status, systolic blood pressure, diabetes mellitus, medication use for diabetes mellitus, eGFR, LDL-cholesterol, triglycerides, hypertension, and medication use for hypercholesterinemia. In conclusion, T4 was positively correlated with albuminuria, and it was completely not consistent with our anticipation. Further study is needed to elucidate the causation association between albuminuria and T4.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global public health problem, affecting 10–16% of the adult population worldwide1. CKD is characterised by low estimated glomerular filtration rate (eGFR) and high albuminuria1, persistent for more than three months1, and is associated with adverse outcomes, irrespective of hypertension2, diabetes3, age4, and sex5.
Persistent proteinuria and/or albuminuria for more than three months is a hallmark of CKD6. It is well established that the kidney is capable of participating in all aspects of peripheral thyroid hormone (TH) metabolism. Intact THs are filtered, reabsorbed and secreted by the kidney. A major route of iodide elimination is by urinary excretion7. Nephrotic syndrome is associated with changes in serum TH levels8. Urinary losses of binding proteins, such as thyroxine binding globulin (TBG), transthyretin or pre-albumin, albumin, and TH binded to them, result in a reduction in serum total thyroxine (TT4) and, sometimes, in total triiodothyronine (TT3) levels9. These hormonal changes are related both to the degree of proteinuria and to serum albumin levels10. Patients often remain euthyroid, because free T4 (FT4) and free T3 (FT3) levels are usually normal10. Thyroid is able to compensate for hormonal urinary losses keeping the patient euthyroid. However, in patients with low thyroid reserve, clinical hypothyroidism (or overt hypothyroidism) can develop11. Similarly, nephrotic syndrome may increase the needs of exogenous levothyroxine in patients with hypothyroidism.
It is well known that serum TT4 and sometimes FT4 were both decreased in nephrotic syndrome7,8,9,10,11,12. Serum TT4 and FT4 were negatively correlated with massive proteinuria. However, the association of albuminuria and thyroid dysfunction is unknown. Our research was to explore the associations between thyroid dysfunction and albuminuria. It is hypothesized that serum TT4 and FT4 would be also negatively correlated with albuminuria.
Methods
Study population
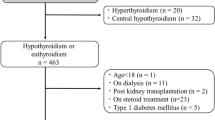
We performed a cross-sectional analysis on the database of the Laboratory Information System of the Clinical Chemistry Laboratory at Nanjing First Hospital of Nanjing Medical University to retrieve results of urinary albumin to creatinine ratio, serum creatinine, and thyroid function tests, which have been performed on 1689 inpatient adults (≥18 yr of age) consecutively referred by nephrology practitioners for routine blood testing over the last 7 yr (from August 2009 to August 2016). Different albuminuria groups were set by the level of albumin to creatinine ratio in urine (ACR) and eGFR according to the KDIGO guideline1. We excluded subjects who were younger than 18 years of age, women who were pregnant (given potential pregnancy-related changes in thyroid function), subjects with urinary traction infection (given potential trasient albuminuria, N = 15), subjects with previous thyroid disease (N = 3), subjects who have a history of thyroid disease or who were receiving concurrent treatment with drugs that could effect on thyroid function (lithium, amiodarone, iodine, methimazole or propylthiouracil, N = 1). We also excluded subjects in which thyrotropin (or thyroid-stimulating hormone, TSH), total T3, free T3, total T4 and free T4 levels were not available (N = 1089). Thus, this study analyzes the remaining 581 patients. Only 15 patients tested anti-thyroid antibody, 16 patients tested reverse T3, so we did not analyze them in the study. Proteinuria was performed in only 72 patients, and the association of proteinuria and thyroid dysfunction was well studied7,8,9,10,11,12, thus, we did not analyze proteinuria in the study.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at Nanjing First Hospital affiliated to Nanjing Medical University (IRB no. 86-025-52271039-21). Informed consents were obtained from all participants when they were admitted to the hospital.
Laboratory measures
Thyroid function and urinary albumin were tested by using electrochemiluminescence assay (Siemens ADVIA Centaur XP, NY, USA). The reference ranges of serum TT4, FT4, TT3, FT3 and TSH in our institute are as follows: for TSH is 0.55–4.78 mIU/L, for TT4 58.1–140.6 mmol/L, for FT4 11.5–22.7 pmol/L, for TT3 0.92–2.79 mmol/L, for FT3 3.5–6.5 pmol/L, respectively. The reference range of thyroid function was set according to the instructions from ADVIA Centaur immunoassay system13. Clinical hyperthyroidism is defined as lower (less than normal value) TSH with higher (more than normal value) FT4, TT4 and/or FT3, TT3; clinical hypothyroidism is defined as higher TSH with lower FT4, TT4; subclinical hyperthyroidism is defined as lower TSH with normal FT4, TT4, FT3 and TT3; subclinical hypothyroidism is defined as higher TSH with normal FT4, TT4, FT3, and TT3; euthyroid sick syndrome is defined as normal TSH with lower FT3, TT3 and/or lower FT4, TT414. Enzymatic method was performed to evaluate serum creatinine (Scr) and urinary creatinine by using OLYMPUS AU5400 automatic biochemical analyzer (Olympus Corporation, Mishima, Japan). The calibrators for the enzymatic method were traceable to an isotope dilution mass spectrometric method for serum creatinine using standard reference methods NIST SRM 96715. All covariates including serum albumin, LDL cholesterol, triglycerides, hs-CRP, smoking status, systolic blood pressure, diabetes mellitus, medication use for diabetes mellitus, hypertension, and medication use for hypercholesterinemia were measured and recorded. Serum albumin, LDL cholesterol and triglycerides were measured by biochemical method, using OLYMPUS AU5400 automatic biochemical analyzer (Olympus Corporation, Mishima, Japan). CRP was measured by rate nephelometry.
Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) four-level race equation16,17. The specific CKD-EPI four-level race GFR estimation equation was shown in Table 1.
Albuminuria was evaluated by ACR which was calculated as the ratio of urinary albumin to urinary creatinine. Patients were assigned into three groups according to the KDIGO guideline 2012: 1normal to mildly increased albuminuria (ACR < 30 mg/g); moderately increased albuminuria (ACR 30–300 mg/g, previously called microalbuminuria); severely increased albuminuria (ACR > 300 mg/g, previously called macroalbuminuria).
Statistical analyses
PASW Statistics 18.0 statistical software (SPSS Inc., Chicago, IL, USA) and Microsoft Office Excel 2003 were used for data analysis. The expression of eGFR equation in PASW: eGFR = EXP(LN(151) − 0.328 * LN(Scr/88.4/0.7) + age * LN(0.993)) (If female and creatinine <0.7); =EXP(LN(151) − 1.210 * LN(Scr88.4/0.7) + age * LN(0.993)) (if female and creatinine >=0.7); =EXP(LN(149) − 0.412 * LN(Scr/88.4/0.9) + age * LN(0.993)) (If male and creatinine <0.9); =EXP(LN(149) − 1.210 * LN(Scr/88.4/0.9) + age * LN(0.993)) (If male and creatinine >=0.9).
Data were collected by mean ± SD. Prevalence of different thyroid diseases among different ACR groups and CKD categories were analyzed by chi-square test. Levels of thyroid hormone were compared among different ACR groups by Kruskal Wallis test. We calculated the prevalence of different thyroid diseases among three ACR groups. Association of albuminuria and thyroid hormone was explored via correlation analysis. P values < 0.05 were considered to be statistically significant.
Results
Characteristics of subjects in different ACR groups
A total of 581 subjects with CKD were enrolled. The causes of CKD cases were chronic glomerulonephritis, asymptomatic hematuria, diabetic nephropathy, polycystic kidney disease, chronic renal failure (unknown or indeterminate aetiology), hypertensive nephropathy, and obstructive nephropathy. There was a significant difference among the three groups in FT4, TT4 (Fig. 1), Scr and eGFR. eGFR in ACR > 300 mg/g group was significantly lower than other groups (P < 0.001) (Fig. 2). There was a positive correlation between FT4, TT4 with ACR. FT4 or TT4 was increased according to the incremental ACR with those of ACR > 300 mg/g being the highest. No significant difference was found among groups in FT3 (P = 0.790), TT3 (P = 0.296), TSH (P = 0.746), sex (P = 0.374) and age (P = 0.735) by Kruskal Wallis test (Table 2).
The prevalence of different thyroidism in each ACR group
Euthyroid sick syndrome and hypothyroidism (subclinical and clinical) were more common in CKD patients. There was a significant difference in the prevalence of different thyroidism among three ACR groups (P = 0.029). Euthyroid sick syndrome and hypothyroidism were both more prevalent than hyperthyroidism in each ACR group. There was an increasing trend for the prevalence of euthyroid sick syndrome with the decrease of the ACR (Table 3).
No significant association of ACR with different clinical categories of thyroid dysfunction was found by Spearman analysis (P = 0.864).
The prevalence of different thyroidism in each CKD stage
There was a significant difference in the prevalence of different thyroidism among 5 groups of CKD stage (P = 0.004). The prevalence of euthyroid sick syndrome in CKD stage 4 and stage 5 group was higher than other groups. There was an increasing trend for the prevalence of euthyroid sick syndrome with the decrease of the eGFR, showed a U-curve (Table 4).
Thyroid hormone correlation with albuminuria
The scatterplot of TT4 associated with ACR (95% CI) was shown in Fig. 3a. Spearman correlation coefficient (r) was 0.165, P = 0.001, indicating that TT4 was significantly positively correlated with ACR.
The scatterplot of FT4 associated with ACR (95% CI) was shown in Fig. 3b. Spearman correlation coefficient (r) was 0.162, P < 0.001, indicating that FT4 was significantly positively correlated with ACR.
TT3, FT3 and TSH were not associated with ACR (P = 0.704, 0.515 and 0.951, respectively).
Albuminuria was an independent variable of FT4 after reduced adjustment for age, sex, serum albumin, hs-CRP, smoking status, systolic blood pressure, diabetes mellitus, medication use for diabetes mellitus (P < 0.001) and full adjustment for all of the above mentioned plus eGFR, LDL cholesterol, triglycerides, hypertension, medication use for hypercholesterinemia (P = 0.006).
Albuminuria was an independent variable of TT4 after reduced adjustment for age, sex, serum albumin, hs-CRP, smoking status, systolic blood pressure, diabetes mellitus, medication use for diabetes mellitus (P = 0.013) and full adjustment for all of the above mentioned plus eGFR, LDL cholesterol, triglycerides, hypertension, medication use for hypercholesterinemia (P = 0.039).
We did not show significant association of the presence of thyroid disease with albuminuria (P = 0.989) by bivariate Logistic regression analysis.
Discussion
In our study, we demonstrated that TT4 and FT4 were significantly different in three ACR groups that classified as <30 mg/g, 30–300 mg/g and >300 mg/g (both P < 0.001). Positive correlation between TT4, FT4 and albuminuria was evaluated by correlation analysis (Spearman correlation coefficient were 0.162 and 0.165, respectively, with P = 0.001 and <0.001). Albuminuria was an independent variable of T4 (TT4 and FT4) after reduced adjustment and full adjustment. In contrast, TT3, FT3 and TSH were not associated with albuminuria. Moreover, we showed that there was a significant difference in the prevalence of different thyroidism among three ACR groups (P = 0.029). Euthyroid sick syndrome and hypothyroidism were more prevalent than hyperthyroidism in CKD. The prevalence of euthyroid sick syndrome was 38.3% in normal albuminuria patient (defined as ACR < 30 mg/g).
Thyroid hormone affects nearly every organ system in the body. T4 is produced only by the thyroid gland, whereas T3, the more biologically active form of thyroid hormone, is produced primarily through local deiodination of T4 by the enzyme T4–5′-deiodinase in other tissues, including the kidney. The kidney contains the D1 isoform of this enzyme, which becomes less active in CKD18. The kidney plays a role in clearance of iodine, TSH, and thyrotropin-releasing hormone. However, some patients with CKD are euthyroid, with normal TSH and free T4 levels. Patients with CKD may have changes in thyroid function tests consistent with the euthyroid sick syndrome; that is, low T4, T3, and TSH concentrations. End stage renal disease (ESRD) patients have decreased levels of free T3. These changes in CKD patients are due to alterations in the peripheral 5′-monodeiodination of T4, reduced levels of plasma proteins that bind T4, the presence of inhibitors of T4 binding to plasma proteins, metabolic acidosis, and effects of medications18. Patients with nephrotic syndromes have urinary losses of proteins that bind thyroid hormones, including thyroxine binding globulin, transthyretin, and albumin. Urinary T4 excretion was measured in patients with proteinuria. One study showed that, it was detectable in the urine in five cases, who had significantly lower serum free T4 and free T3 concentrations than the five patients without detectable urinary T411. This can result in reductions in total plasma T4 and less commonly total T3 levels that are roughly proportional to the severity of hypoalbuminemia and degree of proteinuria. Many such patients remain euthyroid, however, as the result of increased secretion of TSH and thyroid hormone synthesis, albeit clinical hypothyroidism can occur. Heparin and furosemide can inhibit T4 binding to plasma proteins and may transiently elevate free T4 levels18. No previous study focused on the association between thyroid dysfunction and albuminuria. Our study showed that TT4 and FT4 were both higher in macroalbuminuria, which were completely not consistent with our anticipation. There might be several possible reasons for the higher TT4 and FT4 in macroalbuminuria. Firstly, the higher T4 is probably related to glomerular hyperfiltration and hypertension, changes in tubular protein handling, or changes in the structure of glomerular barrier, all of which may increase albuminuria19. The higher T4 (or hyperthyroidism) may result in glomerular hyperfiltration20. Secondly, thyroid gland is able to compensate for hormonal urinary losses keeping the patient euthyroid in macroalbuminuria10. Although TT4 and FT4 were both higher in macroalbuminuria group than microalbuminuria group and nomal albuminuria group, serum TT4 and FT4 did not exceed reference ranges in most patients in our study.
Hypothyroid humans and rats can have an increased transcapillar leaking of the plasma proteins such as albumin, which leads to mild proteinuria and albuminuria19. The albuminuria is considered to be present before the decrease in GFR in hypothyroid patients19, which proved our study by showing that 17.9% of microalbuminuria patients and 22.4% of macroalbuminuria patients had hypothyroidism. Patients with proteinuria have higher TSH levels, consistent with urinary loss of thyroid hormones12. A possible association between subclinical hypothyroidism and albuminuria were examined in 159 people with type 2 diabetes21. Patients with subclinical hypothyroidism had significantly higher levels of ACR than those with euthyroidism. Multivariate logistic regression analyses demonstrated that serum TSH level was an independent risk factor of albuminuria in this study21. However, our study showed that albuminuria was not associated with TSH, attributed to most patients without type 2 diabetes in our study.
Subclinical hypothyroidism and euthyroid sick syndrome are common in chronic kidney disease22,23,24. A study showed that low T3 syndrome was highly prevalent in CKD and was a remarkable finding in early CKD25. An about 60% prevalence of euthyroid sick syndrome (nonthyroidal illness syndrome) was revealed in aged hospitalized patients13. These findings were similar to our results. Our study showed that more than half patients had lower TT4 and/or FT4 across a wide range of ACR (Fig. 1), which resulted in highly prevalence of euthyroid sick syndrome, especially at normal ACR.
Low T3 is the most frequent alteration of the thyroid hormone profile observed in CKD26. This alteration has long been considered an innocent metabolic adaptation to chronic illness. However, low T3 associates with endothelial dysfunction, a harbinger of atherosclerosis, in stage 3 and 4 CKD patients, as well as with cardiovascular disease27, mortality and with a high risk of death in stage 5D CKD patients28.
The strength of this study is the power. To detect a small-moderate correlation (for example r = 0.13), a sample of 500 analyzable subjects will provide 83% power to discover that the correlation is significantly different from there being no correlation (i.e. that the correlation would be zero) at the 0.05 level. The correlation coefficient is more than 0.13 and the sample size of analyzable subjects is more than 500 in this study, and therefore, the power of this study is very strong.
There are some limitations to this study. Firstly, we did not test urinary total protein in our research. Therefore, the association of proteinuria and thyroid dysfunction was not performed. However, the association of proteinuria and thyroid dysfunction was well studied7,8,9,10,11,12. Secondly, the data is based on Chinese patients with CKD, thus, it is not clear whether it is applicable to other racial population. Thirdly, as it is a cross-sectional study, the cause and effect cannot be determined from this data. A selection or systematic bias or other confounding factors may also occur. Fourthly, low case numbers for the different clinical categories of thyroid dysfunction in CKD patients is another limitation, for example, only 14 CKD patients with hyperthyroidism were included in this study.
In conclusion, our results showed that serum TT4 and FT4 were positively correlated with albuminuria, and it was completely not consistent with our anticipation. Albuminuria was not associated with TT3, FT3 and TSH. Further study is needed to elucidate the causal association between albuminuria and T4.
Additional Information
How to cite this article: Du, X. et al. Albuminuria is an independent risk factor of T4 elevation in chronic kidney disease. Sci. Rep. 7, 41302; doi: 10.1038/srep41302 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Eknoyan, G. et al. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl 3, 1–150 (2013).
Mahmoodi, B. K. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 380, 1649–1661 (2012).
Fox, C. S. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 380, 1662–1673 (2012).
Hallan, S. I. et al. Age and association of kidney measures with mortality and endstage renal disease. JAMA. 308, 2349–2360 (2012).
Nitsch, D. et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 346, f324 (2013).
Du, X. & Fan, L. Prevalence of chronic kidney disease in China. Lancet. 380, 213 (2012).
Kaptein, E. M., Feinstein, E. I. & Massry, S. G. Thyroid hormone metabolism in renal diseases. Contrib Nephrol. 33, 122–135 (1982).
Afrasiabi, M. A. et al. Thyroid function studies in the nephrotic syndrome. Ann Intern Med. 90, 335–338 (1979).
Iglesias, P. & Díez, J. J. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 160, 503–515 (2009).
Feinstein, E. I. et al. Thyroid function in patients with nephrotic syndrome and normal renal function. Am J Nephrol. 2, 70–76 (1982).
Fonseca, V. et al. Can urinary thyroid hormone loss cause hypothyroidism? Lancet. 338, 475–476 (1991).
Gilles, R. et al. Thyroid function in patients with proteinuria. Neth J Med. 66, 483–485 (2008).
Reix, N. et al. Thyroid-stimulating hormone and free thyroxine on the ADVIA Centaur immunoassay system: A multicenter assessment of analytical performance. Clin Biochem. 46, 1305–1308 (2013).
Pappa, T. A., Vagenakis, A. G. & Alevizaki, M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 41, 212–220 (2011).
Dodder, N. G. et al. Certification of creatinine in a human serum reference material by GC-MS and LC-MS. Clin Chem. 53, 1694–1699 (2007).
Du, X. et al. Is the Chronic Kidney Disease Epidemiology Collaboration four-level race equation better than the cystatin C equation? Nephrology (Carlton). 17, 407–414 (2012).
Stevens, L. A. et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 79, 555–562 (2011).
Mariani, L. H. & Berns, J. S. The renal manifestations of thyroid disease. J Am Soc Nephrol. 23, 22–26 (2012).
van Hoek, I. & Daminet, S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol. 160, 205–215 (2009).
Ford, H. C. et al. Renal function and electrolyte levels in hyperthyroidism: urinary protein excretion and the plasma concentrations of urea, creatinine, uric acid, hydrogen ion and electrolytes. Clin Endocrinol. 30, 293–391 (1989).
Yasuda, T. et al. Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract. 94, e75–77 (2011).
Lo, J. C. et al. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 67, 1047–1052 (2005).
Carrero, J. J. et al. Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med. 262, 690–701 (2007).
Chonchol, M. et al. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 3, 1296–300 (2008).
Song, S. H. et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant. 24, 1534–1538 (2009).
Zoccali, C. & Mallamaci, F. Thyroid function and clinical outcomes in kidney failure. Clin J Am Soc Nephrol. 7, 12–14 (2012).
Klein, I. & Ojamaa, K. Thyroid hormone and the cardiovascular system. N Engl J Med. 344, 501–509 (2001).
Zoccali, C. et al. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 70, 523–528 (2006).
Acknowledgements
We thank Juncheng Dai (Department of Epidemiology, School of Public Health, Nanjing Medical University) for statistics help. This work was supported by grants from Chinese Society of Nephrology (14050430580), the Foundation of Science and Technology Development Program, Nanjing Medical University (2011NJMU195, 2012NJMU233), Jiangsu Provincial Special Program of Medical Science (BL2014015), the Key Medical Talent Training Program of the Jiangsu Province Health Bureau (RC2011018). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The research was designed by X.D., B.P. and C.C. All authors helped to write the report and commented on the manuscript. X.D. and B.P. analysed the data and advised on statistical issues at the time of the research write up. C.C. was the research administrator; obtained the data; and prepared communications with participating centres, and the data monitoring committee. X.D. and C.C. were funders. X.D., X.H., W.L., Y.Z., X.W. and W.H. were research nurses responsible for recruitment and return of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Du, X., Pan, B., Li, W. et al. Albuminuria is an independent risk factor of T4 elevation in chronic kidney disease. Sci Rep 7, 41302 (2017). https://doi.org/10.1038/srep41302
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41302
This article is cited by
-
Impaired sensitivity to thyroid hormones is associated with albuminuria in the euthyroid population: results from NHANES
Hormones (2024)
-
Associations between the thyroid panel and serum protein concentrations across pregnancy
Scientific Reports (2021)
-
Association between albuminuria and thyroid function in patients with chronic kidney disease
Endocrine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.