Abstract
GDP-mannose pyrophosphorylase (GMP) catalyzed the formation of GDP-mannose, which serves as a donor for the biosynthesis of mannose-containing polysaccharides. In this study, three GMP genes from Dendrobium officinale (i.e., DoGMPs) were cloned and analyzed. The putative 1000 bp upstream regulatory region of these DoGMPs was isolated and cis-elements were identified, which indicates their possible role in responses to abiotic stresses. The DoGMP1 protein was shown to be localized in the cytoplasm. To further study the function of the DoGMP1 gene, 35S:DoGMP1 transgenic A. thaliana plants with an enhanced expression level of DoGMP1 were generated. Transgenic plants were indistinguishable from wild-type (WT) plants in tissue culture or in soil. However, the mannose content of the extracted water-soluble polysaccharides increased 67%, 96% and 92% in transgenic lines #1, #2 and #3, respectively more than WT levels. Germination percentage of seeds from transgenic lines was higher than WT seeds and the growth of seedlings from transgenic lines was better than WT seedlings under salinity stress (150 mM NaCl). Our results provide genetic evidence for the involvement of GMP genes in the biosynthesis of mannose-containing polysaccharides and the mediation of GMP genes in the response to salt stress during seed germination and seedling growth.
Similar content being viewed by others
Introduction
GDP-mannose pyrophosphorylase (GMP, E.C. 2.7.7.13), also known as mannose-1-phosphate guanyltransferase, catalyzes the conversion of mannose-1-phosphate to GDP-mannose. A number of nucleotide sugars such as GDP-L-galactose, GDP-L-fucose and GDP-D-rhamnose are synthesized using GDP-mannose as the precursor1,2. Moreover, GDP-mannose is an important intermediate product related to a wide range of metabolic pathways in plants, such as N-glycosylation3,4 and the synthesis of ascorbic acid (AsA) and polysaccharides1. Insertion of the GMP gene into Saccharomyces cerevisiae restored the viability of alg1 N-glycosylation mutants5. GDP-mannose deficiency, which is caused by GMP deficiency, is responsible for N-glycosylation deficiency, and results in inhibited root growth in the presence of NH4+ 6,7.
In plants, the GMP gene is involved in AsA synthesis and has been shown to improve the tolerance of plants under abiotic stress. For example, in an ozone-sensitive Arabidopsis thaliana mutant (sozi/vct1/cyt1) that contained only 30% of the wild-type (WT) AsA concentration, the VCT1 gene, which encodes a GMP1, was shown to be responsible for AsA deficiency8,9. GMP from rice (Oryza sativa L.) improved salinity stress tolerance in tobacco (Nicotiana tabacum L.)10. A tobacco GMP is involved in tolerance to temperature stress11,12.
Previous studies demonstrated that mannan synthase isolated from plant species such as pea (Pisum sativum L.), fenugreek (Trigonella foenum-graecum L.) and guar (Cyamopsis tetragonoloba L.), used GDP-mannose as a substrate to synthesize a mannan backbone in vitro13,14. The cellulose synthase-like A (CSLA) family, which belongs to the cellulose synthase (CesA) superfamily of glycosyltransferase family 2 (GT2), encodes proteins responsible for mannan polysaccharides by using GDP-mannose as the substrate15,16,17. Transformants of potato (Solanum tuberosum L.) with reduced GMP activity had 30–50% lower mannose content than WT plants4.
Although the vast majority of the characterized GMPs from A. thaliana, rice or other higher plant species have been analyzed, it has been proposed that the involvement of GMPs in AsA synthesis and stress tolerance is conserved. However, it is not possible to predict gene functions of possible GMPs based only on nucleotide or amino acid sequence similarities. For example, a probable GDP-mannose pyrophosphorylase (TAIR number: AT1G74910), predicted by amino acid sequence similarly, lacks GDP-mannose pyrophosphorylase activity18. In this study, we report on the cloning and characterization of three GMPs from D. officinale. We generated 35S:DoGMP1 A. thaliana transgenic lines, studied the relationship between DoGMP1 and mannose content of water-soluble polysaccharides, and assessed the tolerance of these lines to salinity stress. This work can provide insight into understanding the molecular mechanisms of polysaccharide biosynthesis in D. officinale. Furthermore, this work also has implications for the development of abiotic stress-tolerant crops to overcome environmental stress limitations and improve production efficiency in the face of a burgeoning world population.
Materials and Methods
Plant materials and growth conditions
Potted D. officinale plants used to clone genes were grown and maintained in a greenhouse (Guangzhou, Guangdong, China) under natural conditions. The stems of D. officinale (about one year old) were harvested, frozen rapidly in liquid nitrogen and kept at −80 °C until RNA extraction. A. thaliana (ecotype Columbia) was used as the WT in this study. WT and DoGMP1 overexpression lines were cultured in a growth chamber in a 16-h photoperiod (100 μmol m−2s−1) at 22 °C. Plants were grown in pots (8 × 10 cm, diameter × height) filled with soil (topsoil and vermiculite; 1:2) and watered periodically with Hyponex fertilizer (N:P:K = 6-10-5, diluted 1,000-fold; Hydroponic Chemicals Co., Ohio, USA).
Cloning GDP-pyrophosphorylase genes from D. officinale
According to the annotation of unigenes of an in-house transcriptome reference database of sequences19, GDP-mannose pyrophosphorylase unigenes were identified and used to design primers. The total RNA of D. officinale was isolated by using Column Plant RNAout2.0 (Tiandz, Inc., Beijing, China) according to the manufacturer’s protocol. Two μg of total RNA were reverse transcribed for the first-strand cDNA, which served as the template to generate 5′ and 3′ cDNA ends by using M-MLV reverse transcriptase (Promega, Madison, Wisconsin, USA). The SMARTerTM RACE cDNA Amplification Kit (Clontech Laboratories Inc., Mountain View, USA) was used to generate both 5′ and 3′ cDNA ends according to the manufacturer’s protocol. PCR products were purified by a Gel Extraction Kit (Dongsheng Biotech, Guangzhou, China), cloned into the pMD18-T vector (Takara Bio Inc., Dalian, China) and sequenced at the Beijing Genomics Institute (Shenzhen, Guangdong, China). Primer pairs for each gene designed to amplify 3′ and 5′ cDNA regions are listed in Supplementary Table 1.
Isolation and analysis the putative promoters of DoGMPs
To understand the regulatory mechanism of DoGMPs, the Genome Walking Kit (Takara Bio Inc.) was used to clone the putative promoters of DoGMPs according to the user’s manual. Primers specific for each gene were designed by Primer Premier 5.0 (PREMIER Biosoft Palo Alto CA USA) and listed in Supplementary Table 2. The putative promoters were used to analyze the cis-regulatory elements by an on-line prediction soft (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
DoGMP1-YFP plasmid construction and localization analysis
The full-length coding sequences of the DoGMP1 gene (excluding the termination codon) were amplified with a pair of primers (DoGMP1YFPF/DoGMP1YFPR, listed in Supplementary Table 3) introduced as adaptor sequences at the 5′ and 3′ ends according to the pSAT6-EYFP-N1 vector20 sequences. The principles of adaptor sequence design followed In-Fusion® HD Cloning Kit (Clontech Laboratories, Inc.) instructions. The amplified product was inserted downstream of the 35S Cauliflower mosaic virus (CaMV) promoter in the unique NcoI site of the pSAT6-EYFP-N1 vector20 by using the In-Fusion® HD Cloning Kit according to the manufacturer’s instructions. The DoGMP1-YFP recombinant plasmid was verified by DNA sequencing at the Beijing Genomics Institute. Transient transformation was performed with 10 μg of plasmid DNA transferred into mesophyll protoplasts from a 4–5 weeks-old A. thaliana plant by a polyethylene glycol (PEG)-mediated transfection system described by Yoo et al.21. Protoplasts were incubated for 20 h under standard light/dark conditions then yellow fluorescent protein (YFP) was localized via fluorescence microscopy. YFP fluorescence was visualized by a Zeiss LSM 510 confocal microscope (Zeiss, Jena, Germany).
Construction of DoGMP1 overexpression vector and Arabidopsis thaliana transformation
The full-length coding sequences of the DoGMP1 gene (excluding the termination codon) were amplified and cloned into the pCAMBIA1302 vector at the NcoI site, driven by the 35S CaMV promoter. After verification by full sequencing at the Beijing Genomics Institute, the recombinant vector was transformed into Agrobacterium tumefaciens EHA105 by using the freeze-thaw method22 then used for A. thaliana transformation. Transgenic plants were generated by a floral dip transformation method23. The primer pairs designed for construction of 35S:DoGMP1 vector are listed in Supplementary Table 3.
Western blot assay
Seven-day-old A. thaliana seedlings (0.5 g), grown on half-strength Murashige and Skoog medium24 containing 2% sucrose and 0.8% agar (pH 5.7) (basal medium, BM), were harvested and immediately ground in liquid nitrogen with a mortar and pestle. Cells were lysed in 700 μL extraction buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 20% glycerol, 1% Nonidet P-40) containing a protease inhibitor cocktail (Cat. No. 04693132001, Roche, Basel, Switzerland), then centrifuged at 4 °C and 14,000 g for 20 min. The supernatants containing total proteins were fractionated by SDS-PAGE and analyzed on Western blots. Immunoprecipitated recombinant DoGMP1-GFP fusion proteins were visualized on Western blots with an anti-GFP antibody (product code ab290, Abcam, Cambridge, U.K.) and goat anti-rabbit IgG-HRP (catalog number sc-2301, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Measurement of and mannose content of water-soluble polysaccharides
The above-ground parts (leaves, flowers and stems) from two-month-old A. thaliana plants in the reproductive stage were used to determine water-soluble polysaccharide content. Samples were powdered by a DFT-50 pulverizer (Xinno Instrument Equipment Inc., Shanghai, China) after drying in an oven at 85 °C for 6 h. To analyze the mannose content of water-soluble polysaccharides, 0.3 g of powder was pre-extracted with 80% ethanol for 2 h then further extracted with 100 mL of distilled water for 2.5 h at 100 °C. The polysaccharides in the solution were precipitated in 4 volumes of 100% ethanol at 4 °C overnight then centrifuged at 10,000 rpm for 20 min. The residue was re-dissolved in 20 mL of distilled water. The mannose content of the water-soluble polysaccharides was also determined by high performance liquid chromatography (HPLC), as described by He et al.19.
Germination assays under salinity stress
Seeds of all genotypes used in germination assays were grown simultaneously, and harvested and stored under similar conditions. Three transgenic lines of T5 homozygote plants and WT plants were surface sterilized by immersion for 10 min in 1% sodium hypochlorite, and then rinsed six times with sterile distilled water. One hundred surface-sterilized seeds of each line were seeded on plates filled with BM supplemented with NaCl, or not. According to the results of a pre-experiment trial, an optimum concentration of 150 mM was used as the NaCl stress treatment. After 2 days of stratification at 4 °C in the dark, the plates were incubated in a 16-h photoperiod (100 μmol m−2s−1) at 22 °C. Germination, which was considered to have occurred if the radicle emerged from the seed coat, was scored daily for 1–7 days. On the seventh day, seedlings were photographed with a Leica S8 APO stereomicroscope (Leica Microsystems Ltd., Heerbrugg, Switzerland). Seeds sown in BM served as the control. Each experiment was performed in three biological replicates.
Salinity stress treatment for Arabidopsis thaliana seedlings
Sterilized seeds were germinated on BM at 22 °C under a 16-h photoperiod (100 μmol m−2s−1) after stratification for 2 days at 4 °C in the dark. Five-day-old seedlings were transferred to fresh BM supplemented with 150 mM NaCl and cultured at 22 °C under a 16-h photoperiod. Root length was measured and photographs were taken after 7 days. Twelve plants were used in each experiment, and all experiments were repeated three times.
Hydrogen peroxide staining
A. thaliana seedlings (12-d old) that were grown on BM were transferred to fresh BM supplemented with 150 mM NaCl, or not, and then cultured at 22 °C under a 16-h photoperiod. Seedlings transferred to BM served as the control. Seedlings were harvested after 48 h and stained with 3,3′-diaminobenzidine (DAB) (D5637, Sigma-Aldrich)25. Eight plants of each treatment were used in each analysis, and all experiments were repeated three times.
Semi-quantitative RT-PCR
One hundred sterilized seeds of transgenic lines #1 and #3, as well as WT were germinated on BM at 22 °C under a 16-h photoperiod (100 μmol m−2s−1). Total RNA was extracted from seven-day-old A. thaliana seedlings using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Two μg of each RNA sample were reverse transcribed for the first-strand cDNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions after treatment with RNase-free DNase (Takara Bio Inc.) to remove any residual genomic DNA. The DreamTaqTM Green PCR Master Mix Kit (Takara Bio Inc.) was used for amplification. The following thermocycling conditions were applied: initial denaturation at 94 °C for 1 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min; final extension at 72 °C for 10 min in a LabCycler Standard Plus PCR system (SENSOQUEST, Hannah, Germany). The amplified products were separated on a 1.5% agarose gel stained with ethidium bromide (EtBr) and photographed in a Bio Sens SC 710 system. The gene-specific primers for DoGMP1, which was used in semi-quantitative RT-PCR, and the A. thaliana ubiquitin 10 gene (AtUBQ10, TAIR accession number: AT4G05320.2), which was used as the control, are listed in Supplementary Table 4. AtUBQ10 was used as the internal control based on the advice of Zhao et al.26.
Quantitative real-time PCR (qRT-PCR) analysis
The cDNAs described above also used for qRT-PCR analysis. The gene-specific primer pairs were designed for qRT-PCR by online Primerquest software (listed in Supplementary Table 4). qRT-PCR was performed using the SYBR Premix Ex TaqTM Kit (Takara Bio Inc.) in an ABI 7500 Real-time system (ABI, CA, USA). Amplification conditions were 95 °C for 2 min, followed by 40 cycles of amplification (95 °C for 15 s, 60 °C for 1 min) and plate reading after each cycle. AtUBQ10 served as the control based on the recommendation of Zhao et al.26. The gene-specific primers used for qRT-PCR are listed in Supplementary Table 4.
Statistical analyses
All data were analyzed using SigmaPlot12.3 software (Systat Software Inc., San Jose, California, USA) using one-way analysis of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered to be statistically significant.
Results
Analysis of three cloned DoGMP genes from D. officinale
Based on an in-house transcriptome reference database of D. officinale sequences, three GMP genes were identified. Their full-length cDNAs, which were obtained by RACE, were named DoGMP1, DoGMP2 and DoGMP3. The complete DoGMP1 cDNA contains 1528 bp with an open reading frame (ORF) of 1086 bp encoding a protein of 361 amino acid residues with a calculated molecular mass of 39.373 kDa. The complete DoGMP2 cDNA contains 1492 bp with an ORF of 1248 bp encoding a protein of 415 amino acid residues with a calculated molecular mass of 45.875 kDa. DoGMP3 contains an ORF of 1086 bp encoding a protein of 361 amino acid residues with a calculated molecular mass of 39.604 kDa. The full-length cDNAs of DoGMP1-3 were submitted to GenBank with the following accession numbers: DoGMP1, KF195559; DoGMP2, KF195560; and DoGMP3, KP203853.
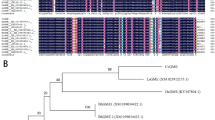
Sequence alignment analysis by ClustalX2 showed that all the GMP proteins from D. officinale, A. thaliana and O. sativa displayed diverse sequence/structure similarity relationships (Supplementary Fig. 1). The conservation of these primary sequences indicated that these proteins had a similar catalytic function. In a further search for conserved domains, GMP proteins were shown to contain four conserved motifs: the pyrophosphorylase signature sequences, a nucleotidyl transferase domain, a metal binding site (D, DxG or D, QxK) and the GMP active site (Supplementary Fig. 1). The conserved VEKP sequence included in the GMP active site is a mannose-1-phosphate binding site of GMP27. To examine the relationship between the three DoGMP proteins and other GMP members in plants, a phylogenetic analysis was performed among GMP protein sequences using MEGA 428. These were divided into two groups: GMPA and GMPB. DoGMP1 and DoGMP3 formed part of the GMPB group while DoGMP2 made up the GMPA group (Fig. 1).
The tree was constructed using MEGA 4 by the neighbor-joining method. GMP proteins used for alignment are as follows: AtGMP1, NP_177629; AtCYT1, NP_001189713; AtGMP3, NP_191118; VvGMP1, XP_002282422; VvGMP2, XP_002283703.1; VvGMP3, XP_002281959.1; SlGMP1, XP_004240924.1; SlGMP2, XP_004246437.1; SlGMP3, XP_004236149.1; OsGMP1, NP_001044795; OsGMP2, NP_001049332.1; OsGMP3, NP_001049673.1; BdGMP1, XP_003564604.1; BdGMP2, XP_003558261; BdGMP3, XP_003558532.1; ZmGMP1, NP_001131394.1; ZmGMP2, NP_001142215.1; ZmGMP3, NP_001142302.1; SiGMP1, XP_004977599.1; SiGMP2, XP_004985259; SiGMP3, XP_004984923.1; SiGMP4, XP_004970565.1.
DoGMP1 protein localized in the cytoplasm
GMP catalyzes the conversion of mannose-1-phosphate to GDP-mannose in the cytoplasm29. The OsGMP protein of O. sativa is also localized in the cytoplasm10. To verify whether DoGMP1 is indeed a cytoplasmic protein, the DoGMP1 protein was fused with YFP to construct a DoGMP1-YFP fusion protein. Transient expression in mesophyll protoplasts of A. thaliana seedlings was observed confirming that DoGMP1 protein is indeed a cytoplasmic protein (Fig. 2).
Analysis of stress-related cis-regulatory elements in the putative promoters of DoGMPs
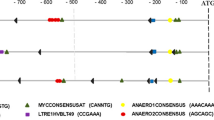
Studies have demonstrated that GMP family members are involved in tolerance to abiotic stresses10. To understand the possible stress-related cis-regulatory elements in the promoter regions of DoGMPs, about 1200, 1000 and 1800 bp of the translation start site of DoGMP1, DoGMP2 and DoGMP3 were obtained namely, respectively. 1 kb of the promoter sequences of each gene was used to predict the stress-related cis-regulatory elements (Supplementary Text 1–3). A low-temperature responsiveness element and defense and stress responsiveness elements were found both in DoGMP1 and in DoGMP2 (Fig. 3). The HSE element, which is involved in heat stress responsiveness, was found both in DoGMP1 and in DoGMP3 (Fig. 3). In addition, the three DoGMPs contained several plant hormone responsiveness elements such as an ethylene responsive element, a salicylic acid (SA) inducible element, a methyl jasmonate (MeJA) inducible element and an abscisic acid (ABA) responsive element (Fig. 3). Plant hormones such as ethylene, SA, MeJA and ABA play important roles during a plant’s response to abiotic stresses30,31,32,33. These results suggest the putative roles of these three DoGMPs in the response of D. officinale to abiotic stresses.
ABRE, a cis-acting element involved in the abscisic acid responsiveness; ARE, a cis-acting regulatory element essential for anaerobic induction; AuxRR-core, a cis-acting regulatory element involved in auxin responsiveness; ERE, an ethylene-responsive element; HSE, a cis-acting element involved in heat stress responsiveness; LTR, a cis-acting element involved in low-temperature responsiveness; Skn-1_motif, a cis-acting regulatory element required for endosperm expression; TC-rich repeats, a cis-acting element involved in defense and stress responsiveness; CCAAT-box, a MYBHv1 binding site; CGTCA-motif, a cis-acting regulatory element involved in MeJA responsiveness; O2-site, a cis-acting regulatory element involved in zein metabolism regulation; WUN-motif, a wound-responsive element; MBS, a MYB-binding site involved in drought induction.
Mannose content of water-soluble polysaccharides increased in 35S:DoGMP1 transgenic lines
The vtc-1 (known as cyt1) mutant of A. thaliana is hypersensitive to abiotic stresses such as ozone stress, sulfur dioxide, ultraviolet B irradiation and salt stress8,34. This indicates that AtCYT1 plays an important role in improving the abiotic stress tolerance of plants. Comparison of the GMP protein sequences in A. thaliana and D. officinale revealed that DoGMP1 was most similar to AtCYT1 and may thus play a similar role. Therefore, to analyze the functional role of the DoGMP1 gene, transgenic A. thaliana plants that constitutively overexpressed the DoGMP1 gene driven by the CaMV 35S promoter (35S:DoGMP1-GFP) were generated (Fig. 4A). Semi-quantitative RT-PCR and qRT-PCR were used to determine DoGMP1 gene expression in transgenic plants. The DoGMP1 gene could not be detected in WT plants but was detected in all three transgenic lines (Fig. 4B,C). Western blot analyses confirmed the expression of DoGMP1-GFP fusion proteins of correct size in all of the transgenic lines (Fig. 4D). No obvious phenotypic changes were observed among WT and transgenic plants when cultured in soil (Fig. 4E).
(A) Schematic presentation of the 35S:DoGMP1 overexpression vector. (B) Analysis of the DoGMP1 gene in WT and transgenic lines by semi-quantified PCR. Total RNA was isolated from 7-day-old WT and homozygous 35S:DoGMP1 transgenic A. thaliana transgenic lines under control conditions. (C) Analysis of the DoGMP1 gene in WT and transgenic lines by qPCR analysis. Total RNA was isolated from 7-day-old WT and s 35S:DoGMP1 transgenic lines under control conditions. Expression levels in transgenic lines were calculated relative to transgenic line #1, which exhibited the lowest transgene expression. (D) Analysis of the DoGMP1-GFP fusion protein expression in WT and transgenic lines by Western blotting. (E) Seedlings of WT and transgenic lines about one month old showed no obvious phenotypic changes. WT, wild-type plant; 35S:DoGMP1 transgenic lines: line #1, line #2 and line #3. Bar = 1 cm.
GDP-mannose, the product of the GMP enzyme, is the mannose donor for the synthesis of mannose-containing polysaccharides. To gain insight into the mannose content of these extracted polysaccharides, mannose content was determined by HPLC, which showed that mannose content in the transgenic lines increased significantly more than in the WT plant (Fig. 5). Over-expression of the DoGMP1 gene resulted in an obvious increase in the content of mannose in A. thaliana plants, suggesting that the DoGMP1 gene is involved in the biosynthesis of water-soluble polysaccharides, which are mannose-containing polysaccharides. Previous studies demonstrated that the backbone of mannan synthase, which is encoded by CSLA family members, is made by using GDP-mannose as the donor15,35. The CSLA family belongs to the CesA superfamily and includes nine CSLA members in A. thaliana: AtCSLA1, AtCSLA2, AtCSLA3, AtCSLA7, AtCSLA9, AtCSLA10, AtCSLA11, AtCSLA14 and AtCSLA1536. Since the mannose content increased significantly when the DoGMP1 gene was overexpressed in A. thaliana, the relative expression level of these nine AtCSLA genes were analyzed. AtCSLA1, AtCSLA7, AtCSLA10, AtCSLA11, AtCSLA14 and AtCSLA15 were significantly down-regulated in all 35S:DoGMP1 transgenic lines (Fig. 6), which indicates that the DoGMP1 gene has a negative role in the transcriptional regulation of AtCSLA genes.
Each data bar represents the mean ± standard deviations (SD) (n = 3). Asterisks indicate significant differences between 35S:DoGMP1 transgenic lines and WT. **, indicates P < 0.01 between WT and transgenic lines by ANOVA/Dunnett’s test. WT, wild-type; 35S:DoGMP1 transgenic lines: line #1, line #2 and line #3. DW, dry weight.
Transcripts were normalized to actin gene (AtUBQ10) expression. Asterisks indicate significant differences between 35S:DoGMP1 transgenic lines and WT. *, indicates P < 0.05, **, indicates P < 0.01 between WT and transgenic lines by ANOVA/Dunnett’s test. Data are means and SD from three biological replicates each with 100 seeds. WT, wild-type; 35S:DoGMP1 transgenic lines: line #1, line #2 and line #3.
Germination of 35S:DoGMP1 transgenic lines improved under salinity stress
Salinity, a critical factor influencing seed germination37, reduces germination rate and delays seed emergence38,39. Soluble sugars or polysaccharides may play important roles in the mechanisms of salt defense40,41. To investigate the effect of salinity stress on the germination of seeds from WT and transgenic lines, seeds were germinated on BM containing 150 mM NaCl (or not) to explore germination ability under salt stress. In the control group, seeds from all three transgenic lines germinated later than WT seeds one day after stratification, although seeds from WT and transgenic lines showed no obvious differences in the following days when germination was scored (Fig. 7A). In contrast, a significant difference in germination was observed between 35S:DoGMP1 lines and WT when MS medium was supplemented with 150 mM NaCl. The three 35S:DoGMP1 transgenic lines exhibited a higher germination percentage than WT seeds on all scoring days (Fig. 7B). In particular, two days after stratification on 150 mM NaCl there was a significant difference in the germination percentage of 35S:DoGMP1 lines #1, #2 and #3 (65.7%, 72.0%, and 69.0%, respectively) compared with 26.0% for WT seeds (Fig. 7B). The seedlings of both WT and the three transgenic lines showed a similar phenotype when germinated on half-strength MS medium without NaCl (Fig. 7C). However, roots of transgenic lines were longer than the roots of WT seedlings and seedling growth was more vigorous in transgenic lines than WT seedlings when seeds were germinated under salinity stress at 7 days after stratification (Fig. 7C). This implies that DoGMP1 plays a role as a positive regulator in seed germination of A. thaliana under salt stress.
Seeds of WT and transgenic lines were cultured on BM (A) without salt or (B) with 150 mM NaCl. (C) Seedlings on the seventh day after stratification from A and B. Asterisks indicate significant differences between 35S:DoGMP1 transgenic lines and WT. *, indicates P < 0.05, **, indicates P < 0.01 between WT and transgenic lines by ANOVA/Dunnett’s test. Each data bar represents the mean ± SD of 100 seeds. Bar = 0.5 mm. WT, wild-type; three 35S:DoGMP1 transgenic lines: line #1, line #2 and line #3.
35S:DoGMP1 transgenic lines grew better than WT plants under salinity stress
To analyze the growth of transgenic seedlings under salinity stress, five-day-old seedlings of 35S:DoGMP1 transgenic A. thaliana versus WT plants were grown on BM containing 150 mM NaCl (or not) to further characterize the response of the over-expressing transgenic plants to abiotic stress at the seedling stage. When the seedlings of WT and transgenic lines were grown under control conditions, no obvious differences were observed in root length (Fig. 8A,C). However, in the presence of 150 mM NaCl, the roots of WT plants were markedly shorter than those of transgenic lines (Fig. 8B,C). The root length of WT seedlings was about 0.75 cm, while that of transgenic lines #1, #2 and #3 was 1.39, 1.33, and 1.24 cm, respectively. The salinity stress test showed that transgenic seedlings could grow better than WT seedlings under salinity stress. These results suggest that DoGMP1 is able to provide A. thaliana seedlings with tolerance to salinity stress.
Seeds of WT and transgenic lines were cultured on half-strength MS medium (A) without salt or (B) with 150 mM NaCl. (C) Seedlings cultured on the 7th day after five-day-old seedlings were transplanted to half-strength MS containing 150 mM NaCl, or not. Each data bar represents the mean ± SD of 12 plants. Asterisks indicate significant differences between 35S:DoGMP1 transgenic lines and WT. *, indicates P < 0.05, **, indicates P < 0.01 between WT and transgenic lines by ANOVA/Dunnett’s test. Bar = 1 cm. WT, wild-type; three 35S:DoGMP1 transgenic lines: line #1, line #2 and line #3.
To investigate whether the better growth of transgenic lines relative to WT was associated with accelerated ROS accumulation, DAB staining was carried out in WT and transgenic lines after control and salt stress treatment. No coloration was observed on the leaves when the seedlings of WT and transgenic lines were cultured in BM while the roots were slightly stained (Fig. 9A). In contrast, the leaves and roots from both WT and transgenic lines under salt stress were strongly stained relative to the control (Fig. 9B). The WT seedlings contained a higher level of H2O2 in leaves and root tips than seedlings of transgenic lines when cultured in BM supplemented with 150 mM NaCl (Fig. 9B).
Discussion
GMP catalyzes the reaction from mannose-1-phosphate to GDP-mannose and uses cofactor Mg2+ as a key switch for effective and continuous enzyme production42,43. Two types of GMP genes, namely GDP-mannose pyrophosphorylase A (GMPA) and GDP-mannose pyrophosphorylase B (GMPB), were found in fungus (Saccharomyces cerevisiae)44,45, pig (Sus scrofa)46, humans (Homo sapiens)29 and a higher plant, rice10. The protein sequences of GMPA and GMPB are very similar, but those of GMPA members are generally longer than those of GMPB members. Three DoGMP genes cloned from D. officinale showed high similarity with GMP sequences of other plant species (Supplementary Fig. 1). DoGMP1 and DoGMP3 belong to the GMPB group while DoGMP2 is a member of the GMPA group (Fig. 1). These three DoGMP proteins are likely to have the same catalytic function similar to other GMP proteins. DoGMP1 was localized in the cytoplasm, as was observed in other species such as Leishmania parasites, Homo sapiens and O. sativa10,47,48.
Studies have demonstrated that GDP-mannose is the active nucleotide sugar that provides mannose for the biosynthesis of mannan polysaccharides by mannosyltransferase49. Transgenic potato plants whose GMP was inhibited by an antisense construct, showed 30–50% less mannose content than WT level4. In the present study, the DoGMP1 gene contributed to the mannose content of water-soluble polysaccharide, consistent with previous studies. This indicates that the DoGMP1 gene plays an important role in mannose-containing polysaccharide synthesis in D. officinale. Feedback regulation is very common in plant metabolism to maintain a balance. For example, carbon metabolites have feedback regulation during photosynthesis50 while amino acids play a role in the feedback regulation of amino acid biosynthetic pathways51. Six genes (AtCSLA1, AtCSLA7, AtCSLA10, AtCSLA11, AtCSLA14 and AtCSLA15) related to mannose-containing polysaccharide synthesis were down-regulated in all of transgenic A. thaliana lines (Fig. 6). The AtCSLA genes were down-regulated significantly when BM was supplemented with 10 mM exogenous mannose (Supplementary Fig. 2). This suggests that high mannose-containing polysaccharide content has a negative feedback regulation of upstream genes.
Salinity stress has negative effects on seed germination and plant growth, and osmotic stress decreases water potential, consequently restricting water uptake by dry seeds and roots52,53. Water uptake during seed germination can be divided into three phases: Phase I, the initial absorption of water (imbibition), is primarily a physical process; Phase II, a plateau with stable water content; Phase III, water uptake by protrusion of the radicle54,55. At the imbibition stage, proteins and polysaccharides are involved in the absorption of water by the dry seed56. As salinity stress builds up, water potential declines, limiting the germination of seeds by negative water potentials. Low molecular weight sugars such as monosaccharides, disaccharides and oligosaccharide serve as an important compatible solute to assist plants in dealing with osmotic stress caused by salinity stress57. Water-soluble polysaccharides may serve as a hydrophilic substance and help dry seeds to initially absorb water. The content of low molecular weight sugars, which were extracted by hot 80% ethanol, in the mature seeds of 35S:DoGMP1 transgenic lines #1–3 was 54.3, 59.9 and 58.6 mg g−1 DW, respectively, while that of WT was 42.3 mg·g−1 DW (Supplementary Fig. 3A). The content of water-soluble polysaccharides in the mature seeds of the three transgenic lines was also significantly higher (35.6, 40.4 and 37.7 mg·g−1 DW, respectively) than that of WT plants (31.6 mg·g−1 DW, Supplementary Fig. 3B). The mature seeds of 35S:DoGMP1 transgenic lines had a higher content of low molecular weight sugars and water-soluble polysaccharides than WT plants. This may help transgenic seeds absorb water from a saline environment at the imbibition phase. Thus, the germination of seeds of transgenic lines was higher than of WT seeds.
Salinity stress not only intervenes in seed germination, but also influences seedling establishment. The decrease in water potential caused by salinity stress results in reduced cell growth, root growth and shoot growth53. The root growth of WT plants was distinctly inhibited by salinity stress (Figs 7C and 8). Salt stress reduces the ability of plants to take water up from the soil58,59. To deal with water stress, plants accumulate compatible solutes such as proline and sugars to reduce the cell water potential and absorb water from soil60. The roots of transgenic plants contained higher levels of low molecular weight sugars and water-soluble polysaccharides than WT plants (Supplementary Fig. 4), which can be used as compatible solutes to reduce cell water potential allowing water to be absorbed from a saline environment. Furthermore, excessive radical oxygen species (ROS) are produced when plants undergo various environmental stresses such as drought, chilling, metal toxicity, UV-B radiation and salinity61. GMPs are involved in the synthesis of AsA, which acts as a non-enzymatic antioxidant playing important roles in plants’ responses to stresses, and serves to eliminate excess ROS in plant cells11,62,63. The AsA content increased significantly in the three transgenic lines (5.31, 5.37 and 6.71 μmol·g−1 FW, respectively; Supplementary Fig. 4) compared to the WT plant (3.70 μmol·g−1 FW). This is likely to have increased ROS scavenging. In DoGMP1-overexpressing plants, H2O2, one form of ROS, was found at low levels than in WT plants under salt stress, as revealed by DAB staining (Fig. 9). These results suggest that DoGMP1 may enhance salt stress tolerance through the up-regulation of water-soluble sugars and AsA, thereby reducing ROS. In contrast, WT plants are unable to mediate the equilibrium of ROS production and scavenging, making the uptake of water from a saline medium very difficult, and subsequently suffering from oxidative damage and water stress, eventually developing a stressed phenotype.
In conclusion, three DoGMP genes were cloned from an important traditional Chinese herb, D. officinale, which contains abundant mannose-containing polysaccharides. The DoGMP1 protein located in the cytoplasm catalyzed the synthesis of GDP-mannose. These results provide evidence for the involvement of the DoGMP1 gene in the biosynthesis of mannose-containing polysaccharides, paving the way for studies on the biosynthesis of bioactive polysaccharides in D. officinale. 35:DoGMP1 transgenic plants showed higher germination and grew better than WT plants under salinity stress. This indicates that the GMP gene can be utilized as a candidate gene for improving abiotic stress tolerance in plants.
Additional Information
How to cite this article: He, C. et al. DoGMP1 from Dendrobium officinale contributes to mannose content of water-soluble polysaccharides and plays a role in salt stress response. Sci. Rep. 7, 41010; doi: 10.1038/srep41010 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Conklin, P. L. et al. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences USA 96, 4198–4203 (1999).
Reiter, W.-D. Biochemical genetics of nucleotide sugar interconversion reactions. Current Opinion in Plant Biology 11, 236–243 (2008).
Barth, C., Gouzd, Z. A., Steele, H. P. & Imperio, R. M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. Journal of Experimental Botany 61, 379–394 (2010).
Keller, R. & Kossmann, J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. The Plant Journal 19, 131–141 (1999).
Benton, B. K., Plump, S. D., Roos, J., Lennarz, W. J. & Cross, F. R. Over-expression of S. cerevisiae G1 cyclins restores the viability of alg1 N-glycosylation mutants. Current Genetics 29, 106–113 (1996).
Kempinski, C. F., Haffar, R. & Barth, C. Toward the mechanism of NH4 + sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase. Plant, Cell & Environment 34, 847–858 (2011).
Lukowitz, W. et al. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences USA 98, 2262–2267 (2001).
Conklin, P. L., Williams, E. H. & Last, R. L. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences USA 93, 9970–9974 (1996).
Conklin, P. L., Pallanca, J. E., Last, R. L. & Smirnoff, N. L-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1 . Plant Physiology 115, 1277–1285 (1997).
Kumar, R. et al. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Molecular Biology 79, 555–568 (2012).
Wang, H.-S., Yu, C., Zhu, Z.-J. & Yu, X.-C. Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Reports 30, 1029–1040 (2011).
Wang, H.-S., Zhu, Z.-J., Feng, Z., Zhang, S.-G. & Yu, C. Antisense-mediated depletion of GMPase gene expression in tobacco decreases plant tolerance to temperature stresses and alters plant development. Molecular Biology Reports 39, 10413–10420 (2012).
Edwards, M., Bulpin, P. V., Dea, I. C. & Reid, J. G. Biosynthesis of legume-seed galactomannans in vitro . Planta 178, 41–51 (1989).
Piro, G., Zuppa, A., Dalessandro, G. & Northcote, D. Glucomannan synthesis in pea epicotyls: the mannose and glucose transferases. Planta 190, 206–220 (1993).
Dhugga, K. S. et al. Guar seed β-mannan synthase is a member of the cellulose synthase super gene family. Science 303, 363–366 (2004).
Goubet, F. et al. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. The Plant Journal 60, 527–538, doi: 10.1111/j.1365-313X.2009.03977.x (2009).
Suzuki, S., Li, L., Sun, Y.-H. & Chiang, V. L. The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa . Plant Physiology 142, 1233–1245 (2006).
Sawake, S. et al. KONJAC1 and 2 are key factors for GDP-mannose generation and affect l-ascorbic acid and glucomannan biosynthesis in Arabidopsis . Plant Cell 27, 3397–3409 (2015).
He, C. et al. Identification of genes involved in biosynthesis of mannan polysaccharides in Dendrobium officinale by RNA-seq analysis. Plant Molecular Biology 88, 219–231 (2015).
Citovsky, V. et al. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta . Journal of Molecular Biology 362, 1120–1131 (2006).
Yoo, S.-D., Cho, Y.-H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2, 1565–1572 (2007).
Weigel, D. & Glazebrook, J. Transformation of Agrobacterium using the freeze-thaw method. CSH protocols 2006, 1031–1036 (2006).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 (1998).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497 (1962).
Ramel, F., Sulmon, C., Bogard, M., Couée, I. & Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biology 9, 28–28 (2009).
Zhao, M. et al. Arabidopsis BREVIPEDICELLUS interacts with the SWI2/SNF2 chromatin remodeling ATPase BRAHMA to regulate KNAT2 and KNAT6 expression in control of inflorescence architecture. PLoS Genetics 11, e1005125, doi: 10.1371/journal.pgen.1005125 (2015).
Tran, S. T., Le, D. T., Kim, Y.-C., Shin, M. & Choi, J.-D. Cloning and characterization of phosphomannose isomerase from Sphingomonas chungbukensis DJ77. BMC Reports 42, 523–528 (2009).
Tamura, K., Dudley, J., Nei, M. & Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology & Evolution 24, 1596–1599 (2007).
Hirayama, H. & Suzuki, T. In Handbook of Glycosyltransferases and Related Genes (eds Naoyuki, Taniguchi et al.) Ch. 155, 1599–1606 (Springer Japan, 2014).
Morgan, P. W. & Drew, M. C. Ethylene and plant responses to stress. Physiologia Plantarum 100, 620–630 (1997).
Vicente, R. S. & Plasencia, J. Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany 62, 3321–3338 (2011).
Creelman, R. A. & Mullet, J. E. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology 48, 355–381, doi: 10.1146/annurev.arplant.48.1.355 (1997).
Chinnusamy, V., Gong, Z. & Zhu, J. K. Abscisic acid-mediated epigenetic processes in plant development and stress responses. Journal of Integrative Plant Biology 50, 1187–1195 (2008).
Veljovic-Jovanovic, S. D., Pignocchi, C., Noctor, G. & Foyer, C. H. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiology 127, 426–435 (2001).
Liepman, A. H., Wilkerson, C. G. & Keegstra, K. Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proceedings of the National Academy of Sciences USA 102, 2221–2226 (2005).
Richmond, T. A. & Somerville, C. R. The cellulose synthase superfamily. Plant Physiology 124, 495–498 (2000).
Katembe, W. J., Ungar, I. A. & Mitchell, J. P. Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Annals of Botany 82, 167–175 (1998).
Meot-Duros, L. & Magné, C. Effect of salinity and chemical factors on seed germination in the halophyte Crithmum maritimum L. Plant and Soil 313, 83–87 (2008).
Jamil, M., Lee, D. B., Jung, K. Y., Ashraf, M., Lee, S. C. & Rha, E. S. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. Journal of Central European Agriculture 7, 273–282 (2006).
Cha-um, S., Charoenpanich, A., Roytrakul, S. & Kirdmanee, C. Sugar accumulation, photosynthesis and growth of two indica rice varieties in response to salt stress. Acta Physiologiae Plantarum 31, 477–486 (2009).
de Lima, R. B. et al. Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydrate Polymers 112, 686–694 (2014).
Diez, M. D. A. et al. Functional characterization of GDP-mannose pyrophosphorylase from Leptospira interrogans serovar Copenhageni. Archives of Microbiology 192, 103–114 (2010).
Fey, S., Elling, L. & Kragl, U. The cofactor Mg2+– a key switch for effective continuous enzymatic production of GDP-mannose using recombinant GDP-mannose pyrophosphorylase. Carbohydrate Research 305, 475–481 (1997).
Donoso, I. et al. Mpg1, a fission yeast protein required for proper septum structure, is involved in cell cycle progression through cell-size checkpoint. Molecular Genetics and Genomics 274, 155–167 (2005).
Muñoz‐Centeno, M. et al. Mpg2 interacts and cooperates with Mpg1 to maintain yeast glycosylation. FEMS Yeast Research 12, 511–520 (2012).
Ning, B. & Elbein, A. D. Cloning, expression and characterization of the pig liver GDP-mannose pyrophosphorylase. European Journal of Biochemistry 267, 6866–6874 (2000).
Carss, K. J. et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of α-dystroglycan. The American Journal of Human Genetics 93, 29–41 (2013).
Davis, A. J. et al. Properties of GDP-mannose pyrophosphorylase, a critical enzyme and drug target in Leishmania mexicana . Journal of Biological Chemistry 279, 12462–12468 (2004).
Reyes, F. & Orellana, A. Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Current Opinion in Plant Biology 11, 244–251 (2008).
Paul, M. J. & Pellny, T. K. Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany 54, 539–547 (2003).
Pratelli, R. & Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. Journal of Experimental Botany 65, 5535–5556 (2014).
Bu, Y., Kou, J., Sun, B., Takano, T. & Liu, S. Adverse effect of urease on salt stress during seed germination in Arabidopsis thaliana . FEBS Letters 589, 1308–1313 (2015).
De Oliveira, A. B., Gomes-Filho, E. & Alencar, N. L. M. Comparison between the water and salt stress effects on plant growth and development. In Responses of Organisms to Water Stress (ed. Akinci, S. ), pp. 67–94 (InTech., 2013).
Bewley J. D., Bradford K. J., Hilhorst H. W. & Nonogaki H. Germination. In Seeds: Physiology of Development, Germination and Dormancy (eds. Bewley, J. D., Bradford, K. J., Hilhorst, H. W. M., Nonogaki, H. ), pp. 133–181 (New York, Springer, 2013).
Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS 15, 763–774 (2011).
Woodstock, L. Seed imbibition: a critical period for successful germination. Journal of Seed Technology 12, 1–15 (1988).
Aquino, R. S., Grativol, C. & Mourão, P. A. Rising from the sea: correlations between sulfated polysaccharides and salinity in plants. PLoS One 6, e18862 (2011).
Munns, R. Comparative physiology of salt and water stress. Plant, Cell & Environment 25, 239–250 (2002).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681 (2008).
Parida, A. K. & Das, A. B. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety 60, 324–349 (2005).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012 (2012).
Zhang, G.-Y. et al. Manipulation of the rice L-galactose pathway: evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance. Plos One 10:e0125870 (2015).
Zhang, Y. Biological Role of Ascorbate in Plants. In Ascorbic Acid in Plants: Biosynthesis, Regulation and Enhancement ( Zhang, Y. ed.), pp. 7–33 (Springer, 2013).
Acknowledgements
This work was supported the Specific Fund Project for Forestry Science and Technology Innovation of Guangdong Province (Project number 2013KJCX014-06), the Forestry Science and Technology Innovation Fund Project of Guangdong province (Project number 2015KJCX040), and the Science and Technology Service Network Initiative of Chinese Academy of Sciences (Project number KFJ-EW-STS-118).
Author information
Authors and Affiliations
Contributions
J.D. conceived the experiments and experimental design. J.L. designed the draft manuscript. C.H. analyzed the data. Z.Y. conducted mannose content analysis and DAB staining. X.W. cloned the DoGMP3 gene. K.W., J.Z., J.T. and X.Z. conducted tissue culture and cultivated plants. C.H., JATdS, X.L., S.Z. and G.M. collectively interpreted the results and wrote all drafts of the manuscript. All 13 authors approved the final draft for submission and take full public responsibility for the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
He, C., Yu, Z., Teixeira da Silva, J. et al. DoGMP1 from Dendrobium officinale contributes to mannose content of water-soluble polysaccharides and plays a role in salt stress response. Sci Rep 7, 41010 (2017). https://doi.org/10.1038/srep41010
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep41010
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.