Abstract
Natural Bacillus isolates generate limited amounts of surfactin (<10% of their biomass), which functions as an antibiotic or signalling molecule in inter-/intra-specific interactions. However, overproduction of surfactin in Bacillus amyloliquefaciens MT45 was observed at a titre of 2.93 g/l, which is equivalent to half of the maximum biomass. To systemically unravel this efficient biosynthetic process, the genome and transcriptome of this bacterium were compared with those of B. amyloliquefaciens type strain DSM7T. MT45 possesses a smaller genome while containing more unique transporters and resistance-associated genes. Comparative transcriptome analysis revealed notable enrichment of the surfactin synthesis pathway in MT45, including central carbon metabolism and fatty acid biosynthesis to provide sufficient quantities of building precursors. Most importantly, the modular surfactin synthase overexpressed (9 to 49-fold) in MT45 compared to DSM7T suggested efficient surfactin assembly and resulted in the overproduction of surfactin. Furthermore, based on the expression trends observed in the transcriptome, there are multiple potential regulatory genes mediating the expression of surfactin synthase. Thus, the results of the present study provide new insights regarding the synthesis and regulation of surfactin in high-producing strain and enrich the genomic and transcriptomic resources available for B. amyloliquefaciens.
Similar content being viewed by others
Introduction
Surfactin is a lipopeptide that is synthesized by non-ribosome peptide synthases (NRPS) encoded by the srfA operon (srfAA, srfAB, srfAC, and srfAD)1. Surfactin comprises a long chain fatty acid tail and a circular heptapeptide containing glutamate acid (Glu), aspartate acid (Asp), valine (Val), and leucine (Leu). Surfactin has been recognized as one of the most promising biosurfactants and has broad application prospects in various industrial fields, including oil recovery, biopesticides, food processing, cosmetic, and pharmaceuticals, reflecting its unique properties, i.e., lower toxicity, higher biodegradability, and effectiveness under extreme conditions2. However, a significant obstacle for the large-scale application of surfactin is its low product yield per cell and the resulting high production costs.
The low yield of surfactin may be attributable to its biosynthetic regulation by a quorum sensing system, in which surfactin synthesis, competence development and sporulation are cross-linked within a complex network of pheromones and pleiotropic regulators. Bacillus cells constantly secrete ComX pheromone into the medium with increasing cell density1. The membrane receptor ComP senses ComX at a critical concentration and autophosphorylates and activates the cognate response regulator ComA. Subsequently, phosphorylated ComA (ComA-P) activates the transcription of the srfA operon after binding to the promoter region. The concentration of ComA-P in the cytoplasm is strongly influenced by several Rap-Phr family regulators, and the expression of the srfA operon is also regulated through several global regulators, including DegU, AbrB, and CodY3,4,5. As a consequence of quorum sensing, surfactin synthesis is dependent on cell density, preventing constant production and limiting overall yields.
In addition to this complex regulation mechanism, only a subpopulation (approximately 10%) of Bacillus subtilis cells sense the ComX pheromone and initiate surfactin production6, which may also reflect the low productivity of surfactin. Surfactin, secreted into the medium, acts as an extracellular signal that triggers another subpopulation of Bacillus cells to conduct cellular differentiation through the production of extracellular matrix6. During prolonged growth, matrix-producing cells undergo sporulation7. The matrix opportunely suppresses surfactin production by blocking the interaction between ComX and ComP without interfering with ComX production6. Therefore, once surfactin-responsive cells commence matrix production, these cells no longer respond to ComX, and no additional cells become surfactin producers6. This mechanism would reasonably explain why most Bacillus species exhibit limited surfactin synthesis.
Surfactin has been also described to act as an antibiotic during interspecific interactions between Bacillus and other species in the same ecosystem through the disruption or disintegration of cell membranes via physicochemical interactions8,9. For instance, surfactin produced by Bacillus sp. H2O-1 was reported to inhibit the growth of sulfate reducing bacteria10; Leu7-surfactin from Bacillus mojavensis showed antibiotic activity against Fusarium verticillioides11; and surfactin from B. amyloliquefaciens 1–45 showed antibacterial activity against Streptomyces species12. When the surfactin concentration is below or near the critical micelle concentration (approximately 0.01–0.025 g/L)13,14, surfactin monomers insert into phospholipid layers in biomimetic membrane systems, inducing mild content leakage15. In contrast, at higher concentrations, surfactin further attacks the phospholipid bi-layer, resulting in membrane solubilisation and vesicle destruction8,9. This mechanism may represent a third reason why most surfactin producers are unable to produce and secrete this antibiotic at high concentrations.
Because low productivity largely limits the commercial application of surfactin2, numerous efforts have been made in recent decades to improve surfactin production by optimizing the fermentation process16,17,18,19,20. However, these efforts have not yet successfully generated commercially viable and profitable surfactin production and will be unable to do so unless the yield of the final product from producer organisms is naturally high2. Therefore, surfactin producers demonstrating enhanced productivity per cell were constructed after screening for overproducing mutants or establishing genetically modified strains21,22. For example, recombinant strains characterized by promoter exchange of the srfA operon were constructed to eliminate surfactin synthesis through quorum sensing and constitutively produce surfactin23. Unfortunately, these attempts have met with limited success, and it remains difficult to meet the needs of industrial applications, reflecting the complex intrinsic regulatory network underlying srfA expression22,23.
In the present study, a Bacillus amyloliquefaciens strain (MT45) producing high levels of surfactin was obtained. Phenotype characterization indicated vigorous surfactin production of this strain independent of cell density, and the high surfactin concentration observed in the culture media provided evidence of the remarkable self-resistance capacity of this bacterium. Therefore, we sought to systemically unravel the biosynthesis and regulatory features underlying the overproduction of surfactin exhibited by this strain. Genome sequencing in combination with global transcriptome analysis was employed, and the results were compared with B. amyloliquefaciens type strain DSM7T, which produces much less surfactin. These results will allow us to better understand the mechanisms underlying surfactin overproduction and provide valuable information regarding potential molecular targets for rational strain improvement.
Results and Discussion
Phenotypic assays for biomass and surfactin production by MT45
Bacillus cells were cultivated and regularly sampled. MT45 grew more slowly during the first 24 h and exhibited a longer logarithmic phase than DSM7T (Fig. 1a). The biomass of MT45 steadily increased through the end of cultivation, reaching a maximum value of 5.6 g/l at 54 h. In contrast, the biomass of DSM7T reached 3.7 g/l at 30 h and remained relatively stable during prolonged fermentation. Surfactin in the culture broth was characterized by LC-MS with standard surfactin as reference. MT45 and DSM7T produced similar surfactin mixtures, comprising homologues with [M + H]+ peaks at 1008.68, 1022.68, 1036.70, 1050.68, and 1064.70, consistent with standard surfactin isomers with fatty acid chains of different lengths (C13, C14, C15, C16, and C17, respectively) (Fig. 1c)10.
The MS/MS fragments of surfactin produced in this study agreed with the surfactin standard (Supplementary Fig. S1) and suggested the sequence as Glu-Leu-Leu-Val-Asp-Leu-Leu. In addition, the retention times of surfactin from MT45 were identical to the standard surfactin from Sigma (Supplementary Fig. S2). Thus surfactins and their homologues were quantified by ultra-high performance liquid chromatogram (UPLC), and the amounts of all homologues were summed to obtain the total surfactin concentration. As shown in Fig. 1d, the maximum concentration of surfactin produced by DSM7T at 36 h was 0.17 g/l, consistent with previous reports describing the production of limited surfactin by most Bacillus strains to act as a signalling molecule in intracellular interactions24,25. In contrast, surfactin produced by MT45 reached a maximum concentration of 2.93 g/l at 48 h and maintained stable levels during cultivation thereafter; thus, MT45 significantly differs from DSM7T in terms of surfactin productivity. Additionally, the high surfactin concentration (>100-fold of CMC) in the culture broth indicated the strong antibiotic resistance capacity of MT45. The specific production rate (qp) of surfactin was calculated, and the maximum qp (g product/g dry cell weight •h) values for MT45 and DSM7T were 0.16 and 0.04, respectively. The efficient production of surfactin by MT45 reflects high metabolic activity rather than high bacterial biomass.
In addition to surfactin, MT45 also produced iturin family lipopeptides with [M + H]+ peaks at 1031.76, 1045.78, 1059.78 (Supplementary Fig. S3), which is identical to bacillomycin D26. However, the concentration of bacillomycin D is much lower than that of surfactin since the peak area of bacillomycin D is only about 3.6% of that of surfactin in the total ion chromatograms. In addition, bacillomycin D was not detected in the culture broth of DSM7T. Neither MT45 nor DSM7T produced fengycin family lipopeptides.
Genomes and comparative genomic analysis
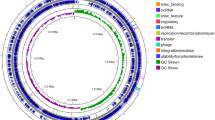
To investigate the efficient biosynthesis features of surfactin in MT45, the entire genome was sequenced, revealing a complete circular genome 3,897,521 bp in length with a GC content of 46.09%. The genome of MT45 is smaller than those of most Bacillus species deposited in the NCBI database (Table 1). The principal features of the MT45 genome are shown in Fig. 2a, in the form of a circular graph. To detect the evolutionary origin of MT45, a neighbour-joining tree was constructed based on multi-genome alignment, including MT45 and the representative genomes of 6 other species in Table 1. The phylogram in Fig. 2d suggests a closer relationship between MT45 and DSM7T.
(a) Circular representation of the MT45 genome. From the outside inwards, circle 1: scale; circles 2 and 3: the predicted CDSs colour-coded according to their functions: cellular process, green; metabolism, pink; information pathway, orange; other functions, red; unknown, black; circle 4: predicted genomic islands; circle 5: unique genes in MT45; circle 6: rRNA; circle 7: tRNA; circle 8: predicted prophages (grey) and gene clusters involved in secondary metabolite synthesis (green); and circles 9 and 10: GC content and GC skew (G+C/G−C), respectively. (b) Global alignment of the bacterial chromosomes constructed using the M-GCAT programme; indels are depicted as red rectangles. (c) GO classification of the unique genes in MT45 and DSM7T. (d) Phylogenetic tree generated via comparison of the genomes of B. amyloliquefaciens MT45, B. amyloliquefaciens DSM7T, B. velezensis FZB42 (BV), B. subtilis DSM10T (BS), B. licheniformis DSM13T (BL), B. pumilus SAFR-032 (BP), and B. cereus ATCC14579T (BC).
Given the enormous differences in surfactin productivity between MT45 and DSM7T, a global alignment of the two bacterial chromosomes was constructed using M-GCAT in combination with Mauve software. As shown in Fig. 2b, three large insertions (longer than 30 kb, depicted as red rectangles) were uniquely observed in the genome of DSM7T, primarily comprising phage-related integrase/recombinase and hypothetical proteins (Supplementary dataset 1). In addition, many unique regions in MT45 were annotated as transporters and antimicrobial peptide resistance-associated genes, separate from phage-related proteins (Supplementary dataset 2). These unique transporters and resistance proteins may contribute to the self-resistance of MT45 against high concentrations of antimicrobial surfactin. According to the pairwise genome comparisons of MT45 and DSM7T, there were 332 and 562 singletons in MT45 and DSM7T, respectively. These sequences were further annotated and analysed using the Blast2Go programme. A total of 298 and 399 unique sequences for MT45 and DSM7T, respectively, were assigned to biological processes, cellular components, and molecular functions (Fig. 2c, Supplementary dataset 3 and 4).
In the biological process category, which is closely associated with cell metabolism activity, 52 singletons in MT45 were uniquely categorized as 4 secondary gene ontology (GO) items, including localization (11 genes), cellular component organization or biogenesis (15 genes), developmental process (10 genes), and response to stimulus (16 genes). Amongst these genes, WV34_01080, WV34_07040, and WV34_04495, categorized under localization, were annotated as ATP-binding cassette (ABC) transporters. Previous studies have implicated ABC transporters in the secretion of antibiotics through the cell membrane, contributing to bacterial self-resistance27. These proteins may assist MT45 in the secretion and resistance of high concentrations of surfactin. In addition, an 8,708-bp gene cluster containing WV34_01100, WV34_01105, WV34_01120, and WV34_01125, categorized under response to stimulus, was annotated as an antimicrobial peptide transporter. This unique gene cluster may be involved in surfactin resistance, as WV34_01125 shares high amino acid sequence identity with SwrC, a membrane protein responsible for the secretion and self-resistance of surfactin28.
To better establish the uniqueness of the regions in strain MT45, a broader comparative genome analysis was performed by comparing the genome of MT45 with 17 other genomes of B. amyloliquefaciens strains (Supplementary Table S1). The phylogram shown in Supplementary Fig. S4 suggested a higher genomic distance of MT45 with other strains. A total of 183 genes were found to be unique in the genome of MT45 (Supplementary dataset 2), including 8 genes encoding ABC transporters and 2 resistance-related genes (WV34_01120 and WV34_01125) which were supposed to be potentially associated with surfactin resistance as mentioned above.
Global transcriptome analysis
RNA-Seq was employed to reveal the transcriptomic features of overproduction of surfactin in MT45 during different growth stages (12, 24, and 36 h), with DSM7T as a comparative control. For simplicity, the transcriptomes obtained at specific time points (in h) were designated M12, M24, and M36 for MT45 and D12, D24, and D36 for DSM7T. More than ten million reads for each sample were generated with clean ratios >82%. The clean reads generated average mapping ratios of 92.87% and 99.47% for the genomes of MT45 and DSM7T, respectively. The mapped genes were classified into 4 groups based on the expression levels indicated by FPKM (Fragments Per Kilobase of exon model per Million mapped Reads) values (Fig. 3a). Using a threshold of FPKM >1 to define potential gene expression, averages of 3,677 and 3,586 expressed genes were observed for MT45 and DSM7T, respectively. Although most genes were medially expressed in both MT45 and DSM7T throughout cultivation, genes expressed at high levels in MT45 were more numerous than those in DSM7T during the 3 different growth stages. The average number of highly expressed genes in MT45 throughout cultivation was 563, while an average of 467 genes were highly expressed in DSM7T. In addition, the average numbers of non-expression and low-expression genes in MT45 were 48 and 578, respectively, which were much lower than those in DSM7T, which had 124 non-expression genes and 861 low-expression genes. Furthermore, the number of non-expression genes in MT45 decreased from 12 to 36 h, in contrast with DSM7T, for which the number of non-expression genes increased over time. This result was consistent with the large numbers of phage-related and/or hypothetical regions in the genome of DSM7T, which are silenced during cultivation (Supplementary dataset 1). Therefore, a smaller genome with less nonessential genes may benefit MT45 with accumulation of more biomass and bioproducts29,30.
(a) Global abundance of the gene expression of MT45 and DSM7T throughout the cultivation period. The transcripts were assessed based on FPKM values: high expression (FPKM ≥ 500), medium expression (10 ≤ FPKM < 500), low expression (1 ≤ FPKM < 10), and non-expression (FPKM < 1). (b) Major differences in metabolic pathways of MT45 compared with DSM7T cultivated for 12 h.
Real-time PCR was performed on the original RNA extracts to confirm the transcriptional profiling data obtained from RNA sequencing. Although there were some differences in the fold-changes for several differentially expressed genes (DEGs) as determined by real-time qPCR and RNA-Seq, the general trends were consistent, supporting the credibility of the RNA-Seq data (Supplementary Table S2). These differences likely result from the use of different methods, a phenomenon that was also observed in previous studies31,32.
To further unravel the influence of transcriptional properties on the metabolic activity of MT45 and DSM7T, a comparative transcriptome analysis was performed at 12 h when specific surfactin production rates were at the maximum values. The distribution of DEGs in metabolic pathways was examined by employing KEGG (Kyoto Encyclopaedia of Genes and Genomes) enrichment. Twenty-five most respondent pathways (P < 0.05) were assigned and categorized into 12 items, comprising 315 up-regulated genes and 83 down-regulated genes (Fig. 3b). The KO numbers and the corresponding pathways are shown in Supplementary dataset 5. Amongst these pathways, signal transduction pathway (two-component system) and membrane transport pathway (including ABC transporters and the phosphotransferase system) contain the greatest number of up-regulated genes.
The two-component system (TCS) is an important signal transduction system comprising a sensor kinase and a response regulator. Bacteria heavily rely on the TCS to detect environmental signals and process information33. Among the DEGs annotated as components of the TCS, the narGHJI operon, involved in nitrogen metabolism, was overexpressed in MT45 with an average FPKM of 1,319.8 but was not expressed in DSM7T. The narGHJI operon is induced under oxygen-limited conditions through Fnr, which is required for the adaptation of B. subtilis to low oxygen34. B. subtilis is able to grow anaerobically via respiration with nitrate as a terminal electron acceptor35. The utilization of nitrate likely occurred in the present study following the depletion of ammonium in the media, with subsequent induction of dissimilatory nitrate metabolism. Therefore, the high expression of narGHJI in MT45 may result in the efficient utilization of nitrogen sources, thus contributing to high surfactin yields. These results are consistent with those of a previous report in which surfactin production in B. subtilis ATCC21332 was strongly enhanced under nitrate metabolism36.
Among the significantly responsive ABC transporters, the glnQHMP operon was assigned as a glutamate transporter. The average FPKM value of glnQHMP in MT45 was 541.2, but it was not expressed in DSM7T. Glutamate is the first amino acid of the heptapeptide sequence in surfactin, and the synthesis of surfactin is initiated from the condensation of glutamate and fatty acid37. High-level glutamate transporter operon expression may provide MT45 with more glutamate for the efficient synthesis of surfactin. More importantly, ABC transporters together with the neighbouring TCS have been recognized as detoxification systems for resistance against antimicrobial peptides in many Firmicutes bacteria38,39. Surfactin works as an antibiotic by attacking the bacterial cell membrane9. Up-regulated ABC transporters are important for the efflux and survival of MT45 in the presence of high concentrations of surfactin9.
Other DEGs were primarily enriched in the citrate cycle, the pentose phosphate pathway, fructose and mannose metabolism, and lipid metabolism. These processes provide essential precursors, such as amino acids, carbon skeleton, NADPH, and ATP, for cell growth and surfactin biosynthesis. Therefore, it is important to analyse these pathways in detail to obtain a better understanding of the high efficiency of surfactin biosynthesis.
Construction of a KEGG pathway map for surfactin biosynthesis
A global surfactin biosynthesis pathway in B. amyloliquefaciens was constructed based on the reported surfactin production process in B. subtilis, as B. amyloliquefaciens and B. subtilis share many common properties only with hydrolases production as major recognized difference40. Levels of individual transcripts were expressed in a heat map and subsequently applied to a metabolic pathway map (Fig. 4, Supplementary dataset 6). Six modules were partitioned according to their functions, specifically glycolysis and tricarboxylic acid41 cycle, NADPH generation, amino acids biosynthesis, fatty acid biosynthesis, modularly enzymatic synthesis of surfactin, and secretion and resistance of surfactin.
The metabolic network was reconstructed based on KEGG pathway analysis. Six modules were partitioned according to function. Transcripts of MT45 and DSM7T are shown near the pathway as a heat map, based on fold-changes in transcript levels relative to DSM7T after 12 h. Colour legend is shown at the top left of the map. Gene abbreviations are shown in Supplementary dataset 6.
Module 1 of the pathway represented sucrose utilization and the subsequent glycolysis pathway and TCA cycle, which provide building precursors for cellular metabolism. The genes involved in the utilization of sucrose, including sacP, murP and sacA, which encode a sugar transporter, permease, and sucrose-6-phosphate hydrolase, respectively, were highly expressed in MT45. Most of the genes involved in glycolysis and the TCA cycle were also up-regulated in MT45, providing carbon skeleton and energy for subsequent metabolism. For example, pyruvate, 2-oxo-glutarate, oxaloacetate, and acetyl-CoA serve as building precursors for the biosynthesis of Val/Leu, Glu/Asp, and fatty acids, respectively.
Module 2 comprised a portion of the pentose phosphate pathway, which primarily generates NADPH and pentose for cell metabolism. ZWF and GNDA, which catalyse the generation of NADPH, were highly expressed in MT45 at 12 h but were not significantly different, suggesting NADPH is not the bottleneck for surfactin production. Module 3 involved the biosynthesis of Glu, Asp, Val, and Leu, which are intrinsic components of surfactin. Higher expression levels of aspartate aminotransferase (AspB and YhdR) in MT45 promote the synthesis of Glu/Asp and subsequent surfactin assembly. In addition, genes for the synthesis of Val and Leu were not expressed as highly in MT45, consistent with a previous report in which srfA expression was negatively regulated through the metabolism of branched chain amino acids3. Fatty acids are another intrinsic component of surfactin, comprising the hydrophobic tail. Most genes in the fatty acid synthesis pathway shown in module 4 were up-regulated in MT45. The biosynthesis of fatty acids is initiated from the building precursor acetyl-CoA. Notably, the genes that participate in acetyl-CoA generation were particularly up-regulated in MT45, which would be highly beneficial for the biosynthesis of fatty acids. The increased fatty acid biosynthesis pathway is likely essential for highly efficient surfactin production. Increased fatty acid biosynthesis may also explain the higher biomass of MT45.
The biosynthesis of surfactin is catalysed through NRPS, initiated from the condensation of fatty acids and Glu. Other constituent amino acids are assembled through the NRPS multi-enzyme complex, comprising adenylation, condensation, and thiolation domains responsible for the activation of amino acids and peptide chain elongation42. As shown in module 5, the srfA operon was highly expressed in MT45 (9.25–48.86-fold compared with DSM7T). Overexpression of the surfactin synthase gene would be closely associated with the high surfactin productivity of MT45.
Amino acids and fatty acids in the cytoplasm are generally utilized to synthesize cell structural elements and various enzymes. Therefore, surfactin biosynthesis inevitably competes with biomass formation. Aminoacyl tRNA synthases play important roles in protein translation. Thus, the relative expression levels of the aminoacyl-tRNA synthases and srfA operon reflect competition for the utilization of amino acids for cell growth or surfactin production. In MT45, the FPKM value of srfAA was >2.99-fold higher than that of glutamyl-tRNA synthase (gltX) (Supplementary Fig. S5b,d), and the expression of aspS (aspartyl-tRNA synthase), valS (valyl-tRNA synthase), and leuS (leucyl-tRNA synthase) was lower than for the other genes of the srfA operon. In contrast, gltX, leuS, and aspS were highly expressed compared with the srfA operon in DSM7T throughout cell growth. The overexpression of aprE, an extracellular alkaline serine protease with an average FPKM of 27,506.3, further supports the preferential usage of amino acids in the cytoplasm of DSM7T for protein synthesis (Supplementary dataset 7). In addition, fatty acids are competitive substrates for the biosynthesis of glycerophospholipids, an essential component of the cell membrane. The expression of srfAA in MT45 was 2.78–13.9-fold higher than that of the genes that convert fatty acids and glycerone phosphate to phosphatidate (Supplementary Fig. S5c,e). Based on these results, additional amino acids and fatty acids may be used to produce surfactin and provide a reasonable explanation for the lower growth rate of MT45 prior to 24 h.
Module 6 comprises the expression of surfactin resistance genes. Reflecting antibiotic activity, self-resistance is essential for the high productivity of surfactin. SwrC, important for the export and self-resistance of surfactin in B. subtilis15, was highly expressed in MT45 with an overall FPKM value of 992.01, indicative of its significant effects on surfactin efflux. In addition, the liaRSFGHI operon, annotated as genes associated with the resistance of daptomycin (a structural analogue of surfactin), was significantly expressed in MT45, particularly for liaH and liaI, with an FPKM value >800 at 36 h (Supplementary dataset 7). The overexpression of liaRSFGHI operon during the late stage of fermentation may help MT45 confront the high concentrations of surfactin; however, this hypothesis requires further investigation. Furthermore, unique transport and resistance genes in the MT45 genome were all expressed (Supplementary dataset 2), supporting their potential antagonistic functions against a high surfactin concentration.
Differentially expressed genes associated with srfA expression
The enormous difference in the surfactin yield between MT45 and DSM7T is significantly influenced by the differential expression of the srfA operon. Therefore, it is necessary to study the regulatory mode of srfA expression in MT45. According to the transcriptome data, expression of srfA operon of MT45 decreased at 24 h and then subsequently increased. Generally, genes with the same or opposing expression tendency as srfA would be positively or negatively associated with srfA expression, respectively4. Therefore, DEGs exhibiting similar or opposing expression tendencies, were selected to further analyse their potential effect on srfA expression. Subsequently, the selected DEGs assigned the function of “regulation of gene expression” in the Subtiwiki database43 were further analysed, including 23 DEGs with similar expression tendencies to srfA and 24 DEGs with opposing expression tendencies (Fig. 5). These DEGs were categorized into 5 subgroups according to their functions: (i) phosphorylation, (ii) sigma factors and their controls, (iii) transcription factors and their controls, (iv) genetic competence, and (v) spore formation.
Arrows indicate positive effects on srfA expression, the transformation of phosphoryl groups, or pentapeptide incorporation. T-bars indicate the negative effects on DNA binding or protein interactions. Bent arrow represents the promoter. ‘P’ in the circle represents the phosphoryl group. Heat maps in (a) and (b) indicate DEGs annotated as regulators that exhibit the same and opposing expression trends as srfA, respectively. Subgroups in (i)–(v) related to phosphorylation, sigma factors and their controls, transcription factors and their controls, genetic competence, and spore formation, respectively. Genes with fold-changes >2, detected by RT-qPCR, are indicated with asterisks.
Among these 47 DEGs potentially associated with srfA expression, we found four positive regulators which is consistent with previous studies. First, as expected, comA and sigA were down-regulated at 24 h and subsequently up-regulated in the prolonged cultivation because ComA and SigA directly bind to the srfA promoter to initiate transcription. In addition, expression of degU in MT45 significantly decreased from 12 to 24 h with a fold-change >2.5 (P < 0.0001). srfA expression is reported to be influenced by DegU-P, as the disruption of degU decreases srfA expression in B. subtilis44. RghR represses the function of Rap proteins, which inhibit the binding of ComA and DegU to target promoters, leading to the inhibition of srfA expression45. In the present study, rghR exhibited the same expression tendency as srfA but an opposing expression tendency to RapG and RapH in MT45, suggesting its positive effects on srfA expression. The transcriptome data in combination with previous reports confirmed the dependability of the analytical method and the results of the present study.
The transition state regulator AbrB was the most significantly down-regulated gene at 24 h, with a fold-change >8.5 (P < 0.0001). This result was consistent with previous study in which abrB was highly expressed from the lag to the exponential phase but dynamically down-regulated when Bacillus cells entered the stationary phase5. B. subtilis expresses another protein, Abh, containing N-terminal DNA-binding domains highly homologous to those of AbrB5. The disruption of abh results in stronger srfA operon derepression than abrB deletion, indicating negative regulation of srfA expression by Abh46. However, AbrB binding to the srfA promoter in the abh deletion strain was retained, whereas Abh binding was weak on the abrB deletion background46. Therefore, the effects of abh–abrB on sfrA expression remain unclear. The expression of abh differed from abrB and remained relatively stable in MT45 throughout cultivation. Thus, AbrB, unlike Abh, may positively regulate srfA expression, reflecting its similar expression tendency as srfA, although the molecular mechanism remains unknown.
DEGs, which would be negatively associated with srfA expression of MT45, were shown in Fig. 5b. First, the transcriptome data suggested the suppression of srfA expression through multiple Rap proteins, i.e., RapH1, RapH3, and RapC, which are aspartate phosphatases controlling ComA activity and negatively influence surfactin production45. CodY was also negatively associated with srfA expression, consistent with the results of previous studies in which CodY repressed srfA expression through direct binding to the srfA promoter46,47. A recent study showed that deletion of codY could significantly improve the surfactin production in B. subtilis48, indicating that CodY is a negative regulator of srfA. Another potential repressor is Spx, which regulates gene expression in B. subtilis in response to oxidative stress through interactions with the C-terminal domain of RNA polymerase (RNAP) alpha subunit (α-CTD)49. RNAP interacts with the −35 region of srfA, assisted by ComA-P, to fully activate transcription. Furthermore, ComA-P interacts with the RNAP α-CTD in a region previously shown to interact with Spx49. Therefore, Spx suppresses srfA expression by blocking the interaction between ComA and RNAP in the promoter region via competition for an overlapping site in the α-CTD49. The up-regulated expression of SinI at 24 h is reasonable because SinI positively regulates matrix production, which suppresses the constitutive synthesis of surfactin25. Therefore, SinI negatively and indirectly regulates srfA expression.
According to the transcriptome and qPCR data, another potential repressor of srfA is PerR. This result differs from a previous report in which PerR positively regulated sfrA expression in B. subtilis44. The mechanism through which PerR affacts srfA remains unclear, but two PerR boxes were identified in the srfA promoter-distal region near the ComA box, indicating PerR may interact with ComA to influence the activation of srfA expression44. In addition, these disparities may reflect the use of different culture media and harvest time points44,50. Besides, srfA was reported recently to be positively regulated by PhoP under phosphate-limiting conditions in B. subtilis51. However, in the present study, phoP showed an opposing expression tendency to that of srfA, suggesting PhoP may be negatively associated with srfA expression under the culture conditions applied herein (phosphate enrichment).
Unlike MT45, the expression of srfA operon in DSM7T continued to decline from 12 to 36 h (Supplementary Fig. S6). However, we obtained generally similar regulatory DEGs in DSM7T with those in MT45. For instance, RghR and AbrB would positively regulated srfA expression since their expressions were down regulated across the cultivation, and RapC, RapF, RapH, PerR, SinR, and Spx would negatively regulated srfA expression since their expressions were continually up regulated. However, the effects of CodY, PhoP, and ComA on srfA expression is ambiguous in DSM7T, since their functions seem to be inconsistent with our hypothesis. The results obtained here further suggest that surfactin synthesis may be differentially regulated in minor and strong surfactin producers or in different Bacillus species and under different culture conditions23. Other DEGs (shown in Fig. 5) associated with competence development and spore formation also indicated the complexity of the transcriptional regulation of srfA and the cross-linked relationship between surfactin production and the two processes.
Therefore, in combination with the transcriptional data in this study and the regulators reported previously, we showed the potential genes and regulators that would be positively or negatively associated with srfA expression. Other DEGs, predicted based on transcriptional tendency, represent potential regulators and targets for the rational design of surfactin producers. In further studies, the function of these genes should be verified in a more comprehensive way, including gene expression and construction of gene deletion mutant, and applied to the artificial design of improving industrial surfactin production.
Overall, the results of the present study provide an integrated omics profile of a surfactin high-producing strain, facilitating the genomic and transcriptomic comparison of minor and strong surfactin producers, and reveal differences in srfA operon expression, precursor redirection, and antibiotic resistance capacity that significantly influence surfactin productivity. These findings will enrich the available genetic resources for surfactin-producing Bacillus and may provide fundamental information for future studies to artificially construct industrial strains via genetic modification or pathway engineering.
Materials and Methods
Microorganisms and growth conditions
B. amyloliquefaciens MT45 with high surfactin productivity was isolated from Chinese Maotai Daqu (Renhuai, China), and stored at China General Microbiology Culture Collection Centre (CGMCC: 12593). B. amyloliquefaciens type strain DSM7T was obtained from DSMZ (German Collection of Microorganisms and Cell Cultures). Bacillus cells were cultivated at 30 °C in 500 ml flasks, containing 100 ml of minimum media (0.8% NH4NO3, 0.03 M KH2PO4, 0.04 M Na2HPO4, 7 μM CaCl2, 4 μM FeSO4, 100 μM MgSO4, 4 μM ethylene diamine tetraacetic acid) supplied with 4% sucrose (MMS media).
Cell growth and surfactin production assays
To determine dry cell weight, a cell culture aliquot was sampled into a pre-weighed sampling tube and centrifuged at 8,000× g for 10 min. Subsequently, the supernatant was removed and the cell pellet was washed three times with sterile saline solution. The washed cell pellet was dried for at least 4 h to a constant weight at 105 °C. For surfactin determination, cell-free culture broth was adjusted to pH 2.0 using 6 M HCl and centrifuged at 10,000× g for 30 min. Crude surfactin was obtained after removing the supernatant and re-dissolving the pellet in 100% methanol. Surfactin composition and other potential lipopeptides were characterized by LC-MS as previously reported12. The quantitative analysis of surfactin was performed on Waters UPLC H-class system equipped with a binary solvent delivery system and an auto-sampler. The chromatographic separation was performed on a Waters Acquity C18 column (50 mm × 2.1 mm, 1.7 μm particle). Injection volume was 5 μL. The mobile phase consisted of solvent A (HPLC grade water containing 0.1% formic acid) and solvent B (HPLC grade methanol). Elution was performed by linear biphasic gradient of 85–100% solvent B over 6 min, at a flow rate of 0.3 mL/min. The elution pattern was monitored by determining absorbance at 215 nm. The concentration of surfactin was analysed and quantified by Empower Version 3.0, using a calibration curve made by surfactin standards (Sigma Chemicals, St. Louis, MO). Then the total surfactin concentration was calculated by summing up the concentration of each congener. All the experiments were performed in triplicate.
Genomic sequencing and annotation
MT45 cells were cultivated for 24 h and subsequently harvested through centrifugation at 10,000× g for 5 min at 4 °C. Genomic DNA was immediately extracted as previously described52. The MT45 genome was sequenced using the whole-genome shotgun method on an Illumina Miseq platform. Four paired-end/mate-paired sequencing libraries were constructed with insert sizes of 450 bp, 700 bp, 3 kb, and 8 kb. The reads were assembled into contigs and scaffolds employing the de novo assembler Newbler53 and SSPACE54. Gaps were closed using the GapCloser programme55 combined with long-range PCR using Phusion polymerase from New England Biolabs Inc. (Beverly, MA, USA) and subsequent Sanger sequencing (Personalbio, Shanghai, China). The genome sequence of MT45 was deposited in GenBank under the Whole Genome Shotgun project [GenBank: CP011252].
The prediction of protein-encoding sequences was accomplished by employing both the MAKER pipeline prediction system and Glimmer 356. Functional annotation was carried out using the BLASTP search tool with B. amyloliquefaciens DSM7T and B. subtilis 168 as references, as well as the non-redundant protein database (nr) of GenBank (parameters: E-value: 1E-5, coverage >60%, identity >50%). Each gene was functionally classified into Cluster of Orthologous Genes (COG) categories by performing an RPS-BLAST search against the COG database with an E value of 1E-557. Genes for tRNAs and rRNAs were predicted with tRNAscan-SE and RNAmmer 1.2 Server, respectively58,59. Horizontally transferred genomic islands (GIs) were identified with IslandViewer 360, using Seq-Word Sniffer tools61 to combine the prediction results. Prophage regions were predicted using the PHAST server62. The circular map and the graphic representation of genome-compared orthologous genes were generated using Circos. Global alignment of whole genome sequences of MT45 and DSM7T was performed using M-GCAT software63.
Sequence comparison and construction of an evolutionary tree
Genomic comparison of MT45 and DSM7T was performed using Mauve software, employing Progressive Mauve sequence alignment using default parameters. The resulting unique genes in MT45 and DSM7T were functionally classified into GO categories using the Blast2Go programme. To construct an evolutionary tree, a family containing 7 closely related Bacillus genomes was generated using Orthomcl software to obtain the protein sequences with a strict 1:1:1 ratio. Subsequently, Muscle (version 3.8.31) was employed to compare the protein sequences using default parameters. No matching portions of the alignments were removed using Gblock (version 0.91 b). Finally, the phylogenetic tree was constructed using the PHYLIP package represented in Newick format.
Transcriptomic analysis using RNA-seq
For RNA extraction, Bacillus cells were cultivated for 12, 24, and 36 h and harvested after centrifugation at 10,000× g for 5 min at 4 °C. The resulting pellets were immediately frozen in liquid nitrogen. Total RNA was extracted using an RNeasy Mini Kit (QIAGEN, GmBH, Germany) according to the manufacturer’s instructions. RNA quality was evaluated using an Agilent Bioanalyser 2100 system (Agilent Technologies, Santa Clara, CA, USA). Ribosomal RNAs were removed using the Ribo-Zero rRNA Removal Kit (Epicentre) for Gram-positive organisms prior to sequencing analysis. Subsequently, RNA was fragmented and used as a template for randomly primed PCR.
Strand-specific cDNA libraries were prepared employing standard techniques for subsequent Illumina sequencing using the mRNA-seq Sample Prep Kit (Illumina). The cDNA libraries were sequenced on an Illumina HiSeq 2500 according to the manufacturer’s instructions. Sequencing raw reads were pre-processed after filtering out rRNA reads, sequencing adapters, short-fragment reads and other low-quality reads. The remaining clear reads were mapped to the reference genome of MT45 or DSM7T using Bowtie2 software based on the local alignment algorithm64. The expression levels were normalized to the library and the gene length by calculating the FPKM value65. Differential expression of all of transcripts was quantified using DESeq software66. The method of FDR (False Discovery Rate) control was used to correct the results for multiple hypothesis testing67. Significant DEGs were screened based on an FDR threshold of ≤ 0.001, and a |log2 Fold change| value ≥ 1. KOBAS software (KOBAS, Surrey, UK) was applied to test the statistical enrichment of DEGs in KEGG pathways using hypergeometric tests with a P value cut-off of 0.05. The RNA sequence data has been deposited to DDBJ (DNA Data Bank of Japan) with Accession ID DRA005269.
Reconstruction of the KEGG pathway map
Functional annotation descriptions were assigned using BLASTP68 in conjunction with the KEGG database (E-value cut-off of 1E-10). A metabolic network was reconstructed using KEGG mapper (http://www.genome.jp/kegg/) with the KO numbers and gene abbreviations shown in Supplementary dataset 6. Subsequently, the KEGG map was manually redrawn using Adobe Illustrator CC 2014 (Adobe Systems). Heat maps of the expressed genes involved in surfactin biosynthesis were generated using the heatmap.2 function from the Gplots package in R statistical software v. 3.1.069. Statistical testing for gene expression was performed in R with DESeq using the no replicate method (Supplementary dataset 8)66.
qRT - PCR assay
Real-time PCR was performed on the same RNA extracts using in RNA-seq to confirm the transcriptional data obtained from Illumina sequencing as well as to study the expression of srfA operon and its regulatory genes in MT45 and DSM7. Reverse transcription was performed using the QuantiNova Reverse Transcription Kit (QIAGEN, Hilden, Germany) with total RNA as template. The transcription levels of genes were detected by quantitative real-time PCR (qRT-PCR) with BIO-RAD CFX96 Touch q-PCR system and all regents for qPCR were from BIO-RAD Laboratories, Inc. (Hercules, CA, USA). The relative transcription levels of target genes were quantified by the 2-ΔΔCT method using recA gene as an internal control. Each qPCR reaction was performed in 20 μL volume containing 10 μL of SsoFast EvaGreen Supermix, 0.4 μL of each primer, 1 μL of template cDNA and 8.2 μL of RNase-free H2O. The amplification conditions were as follows: preheating at 95 °C for 2 min and then 40 cycles of 95 °C for 5 s, 60 °C for 15 s. Three technical replicates were carried out for each target gene.
Additional Information
How to cite this article: Zhi, Y. et al. Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci. Rep. 7, 40976; doi: 10.1038/srep40976 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Nakano, M. M. et al. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J. Bacteriol. 173, 1770–1778 (1991).
Mukherjee, S., Das, P. & Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 24, 509–515, doi: 10.1016/j.tibtech.2006.09.005 (2006).
Serror, P. & Sonenshein, A. L. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178, 5910–5915 (1996).
Ogura, M., Yamaguchi, H., Yoshida, K.-i., Fujita, Y. & Tanaka, T. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 29, 3804–3813, doi: 10.1093/nar/29.18.3804 (2001).
Strauch, M. A. et al. Abh and AbrB Control of Bacillus subtilis Antimicrobial Gene Expression. J. Bacteriol. 189, 7720–7732, doi: 10.1128/jb.01081-07 (2007).
Lopez, D., Vlamakis, H., Losick, R. & Kolter, R. Paracrine signaling in a bacterium. Genes Dev. 23, 1631–1638, doi: 10.1101/gad.1813709 (2009).
Vlamakis, H., Chai, Y., Beauregard, P., Losick, R. & Kolter, R. Sticking together: building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 11, 157–168, doi: 10.1038/nrmicro2960 (2013).
Shen, H.-H., Thomas, R. K., Penfold, J. & Fragneto, G. Destruction and Solubilization of Supported Phospholipid Bilayers on Silica by the Biosurfactant Surfactin. Langmuir 26, 7334–7342, doi: 10.1021/la904212x (2010).
Deleu, M. et al. Effects of surfactin on membrane models displaying lipid phase separation. Biochim. Biophys. Acta 1828, 801–815, doi: 10.1016/j.bbamem.2012.11.007 (2013).
Korenblum, E. et al. Purification and characterization of a surfactin-like molecule produced by Bacillus sp H2O-1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol. 12, 252–265, doi: 25210.1186/1471-2180-12-252 (2012).
Snook, M. E., Mitchell, T., Hinton, D. M. & Bacon, C. W. Isolation and Characterization of Leu7-Surfactin from the Endophytic Bacterium Bacillus mojavensis RRC 101, a Biocontrol Agent for Fusarium verticillioides. J. Agric. Food Chem. 57, 4287–4292, doi: 10.1021/jf900164h (2009).
Zhi, Y., Wu, Q., Du, H. & Xu, Y. Biocontrol of geosmin-producing Streptomyces spp. by two Bacillus strains from Chinese liquor. Int. J. Food Microbiol. 231, 1–9, doi: 10.1016/j.ijfoodmicro.2016.04.021 (2016).
Carrillo, C., Teruel, J. A., Aranda, F. J. & Ortiz, A. Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim. Biophys. Acta 1611, 91–97, doi: 10.1016/S0005-2736(03)00029-4 (2003).
Cooper, D. G., Macdonald, C. R., Duff, S. J. B. & Kosaric, N. Enhanced Production of Surfactin from Bacillus subtilis by Continuous Product Removal and Metal Cation Additions. Appl. Environ. Microbiol. 42, 408–412 (1981).
Li, X. et al. Overexpression of specific proton motive force-dependent transporters facilitate the export of surfactin in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 1–11 (2015).
Ohno, A., Ano, T. & Shoda, M. Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus subtilis in solid state fermentation. Biotechnol. Bioeng. 47, 209–214 (1995).
Jacques, P. et al. In Twentieth Symposium on Biotechnology for Fuels and Chemicals. 223–233 (Springer) (1998).
Wei, Y.-H. & Chu, I.-M. Mn2+ improves surfactin production by Bacillus subtilis. Biotechnol. Lett. 24, 479–482 (2002).
Wei, Y. H., Wang, L. F. & Chang, J. S. Optimizing iron supplement strategies for enhanced surfactin production with Bacillus subtilis. Biotechnol. Prog. 20, 979–983 (2004).
Abdel-Mawgoud, A. M., Aboulwafa, M. M. & Hassouna, N. A.-H. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 150, 289–303, doi: 10.1007/s12010-008-8153-z (2008).
Gong, G. et al. Enhanced production of surfactin by Bacillus subtilis E8 mutant obtained by ion beam implantation. Food Technol. Biotechnol. 47, 27 (2009).
Jung, J. et al. Improvement of surfactin production in Bacillus subtilis using synthetic wastewater by overexpression of specific extracellular signaling peptides, comX and phrC. Biotechnol. Bioeng. 109, 2349–2356, doi: 10.1002/bit.24524 (2012).
Willenbacher, J. et al. Substitution of the native srfA promoter by constitutive P in two B. subtilis strains and evaluation of the effect on Surfactin production. J. Biotechnol. 224, 14–17, doi: 10.1016/j.jbiotec.2016.03.002 (2016).
Arima, K., Kakinuma, A. & Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillussubtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 31, 488–494 (1968).
López, D., Vlamakis, H., Losick, R. & Kolter, R. Paracrine signaling in a bacterium. Genes Dev. 23, 1631–1638, doi: 10.1101/gad.1813709 (2009).
Caboche, S. et al. NORINE: a database of nonribosomal peptides. Nucleic Acids Res. 36, D326–D331, doi: 10.1093/nar/gkm792 (2008).
Mendez, C. & Salas, J. A. The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Res. Microbiol. 152, 341–350, doi: 10.1016/s0923-2508(01)01205-0 (2001).
Tsuge, K., Ohata, Y. & Shoda, M. Gene yerP, involved in surfactin self-resistance in Bacillus subtilis. Antimicrob. Agents Chemother. 45, 3566–3573, doi: 10.1128/aac.45.12.3566-3573.2001 (2001).
Morimoto, T. et al. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 15, 73–81 (2008).
Baumgart, M. et al. Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl. Environ. Microbiol. 79, 6006–6015 (2013).
Suarez-Vega, A. et al. Characterization and Comparative Analysis of the Milk Transcriptome in Two Dairy Sheep Breeds using RNA Sequencing. Sci. Rep. 5, doi: 10.1038/srep18399 (2015).
Zhang, N. et al. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics 16, 1–20, doi: 10.1186/s12864-015-1825-5 (2015).
Podgornaia, A. I. & Laub, M. T. Determinants of specificity in two-component signal transduction. Curr. Opin. Microbiol. 16, 156–162, doi: 10.1016/j.mib.2013.01.004 (2013).
Reents, H., Münch, R., Dammeyer, T., Jahn, D. & Härtig, E. The Fnr Regulon of Bacillus subtilis. J. Bacteriol. 188, 1103–1112, doi: 10.1128/jb.188.3.1103-1112.2006 (2006).
Nakano, M. M., Dailly, Y. P., Zuber, P. & Clark, D. P. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J. Bacteriol. 179, 6749–6755 (1997).
Davis, D. A., Lynch, H. C. & Varley, J. The production of Surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme Microb. Technol. 25, 322–329, doi: 10.1016/S0141-0229(99)00048-4 (1999).
Koglin, A. et al. Structural basis for the selectivity of the external thioesterase of the surfactin synthetase. Nature 454, 907–U968, doi: 10.1038/nature07161 (2008).
Dintner, S., Heermann, R., Fang, C., Jung, K. & Gebhard, S. A sensory complex consisting of an ATP-binding cassette transporter and a two-component regulatory system controls bacitracin resistance in Bacillus subtilis. J. Biol. Chem. 289, 27899–27910, doi: 10.1074/jbc.M114.596221 (2014).
Dintner, S. et al. Coevolution of ABC transporters and two-component regulatory systems as resistance modules against antimicrobial peptides in Firmicutes Bacteria. J. Bacteriol. 193, 3851–3862, doi: 10.1128/jb.05175-11 (2011).
Priest, F. G., Goodfellow, M., Shute, L. A. & Berkeley, R. C. W. Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Bacteriol. 37, 69–71, doi: 10.1099/00207713-37-1-69 (1987).
Doroghazi, J. R. et al. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat. Chem. Biol. 10, 963–968, doi: 10.1038/nchembio.1659 (2014).
Sieber, S. A. & Marahiel, M. A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 105, 715–738, doi: 10.1021/cr0301191 (2005).
Michna, R. H., Commichau, F. M., Toedter, D., Zschiedrich, C. P. & Stuelke, J. SubtiWiki-a database for the model organism Bacillus subtilis that links pathway, interaction and expression information. Nucleic Acids Res. 42, D692–D698, doi: 10.1093/nar/gkt1002 (2014).
Hayashi, K., Ohsawa, T., Kobayashi, K., Ogasawara, N. & Ogura, M. The H2O2 stress-responsive regulator PerR positively regulates srfA expression in Bacillus subtilis. J. Bacteriol. 187, 6659–6667, doi: 10.1128/jb.187.19.6659-6667.2005 (2005).
Hayashi, K., Kensuke, T., Kobayashi, K., Ogasawara, N. & Ogura, M. Bacillus subtilis RghR (YvaN) represses rapG and rapH, which encode inhibitors of expression of the srfA operon. Mol. Microbiol. 59, 1714–1729, doi: 10.1111/j.1365-2958.2006.05059.x (2006).
Chumsakul, O. et al. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res. 39, 414–428, doi: 10.1093/nar/gkq780 (2011).
Serror, P. & Sonenshein, A. L. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178, 5910–5915 (1996).
Coutte, F. et al. Modeling leucine’s metabolic pathway and knockout prediction improving the production of surfactin, a biosurfactant from Bacillus subtilis. Biotechnology journal 10, 1216–1234 (2015).
Zhang, Y., Nakano, S., Choi, S. Y. & Zuber, P. Mutational analysis of the Bacillus subtilis RNA polymerase alpha C-terminal domain supports the interference model of Spx-dependent repression. J. Bacteriol. 188, 4300–4311, doi: 10.1128/jb.00220-06 (2006).
Hatfield, G. W., Hung, S. P. & Baldi, P. Differential analysis of DNA microarray gene expression data. Mol. Microbiol. 47, 871–877 (2003).
Salzberg, L. I. et al. Genome-wide analysis of phosphorylated PhoP binding to chromosomal DNA reveals several novel features of the PhoPR-mediated phosphate limitation response in Bacillus subtilis. J. Bacteriol. 197, 1492–1506, doi: 10.1128/jb.02570-14 (2015).
Hoffman, C. S. & Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformaion of Escherichia coli. Gene 57, 267–272, doi: 10.1016/0378-1119(87)90131-4 (1987).
Miller, J. R., Koren, S. & Sutton, G. Assembly algorithms for next-generation sequencing data. Genomics 95, 315–327, doi: 10.1016/j.ygeno.2010.03.001 (2010).
Boetzer, M., Henkel, C. V., Jansen, H. J., Butler, D. & Pirovano, W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27, 578–579, doi: 10.1093/bioinformatics/btq683 (2011).
Boetzer, M. & Pirovano, W. Toward almost closed genomes with GapFiller. Genome Biol. 13, 9, doi: 10.1186/gb-2012-13-6-r56 (2012).
Delcher, A. L., Harmon, D., Kasif, S., White, O. & Salzberg, S. L. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27, 4636–4641, doi: 10.1093/nar/27.23.4636 (1999).
Tatusov, R. L. et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, doi: 10.1186/1471-2105-4-41 (2003).
Schattner, P., Brooks, A. N. & Lowe, T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689, doi: 10.1093/nar/gki366 (2005).
Lagesen, K. et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35, 3100–3108, doi: 10.1093/nar/gkm160 (2007).
Dhillon, B. K. et al. IslandViewer 3: more flexible, interactive genomic island discovery, visualization and analysis. Nucleic Acids Res. 43, W104–W108, doi: 10.1093/nar/gkv401 (2015).
Bezuidt, O. K. Oleg ON Reva, and Gipsi Lima Mendez. SeqWord Gene Island Sniffer: A program to study the lateral genetic exchange among bacteria. World Academy of Science, Engineering and Technology 58, 410–415 (2009).
Zhou, Y., Liang, Y., Lynch, K. H., Dennis, J. J. & Wishart, D. S. PHAST: A Fast Phage Search Tool. Nucleic Acids Res. 39, W347–W352, doi: 10.1093/nar/gkr485 (2011).
Treangen, T. J. & Messeguer, X. GCAT: interactively and efficiently constructing large-scale multiple genome comparison frameworks in closely related species. BMC Bioinformatics 7, doi: 10.1186/1471-2105-7-433 (2006).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–U354, doi: 10.1038/nmeth.1923 (2012).
Frazee, A. C. et al. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat Biotech 33, 243–246, doi: 10.1038/nbt.3172 (2015).
Wang, L. K., Feng, Z. X., Wang, X., Wang, X. W. & Zhang, X. G. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138, doi: 10.1093/bioinformatics/btp612 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate-A pratical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological 57, 289–300 (1995).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402, doi: 10.1093/nar/25.17.3389 (1997).
Ota, S. et al. Highly efficient lipid production in the green alga Parachlorella kessleri: draft genome and transcriptome endorsed by whole-cell 3D ultrastructure. Biotechnol. Biofuels 9, 10, doi: 10.1186/s13068-016-0424-2 (2016).
Acknowledgements
The work was supported by the National High Technology Research and Development Program of China (2013AA102108), National Natural Science Foundation of China (31371822, 31530055), Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06), Research and Innovation Program for Graduate Students of Jiangsu Province, China (2014 KYLX_1144).
Author information
Authors and Affiliations
Contributions
Y.Z., Q.W., and Y.X. designed the research. Y.Z. performed the experiments. Y.Z., Q.W., and Y.X. performed the analysis and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhi, Y., Wu, Q. & Xu, Y. Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci Rep 7, 40976 (2017). https://doi.org/10.1038/srep40976
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40976
This article is cited by
-
Metabolic cooperation between conspecific genotypic groups contributes to bacterial fitness
ISME Communications (2023)
-
Genome Sequencing and Genetic Engineering Reveal the Contribution of Bacitracin Produced by Bacillus paralicheniformis CPL618 to Anti-Staphylococcus aureus Activity
Current Microbiology (2023)
-
Spent coffee grounds as feedstock for the production of biosurfactants and the improved recovery of melanoidins
World Journal of Microbiology and Biotechnology (2023)
-
A review on surfactin: molecular regulation of biosynthesis
Archives of Microbiology (2023)
-
Entrapment and Delivery of Doxorubicin: Employing a Permeable Lipopeptide-Based Hydrogel as an Efficient Cationic Binder
Applied Biochemistry and Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.