Abstract
In order to examine the difference in brain structure between obese and normal weight individuals, and to explore the relationship between the neuroanatomical changes and impulsivity traits, this study used a voxel-based morphometry method to examine gray matter (GM) volume alterations related to impulsive personality traits in obese individuals relative to normal weight. Eighty adults that completed the UPPS-P Impulsive Behavior Scale were analyzed. Possible GM volume alterations were first analyzed at the whole brain level, and then the relationship between regional GM volume differences and UPPS-P scores were examined in selected regions of interest. Reduced GM volumes were found in the frontal and limbic regions in the obese group compared to normal weight individuals. In the normal weight group, lack of perseverance was negatively correlated with GM volume in the anterior cingulate cortex, and negative urgency was negatively correlated with GM volume in the insula. In the obese group, sensation seeking was negatively correlated with GM volume in the left amygdala and right pallidum. These findings might improve our understanding of the relationship between lack of perseverance, negative urgency, and sensation seeking and body weight fluctuations.
Similar content being viewed by others
Introduction
Obesity is a major health hazard of modern society and promotes co-morbid diseases1,2. For example, it is documented that a body mass index (BMI) of 30–35 kg/m2 reduces life expectancy by two to four years3. Overconsumption of calorie-dense foods, depression and anxiety, side effects of pharmaceuticals, or genetics all may be causal factors for obesity4. Impulsive personality trait is also documented to contribute to obesity5. Impulsivity towards food has been indicated for increased food intake in obese people, and appears more pronounced in people with binge eating disorder2,6,7,8. An impulsive personality predicts a heightened food intake and body fat in women9,10. Urgency is negatively related to self-control on eating11,12. Negative urgency is associated with food addiction directly, and this link is responsible for the relationship between food addiction and BMI13. Lack of perseverance is related to weight fluctuation12. Overweight and obese people have higher levels of urgency, lack of perseverance and sensation seeking14. Neuroanatomical investigations on personal impulsivity traits on the presentation of obesity may help inform the neural basis of impulsivity and ultimately benefit obesity prevention.
Neuroimaging studies reveal that impulsivity involves brain regions important in reward reinforcement and response inhibition. Brain anatomical studies have identified gray matter (GM) atrophy in impulsive individuals in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), medial prefrontal cortex, and amygdala15. A study notes that a smaller OFC volume in healthy subjects relates to high impulsivity16. Functional neuroimaging evidence also pinpoints several brain regions corresponding to impulsiveness, including the OFC, inferior frontal gyrus, ventrolateral and dorsolateral prefrontal cortices, ACC, amygdala, ventral pallidum, insula and hippocampus17,18,19,20. In addition, pallidum activation during reward anticipation has been demonstrated to be correlated with impulsivity in alcoholics21.
Structural imaging studies also have uncovered lower total GM volumes and reduced regional GM volumes in the OFC of the obese relative to lean controls11,12. Moreover, Yokum et al. found that BMIs are correlated with volume changes in brain regions involved in reward processing and somatosensory processing22, whereas reduced regional GM volumes in the prefrontal cortex are correlated with higher rates of BMI increase22. Impulsivity plays an important role in weight gain. Maayan et al. reported that obese individuals are characterized by increased disinhibition and reduced cognitive control, and that both traits are correlated with reduced GM volumes in the OFC23. Ralph et al. observed an enlarged amygdala in obese subjects, which implicated the importance of the hedonic effect in the regulation of feeding24. These findings suggest that volumetric brain measures are useful to characterize the neurobiological underpinnings of obesity and that brain structural volumes are associated with certain disease-specific features (e.g., BMI). In the context of the current study, there is a knowledge gap in the link between brain structural alterations and impulsive traits in the presentation of adult obesity.
The objectives of this investigation are to examine the differences in brain structures between obese and normal weight individuals, and to explore the relationship between neuroanatomical changes and impulsivity traits. We hypothesize that lack of perseverance, negative urgency and sensation seeking has different links with these neuroanatomical alterations in normal weight and obese groups.
Results
Participants’ demographic characteristics
The demographic characteristics for the normal weight and obese groups are summarized in Table 1. The groups were matched for gender, handedness, depression status, cognitive restraint on eating and disinhibition of control. However, the obesity subjects had higher hunger scores than the normal weight group. Given that the age of the two groups was significantly different, it was used as a covariate in a further analysis.
Trait impulsivity measures
There were no significant between group differences in lack of premeditation, lack of perseverance, sensation seeking, negative urgency and positive urgency (Table 1).
MRI Imaging analysis
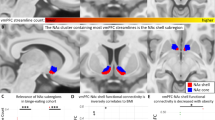
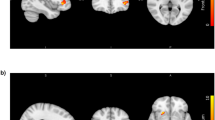
The obese group had significantly lower GM volumes than the normal weight group in the left inferior frontal gyrus (BA 13), bilateral insula (BA 13), left pyramis, inferior semi-lunar lobule and cerebellar tonsil, bilateral medial frontal gyrus (BA 10), right anterior cingulate cortex (BA 32), bilateral thalamus and left middle frontal gyrus (BA 6) (Table 2, Fig. 1). On the other hand, the obese group showed significantly higher GM volumes in the left inferior occipital gyrus and middle occipital gyrus than in the normal weight group (Table 2, Fig. 1).
Correlation analyses between GM and personality
Lack of perseverance was negatively correlated with GM volume in the anterior cingulate in the normal weight group (R = −0.372, p = 0.009) but not in the obesity group. Negative urgency was negatively correlated with GM volume within the insula in the normal weight group (R = −0.364, p = 0.010) but not in the obesity group. Sensation seeking was negatively correlated with GM volume in the left amygdala (R = −0.414, p = 0.010) and right pallidum (R = −0.448, p = 0.010) in the obese group.
Discussion
In the current study, we examined the difference in brain structures between obese and normal weight individuals, and explored the relationship between neuroanatomical changes and impulsivity traits. The imaging analysis found a significant group difference in brain regions modulating impulsivity such as the ventromedial prefrontal cortex, anterior cingulate cortex, insula and thalamus. The anterior cingulate cortex and insula, which constitute the salience network, possess a significant link with a lack of perseverance and negative urgency in the normal group respectively. In addition, the left amygdala and right pallidum showed a close relationship with sensation seeking in the obese group. These results showed a different relationship between these neuroanatomical differences and impulsivity traits in normal weight and obese individuals.
In the current study, we did not find a significant group difference in cognitive restraint, disinhibition, and depression status. Some study indicated that obese subjects had poor cognitive control and high disinhibition23. However, another study also documented that obese subjects may attempt to curb the intake of high-calorie foods because they are aware of the weight gain effect25. However, in our study, there were 19 subjects’ BMIs that ranged from 30 to 35, 8 subjects’ BMIs that ranged from 35 to 40, and 4 subjects’ BMIs that ranged from 40 to 43.40. The missing significant group difference in cognitive restraint and disinhibition may be partly related to the low ratio of morbid obesity. A longitudinal study suggested that baseline obesity increased the risk of depression, and depression also promoted the odds for being overweight26. We need more information about the participant’s history of being obese to explain the depression and weight status. We discuss this in the limitation parts.
In line with previous studies27,28,29,30,31, the obese group showed decreased GM volumes in the frontal, limbic and cerebellum cortices. A previous study demonstrated that the anterior insula takes part in processing the taste, smell, texture, and fat content of foods27. One PET study documented that obese individuals have greater response to food tastes than lean individuals28. The insula, anterior cingulate cortex and medial frontal gyrus receive various homeostatic and salience information. Some studies found that the response of the ACC was negatively correlated with disinhibition, and obese individuals had less activation in the ACC than normal-weight participants29,30. GM volume of the thalamus has been suggested to be negatively associated with body fat content31. These findings showed decreased brain structures responding to sensory and salience processing of food in obese individuals, which were consistent with the reward-deficiency theory32.
In the current study, lack of perseverance was negatively correlated with GM volume in the ACC in the normal weight group. Lack of perseverance refers to failing to maintain focus on difficult or boring tasks33. It possesses the important relationship in decision making, for example, subjects with a low perseverance score learn more slowly in choosing from the good decks during the gambling task34. The ACC is involved in attention control and decision-making29,35,36. It plays a key role in cognitive control and reward expectation during food intake37,38. The negative association between lack of perseverance and GM volume is consistent with the items mentioned above. However, for the obese subjects, this link may be broken, suggesting that there is a change in the neural mechanism underlying cognitive control in obese people.
Negative urgency was negatively correlated with GM volume in the insula in the normal weight group but not in the obesity group. Negative urgency refers to losing control over their behavior when experiencing strong negative emotions33. Individuals with a high level of negative urgency had a high tendency to engage in addictive behaviors, such as consumption of alcohol and drugs39,40. Negative urgency is significantly associated with food addiction directly, and this link is also responsible for their relationship between food addiction and BMI13. A wealth of neuroimaging data on eating behavior indicated that difficulties in the regulation of food intake may be related to aberrant brain function in the insular cortex41. The insula expresses the feeling state that modulates motivational behavior in conjunction with bodily homeostasis42. In addition, an fMRI study found that the response in the insula underlies emotional processing in working memory43 and decision making. For example, its activity is related to the extent of risky decisions during the gambling task44. There is a negative correlation between negative urgency and GM volume in the insula in normal weight subjects, which suggests that the structure of the insula is altered in the obesity group. The insula may fail to regulate the negative emotion causing risky decisions.
Sensation seeking was negatively correlated with the GM volume in the left amygdala and right pallidum in the obese group. Sensation seeking is a personality trait to search for new experiences and feelings. It is associated with a tendency to strengthen the impact of rewards to food. Overweight and obese persons have higher levels of sensation seeking14. This has been positively correlated with preferences for unhealthy foods45. Amygdala and pallidum are implicated in the rewarding effect of food. Amygdala is involved in the conditioned response to food, and pallidum selectively responds to a cue predicting reward availability46. In line with the previous study, we found that GM volumes of the amygdala and pallidum were reduced in the obese group47, suggesting of a modulation of the alterations of the conditional response to food in obese people. The negative association between sensation seeking and GM volume of the amygdala and pallidum may further indicate that the conditional regulation is more important in obese people.
Limitations
It is important to consider the limitations of this study. First, this study was designed to compare the GM volume alterations in obese subjects and to explore the relationship between the possible change and impulsivity traits. There is no group difference in cognitive restraint, disinhibition, and depression status, which may be partly related to the low ratio of morbid obesity and relatively small sample size. Studies with a larger body of subjects will increase the statistical power. As a cross-sectional design, the present study cannot infer causality of the relationships between GM volume alterations and impulsivity traits, which may be bi-directional and related to some latent variables. Further longitudinal studies may be required to improve the understanding of the link between GM volume alterations and impulsivity traits. Second, there is a significant group difference in age. The previous study has documented that there is an age difference in the impulsivity trait48. Although age is included in the analysis as a covariate, the potential influence of age should be further noted. For example, what is the relationship between BMI and impulsivity change modulated by age? We need more information to answer this question. Further studies may be required to answer this question.
Conclusion
In the current study, we investigated the difference in brain structures between obese and normal weight individuals, and explored the relationship between the neuroanatomical changes and impulsivity traits. Compared with normal weight controls, obese subjects showed reduced GM volume in cortices responding to reward and salience encoding. The impulsivity traits showed different relationships with brain regions between obese and normal weight control patients.
Methods
Participants and MRI acquisition
MRI data were obtained from an open data website (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html), provided by the Center for Advanced Brain Imaging of the Nathan S. Kline Institute49. The imaging data of eighty adults (18–55 years old) were analyzed in the current study. The participants were initially classified as normal weight adults (n = 49, mean BMI = 21.87, SE = 0.29) and obese (n = 31, mean BMI = 34.38, SE = 0.69) according to their BMI following the International Obesity Task Force (IOTF) criteria14,15. All of the subjects possessed no history of psychiatric disorders or any neurological illnesses. Informed written consent was obtained prior to the image scans. The scans were conducted in accordance with the Institutional Review Board guidelines from the Center for Advanced Brain Imaging of the Nathan S. Kline Institute and in compliance with the Declaration of Helsinki49.
Participants were scanned in a 3.0 T whole body MRI scanner (Siemens MAGNETOM TrioTim Syngo MR). A T1-weighted 3D volume was acquired for each participant using a T1-weighted 3D-turbo-gradient echo sequence in sagittal orientation with a 0.94 × 0.94 × 1.0 mm resolution (200 Transverse slices, FOV = 240 × 240 mm2, matrix 256 × 256), TR = 2500 ms, TE = 3.5 ms, TI = 1200 ms, slice thickness = 1 mm and Flip angle = 8°. The sequence was optimal for reducing field inhomogeneity, susceptibility artifacts and motion sensitivity.
Measure of impulsivity
Impulsivity scores were assessed with a UPPS-P Impulsive Behavior Scale. The UPPS-P Impulsive Behavior Scale33,50 is a 59-item inventory. It was designed to measure five distinct personality pathways characterizing impulsive behaviors: sensation seeking, perseverance, premeditation, negative urgency and positive urgency. We used the scores of the sum of each of these five UPPS–P dimensions for the analyses51. In addition, depression scores were assessed with Beck Depression Inventory-II52. Eating behavior scores were evaluated with the Three Factor Eating Questionnaire53 during fasting state.
GM Volumetric Analysis
All MPRAGE images were pre-processed and analyzed using SPM8 (http://www.fil.ion.ucl. ac.uk/spm). In order to screen for artifacts or gross anatomical abnormalities, each MR image was first displayed in SPM8. Images were reoriented manually to be set to the anterior commissure for better registration. MPRAGE images of each subject were then spatially normalized to the standard T1 Montreal Neurological Institute template and segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using the tissue classification algorithm in SPM8. The segmented partitions were subsequently normalized to their respective standard templates. The normalized, segmented gray matter images were then modulated by calculating the Jacobian determinants derived from the special normalization step, and multiplying each voxel by the relative change in volume54. Finally, images were smoothed with a 3-D Gaussian filter of 8 mm3 full width at half maximum (FWHM) to increase the signal to noise ratio.
ROI analysis
The anatomic region of interest (ROI) was anatomically selected based on previous evidence of its involvement in adult obesity, including the OFC, amygdala, and pallidum18,20. ROIs were created by the Wake Forest University (WFU) PickAtlas toolbox55.
Statistical Analyses
Two groups of subjects, normal weight (BMI < 25) and obese (BMI > 30), were assessed for their GM volume values. The voxel-wise two-sample t-tests were performed to compare the group differences in GM volumes. The resulting statistical maps with a significant level of p = 0.005, cluster size > 50 were identified as activations51. The GM volume values of the significant activations and each ROI (OFC, amygdala and pallidum) were calculated for each subject. To compare structural brain alterations related to impulsive personality traits between the two groups, we used a partial correlation analysis with age, gender and handiness as covariates to analyze the relationship between regional GM volumes of ROIs and the scores of each impulsive personality traits scale (lack of premeditation, lack of perseverance, sensation seeking, negative urgency, and positive urgency). The correlation with a significance of p = 0.010 was further discussed.
Additional Information
How to cite this article: Brain Structural Differences between Normal and Obese Adults and their Links with Lack of Perseverance, Negative Urgency, and Sensation Seeking. Sci. Rep. 7, 40595; doi: 10.1038/srep40595 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Pi-Sunyer, X. The medical risks of obesity. Postgraduate medicine 121, 21–33, doi: 10.3810/pgm.2009.11.2074 (2009).
Flegal, K. M., Graubard, B. I., Williamson, D. F. & Gail, M. H. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama 298, 2028–2037, doi: 10.1001/jama.298.17.2028 (2007).
Collaboration, P. S. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. The Lancet 373, 1083–1096 (2009).
Zhang, Y., M von Deneen, K., Tian, J., S Gold, M. & Liu, Y. Food addiction and neuroimaging. Current pharmaceutical design 17, 1149–1157 (2011).
Provencher, V. et al. Personality traits in overweight and obese women: associations with BMI and eating behaviors. Eat Behav 9, 294–302, doi: 10.1016/j.eatbeh.2007.10.004 (2008).
Schag, K., Schönleber, J., Teufel, M., Zipfel, S. & Giel, K. E. Food-related impulsivity in obesity and Binge Eating Disorder – a systematic review. Obesity Reviews 14, 477–495, doi: 10.1111/obr.12017 (2013).
Meule, A. & Platte, P. Facets of impulsivity interactively predict body fat and binge eating in young women. Appetite 87, 352–357, doi: 10.1016/j.appet.2015.01.003 (2015).
Fischer, S., Settles, R., Collins, B., Gunn, R. & Smith, G. T. The role of negative urgency and expectancies in problem drinking and disordered eating: testing a model of comorbidity in pathological and at-risk samples. Psychology of Addictive Behaviors 26, 112 (2012).
Guerrieri, R. et al. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite 49, 66–73, doi: 10.1016/j.appet.2006.11.008 (2007).
Sutin, A. R., Ferrucci, L., Zonderman, A. B. & Terracciano, A. Personality and obesity across the adult life span. Journal of personality and social psychology 101, 579–592, doi: 10.1037/a0024286 (2011).
Dir, A. L., Karyadi, K. & Cyders, M. A. The uniqueness of negative urgency as a common risk factor for self-harm behaviors, alcohol consumption, and eating problems. Addictive behaviors 38, 2158–2162 (2013).
Mobbs, O., Ghisletta, P. & Van der Linden, M. Clarifying the role of impulsivity in dietary restraint: A structural equation modeling approach. Personality and Individual Differences 45, 602–606 (2008).
Murphy, C. M., Stojek, M. K. & MacKillop, J. Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite 73, 45–50, doi: http://dx.doi.org/10.1016/j.appet.2013.10.008 (2014).
Mobbs, O., Crépin, C., Thiéry, C., Golay, A. & Van der Linden, M. Obesity and the four facets of impulsivity. Patient Education and Counseling 79, 372–377 (2010).
O’Callaghan, C., Shine, J. M., Hodges, J. R., Lewis, S. J. & Hornberger, M. Neural substrates of impulse control: Insights from neurodegenerative disease. Frontiers in Human Neuroscience, doi: 10.3389/conf.fnhum.2013.212.00076.
Matsuo, K. et al. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping 30, 1188–1195, doi: 10.1002/hbm.20588 (2009).
Lee, T. M. et al. Neural correlates of traditional Chinese medicine induced advantageous risk-taking decision making. Brain and cognition 71, 354–361 (2009).
Love, T. M., Stohler, C. S. & Zubieta, J.-K. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Archives of general psychiatry 66, 1124–1134 (2009).
Park, S. Q. et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. The Journal of neuroscience 30, 7749–7753 (2010).
Horn, N. R., Dolan, M., Elliott, R., Deakin, J. F. & Woodruff, P. W. Response inhibition and impulsivity: an fMRI study. Neuropsychologia 41, 1959–1966 (2003).
Beck, A. et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological psychiatry 66, 734–742 (2009).
Yokum, S., Ng, J. & Stice, E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. International journal of obesity (2005) 36, 656–664, doi: 10.1038/ijo.2011.175 (2012).
Maayan, L., Hoogendoorn, C., Sweat, V. & Convit, A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring, Md.) 19, 1382–1387, doi: 10.1038/oby.2011.15 (2011).
Widya, R. L. et al. Increased amygdalar and hippocampal volumes in elderly obese individuals with or at risk of cardiovascular disease. Am J Clin Nutr 93, 1190–1195, doi: 10.3945/ajcn.110.006304 (2011).
Lindroos, A. K. et al. Dietary intake in relation to restrained eating, disinhibition, and hunger in obese and nonobese Swedish women. Obesity research 5, 175–182 (1997).
Luppino, F. S., de Wit, L. M., Bouvy, P. F. et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry 67, 220–229, doi: 10.1001/archgenpsychiatry.2010.2 (2010).
Dagher, A. Functional brain imaging of appetite. Trends in Endocrinology & Metabolism 23, 250–260 (2012).
DelParigi, A., Chen, K., Salbe, A. D., Reiman, E. M. & Tataranni, P. A. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. NeuroImage 24, 436–443, doi: http://dx.doi.org/10.1016/j.neuroimage.2004.08.035 (2005).
Tang, D. W., Fellows, L. K., Small, D. M. & Dagher, A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiology & Behavior 106, 317–324, doi: http://dx.doi.org/10.1016/j.physbeh.2012.03.009 (2012).
Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324, 646–648, doi: 10.1126/science.1168450 (2009).
Katulska, K., Wykrętowicz, M. & Piskorski, J. Brain segmentation unmasks association between body composition and central nervous system structures. Journal of Medical Science 83, 127–131 (2016).
Blum, K., Thanos, P. K. & Gold, M. S. Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in Psychology 5, 919, doi: 10.3389/fpsyg.2014.00919 (2014).
Dvorak, R. D., Pearson, M. R. & Kuvaas, N. J. The five-factor model of impulsivity-like traits and emotional lability in aggressive behavior. Aggressive behavior 39, 222–228, doi: 10.1002/ab.21474 (2013).
Zermatten, A., Van der Linden, M., d’Acremont, M., Jermann, F. & Bechara, A. Impulsivity and decision making. The Journal of nervous and mental disease 193, 647–650 (2005).
Bush, G. et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences 99, 523–528, doi: 10.1073/pnas.012470999 (2002).
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L. & Brownell, K. D. Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience, doi: 10.1093/scan/nst059 (2013).
Hon, N., Epstein, R. A., Owen, A. M. & Duncan, J. Frontoparietal activity with minimal decision and control. J Neurosci 26, 9805–9809, doi: 10.1523/jneurosci.3165-06.2006 (2006).
Hinkle, W., Cordell, M., Leibel, R., Rosenbaum, M. & Hirsch, J. Effects of Reduced Weight Maintenance and Leptin Repletion on Functional Connectivity of the Hypothalamus in Obese Humans. PLoS ONE 8, e59114, doi: 10.1371/journal.pone.0059114 (2013).
Verdejo-García, A., Bechara, A., Recknor, E. C. & Pérez-García, M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug and Alcohol Dependence 91, 213–219, doi: http://dx.doi.org/10.1016/j.drugalcdep.2007.05.025 (2007).
Settles, R. E. et al. Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of abnormal psychology 121, 160 (2012).
Brooks, S. J., Cedernaes, J. & Schiöth, H. B. Increased Prefrontal and Parahippocampal Activation with Reduced Dorsolateral Prefrontal and Insular Cortex Activation to Food Images in Obesity: A Meta-Analysis of fMRI Studies. PLoS ONE 8, e60393, doi: 10.1371/journal.pone.0060393 (2013).
Singer, T., Critchley, H. D. & Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends in cognitive sciences 13, 334–340 (2009).
Levens, S. M. & Phelps, E. A. Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. Journal of cognitive neuroscience 22, 2790–2803 (2010).
Xue, G., Lu, Z., Levin, I. P. & Bechara, A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage 50, 709–716 (2010).
Logue, A. & Smith, M. E. Predictors of food preferences in adult humans. Appetite 7, 109–125 (1986).
Root, D. H., Melendez, R. I., Zaborszky, L. & Napier, T. C. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Progress in Neurobiology 130, 29–70, doi: http://dx.doi.org/10.1016/j.pneurobio.2015.03.005 (2015).
Shott, M. E. et al. Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes 39, 214–221, doi: 10.1038/ijo.2014.121 (2015).
Steinberg, L. et al. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental psychology 44, 1764 (2008).
Nooner, K. B. et al. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Frontiers in Neuroscience 6, doi: 10.3389/fnins.2012.00152 (2012).
Cyders, M. A. et al. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychological assessment 19, 107–118, doi: 10.1037/1040-3590.19.1.107 (2007).
Moreno-Lopez, L., Soriano-Mas, C., Delgado-Rico, E., Rio-Valle, J. S. & Verdejo-Garcia, A. Brain structural correlates of reward sensitivity and impulsivity in adolescents with normal and excess weight. PLoS One 7, e49185, doi: 10.1371/journal.pone.0049185 (2012).
Beck, A. T., Steer, R. A. & Brown, G. K. Beck depression inventory-II. San Antonio, TX, 78204–72498 (1996).
Stunkard, A. J. & Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research 29, 71–83 (1985).
Good, C. D. et al. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 14, 21–36, doi: http://dx.doi.org/10.1006/nimg.2001.0786 (2001).
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
Author information
Authors and Affiliations
Contributions
J.-L.C. and H.-P.L. designed the study. H.-F.W., B.-H.W. did the data analysis and statistical analysis. H.-F.W. and B.-H.W. wrote the main manuscript text. J.-L.C. and H.-P.L. did the manuscript editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, H., Wen, B., Cheng, J. et al. Brain Structural Differences between Normal and Obese Adults and their Links with Lack of Perseverance, Negative Urgency, and Sensation Seeking. Sci Rep 7, 40595 (2017). https://doi.org/10.1038/srep40595
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40595
This article is cited by
-
Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions
Molecular Psychiatry (2023)
-
Abnormalities in deep-brain morphology and orbitofrontal cortical thinning relate to reward processing and body mass in adolescent girls
International Journal of Obesity (2022)
-
Advances in the Neurobiology of Food Addiction
Current Behavioral Neuroscience Reports (2021)
-
Impulsivity and body fat accumulation are linked to cortical and subcortical brain volumes among adolescents and adults
Scientific Reports (2019)
-
Fibroblast growth factor 21 deficiency aggravates obesity-induced hypothalamic inflammation and impairs thermogenic response
Inflammation Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.