Abstract
Non-noble metal nanoparticles are becoming more and more important in catalysis recently. Cu/CuO nanoclusters on highly ordered TiO2 nanotube arrays are successfully developed by a surfactant-free photoreduction method. This non-noble metal Cu/CuO-TiO2 catalyst exhibits excellent catalytic activity and stability for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) with the presence of sodium borohydride (NaBH4). The rate constant of this low-cost Cu/CuO based catalyst is even higher than that of the noble metal nanoparticles decorated on the same TiO2 substrate. The conversion efficiency remains almost unchanged after 7 cycles of recycling. The recycle process of this Cu/CuO-TiO2 catalyst supported by Ti foil is very simple and convenient compared with that of the common powder catalysts. This catalyst also exhibited great catalytic activity to other organic dyes, such as methylene blue (MB), rhodamine B (RhB) and methyl orange (MO). This highly efficient, low-cost and easily reusable Cu/CuO-TiO2 catalyst is expected to be of great potential in catalysis in the future.
Similar content being viewed by others
Introduction
Nitroaromatic compounds are very important industrial chemicals nowadays and are widely used in the manufacture of dyes, plastics, pesticides, and explosives1. The release of these compounds in natural water will cause serious environmental pollution since that most of them are considered as potential toxicity to organisms2. 4-nirtophenol (4-NP) is one of the most common nitroaromatic compounds in industrial effluents and has been classified as priority pollutant by the US Environmental Protection Agency3,4. However, 4-NP is very stable in the environment and hardly to be biodegraded. An efficient and environment friendly method to remove them from waste water is the direct reduction of 4-NP in the presence of NaBH4 and catalyst to 4-aminophenol (4-AP), which is a very important intermediate for the manufacture of analgesic and antipyretic drugs5. It is highly desirable to find an efficient and eco-friendly catalyst for this reduction.
Noble metal nanoparticles such as Au, Ag, Pt, Pd and their alloys are commonly used as catalysts for the reduction of 4-NP in industry because of the high catalytic activity6,7,8,9,10,11,12,13,14, but the expensiveness and rareness of noble metals limit their extensive applications in catalysis. Design and fabrication of non-noble metal catalyst with high activity is becoming increasingly important. Copper (Cu) based composites are receiving more and more attention due to their relatively low cost, large abundance and great catalytic activity15,16,17,18. Recent reports have shown that Cu15,19,20, Cu2O16,21,22 and CuO17,23,24 nanostructures exhibited excellent catalytic activities comparable to or even higher than that of noble metals. But the aggregation of metal nanoparticles during reaction usually leads to the rapid decrease of catalytic activity25,26 and the recycle process of these small catalysts by repeating centrifuging and washing is time-consuming and inefficient15. These Cu-based catalysts are still suffering from the stability and reusability problems. It is highly demanded to design a nano-catalyst with high activity that can prevent the aggregation and also be easily recycled.
Herein, we fabricate Cu/CuO-TiO2 catalyst by decorating non-noble Cu/CuO nanoclusters on highly ordered TiO2 nanotube (NT) arrays through a surfactant-free photoreduction method27,28. This novel catalyst exhibits several advantages in catalytic application: (1) cost-efficient. These Cu/CuO nanoclusters based catalyst are much cheaper than noble metals; (2) high activity. This catalyst shows excellent catalytic activity towards 4-NP attributed to the sufficient “clean” surfaces of Cu/CuO nanoclusters provided by the surfactant-free photoreduction method; (3) great stability. This Cu/CuO-TiO2 catalyst exhibits excellent stability because the in-situ formed nanoclusters are firmly combined with the TiO2 NT substrate, which prevents the aggregation and loss of the nanoclusters effectively; (4) easy to recycle. The recycle process of Cu/CuO-TiO2 is simple by only removing the TiO2/Ti foil supported catalysts out of the reaction media with tweezers and rinsed with DI water; (5) universal. The Cu/CuO-TiO2 catalyst also exhibits great catalytic activity to other dyes including methylene blue (MB), rhodamine B (RhB) and methyl orange (MO).
Materials and Methods
Materials
The Ti foil was purchased from Sigma-Aldrich (99.7%, 0.127 mm). Ethylene glycol (EG), sodium borohydride (NaBH4), copper chloride (CuCl2), chloroauric acid (HAuCl4), and palladium chloride (PdCl2) were from Sinopharm Chemical Reagent Co., Ltd, China. Ammonia fluoride (NH4F), 4-NP, MB, RhB, MO, silver nitrate (AgNO3) and ethanol were obtained from Beijing Regent Co. China. Deionized (DI) water was used throughout the experiments with a resistivity of 18.2 MΩ cm.
Instrument
Constant voltage for anodization of Ti was conducted on a SAKO DC power supply. The photoreduction was performed on a 300 W Xe lamp illumination. X-ray diffraction (XRD) data were collected by a D8 advanced Bragg-Brentano diffractometer (Bruker AXS, Germany). Morphologies were characterized by a JEM-6700F (JEOL, Japan) scanning electron microscope (SEM). Transmission electron microscopy (TEM) images were acquired by a JEM-2100F transmission electron microscope (JEOL, Japan). X-ray photoelectron spectroscopy (XPS) data were acquired with an ESCALAB-250 instrument (Thermo Fisher Scientific, USA). The UV-Vis adsorption spectra were recorded on a USB4000 UV–Vis spectrophotometer (Ocean Optics Inc., US).
Preparation of TiO2 Nanotube
Two-step anodization of Ti foil was carried out to fabricate the highly ordered TiO2 nanotube (NT) arrays29,30. Firstly, the Ti foil was degreased by sonication in acetone and ethanol, followed by rinsing with water and drying with nitrogen. Then, anodization was carried out using a conventional two-electrode system. Ti foil was working as anode and Pt gauze as cathode. The electrolyte was ethylene glycol including 0.3 wt% NH4F and 2 vol% DI water. The temperature of the reaction was kept at 25 °C by a circulating water bath. The Ti foil was first anodized under a constant voltage of 60 V for 1 h, leading to the formation of irregular TiO2 NT arrays. These irregular arrays were ultrasonically removed in DI water and the same Ti foil was anodized again under 40 V for 1 h. After this second anodization, highly ordered and regular TiO2 NT arrays were formed on top of the Ti foil. Compared to the irregular NT arrays, these two-step-fabricated highly ordered TiO2 NT arrays can provide greater absorption of incident light31 and offer more suitable nucleation sites for metal nanoparticles during the photoreduction as discussed in our previous work30. The anodized TiO2 substrates were rinsed with ethanol, dried with pure nitrogen and finally annealed in air at 480 °C for 3 h with a heating rate of 5 °C/min30,32.
Cu/CuO Nanoclusters Decoration
In-situ surfactant-free photoreduction method was carried out for the decoration of “clean” Cu/CuO nanoclusters on top of the ordered TiO2 NT arrays27,28. First, different concentration of CuCl2 aqueous (0.02 mM, 0.1 mM and 0.5 mM) contained 5 vol% ethanol were prepared and saturated with N2. Then, TiO2 NT substrates were soaked in these CuCl2 aqueous for 30 min for the absorption of Cu2+ onto the surface. Finally, it was irradiated in-situ with a 300 mW/cm2 white light for 90 min to reduce the absorbed Cu2+ into Cu by the photocatalysis of TiO2. The newly formed Cu nanoclusters are very easy to be oxidized exposed in air and thin CuO passivation layers are evenly formed on the surfaces. The as-prepared Cu/CuO-TiO2 samples were rinsed with DI water, dried with nitrogen flow. The sample prepared in 0.02 mM, 0.1 mM and 0.5 mM CuCl2 solution is denoted as C-0.02, C-0.1 and C-0.5, respectively.
Other noble metal nanoparticles, such as Au, Ag and Pd were also prepared onto the TiO2 NT arrays for comparison using the same photoreduction method in 0.1 mM HAuCl4, AgNO3 and PdCl2, respectively.
Catalysis Procedures
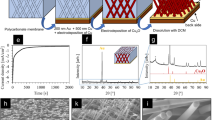
The reduction of 4-NP to 4-aminophenol (AP) in the presence of NaBH4 was performed to test the catalytic activity of Cu/CuO-TiO2 catalyst. Typically, the reaction was carried out in a quartz cuvette at room temperature under stirring and monitored by a UV-vis spectrophotometer. 0.25 ml 4-NP aqueous solution (1 mM) and 0.25 ml freshly prepared NaBH4 (100 mM) were mixed with 2 ml DI water. Subsequently, a 0.6 × 0.6 cm2 Cu/CuO-TiO2 catalyst was soaked in the solution and the absorption spectrum was recorded every 0.5 minute. Excess of NaBH4 was used to eliminate the influence of BH4− on the reaction. In the recycle test, the catalyst was easily taken out of the solution by tweezers, rinsed with DI water, dried with pure N2 and reused for the second cycle directly. Figure 1 shows the fabrication and reusability schematic of the catalyst for the 4-NP reduction with NaBH4. Reduction of other organic dyes, such as MB, RhB and MO were also performed using Cu/CuO-TiO2 as catalyst in the same condition excepted for the concentration, which were adjusted to 0.1 mM to avoid the over-ranging during UV-vis spectrophotometer monitored.
Results and Discussion
Characterization
SEM and XRD characterizations were firstly carried out to investigate the morphologies and compositions of Cu/CuO-TiO2 catalysts fabricated in different concentration of CuCl2. SEM images of TiO2 nanotube arrays without Cu/CuO decoration are shown in Supplementary Fig. S1. Figure 2a,b and c are the top view SEM images of C-0.02, C-0.1 and C-0.5, respectively. The concentration of CuCl2 shows significant influence on the morphology of the catalyst. The size distributions of Cu/CuO on these catalysts are shown in Supplementary Fig. S2. For catalyst C-0.02, Cu/CuO nanoclusters with average size of approximate 81 nm are formed and dispersed on top of TiO2 substrate after photoreduction in 0.02 mM CuCl2 as shown in Fig. 2(a). Increasing the concentration of CuCl2 to 0.1 mM led to the formation of larger Cu/CuO nanoclusters with average size of 158 nm consisted of small nanoparticles with size of 50~80 nm. The amount of nanoclusters distributed on TiO2 is also increased apparently as in Fig. 2(b). Further increase the concentration of CuCl2 to 0.5 mM, Cu/CuO nanocrystal instead of nanoclusters with average size of more than 220 nm are formed in large amount and covered the surface of TiO2 NT arrays mostly as observed in Fig. 2(c). The XRD patterns of TiO2, C-0.02, C-0.1 and C-0.5 are shown in Fig. 2(d). The diffraction peaks of Ti and anatase30,33,34 are clearly observed on all the samples. No obvious peaks assigned to Cu are observed on TiO2 substrate or catalyst C-0.02 with very small size and amount of Cu nanoclusters. Weak diffraction peaks at 43.6° and 50.7° assigned to Cu are observed for catalyst C-0.1. Catalyst C-0.5 shows much clearly diffraction peaks of Cu because of the large size and crystallinity of the Cu15. There are no diffraction peaks of copper oxide shown in Fig. 2(d) because of the tiny amount of CuO formed on the surfaces of Cu nanoclusters.
Figure 3(a) shows the TEM image of a Cu/CuO nanoparticle scratched off the TiO2 nanotube. Slight amount of the residual TiO2 are observed around the particle as marked. The size of the nanoparticle is about 70 nm, which is in agreement with the SEM images in Fig. 2(b). A thin layer of CuO on the surface of the nanoparticle is also observed as expected. High resolution TEM image of the marked region is shown in Fig. 3(b) and the crystal lattice fringes of Cu and CuO are clearly observed. The measured lattice spacing inside the nanoparticle is 0.182 nm corresponding to the (200) plane of Cu19 and the lattice spacing on the edge of the nanoparticle is 0.234 nm corresponding to the (111) plane of CuO35. The TEM characterization certainly proved that the surfaces of Cu were oxidized in air and formed a CuO protective layer with thickness of about 5 nm.
XPS measurement was further performed to confirm the surface composition and the elemental chemical states of the catalyst. Figure 4 shows the XPS spectra of C-0.1. The full XPS survey spectrum shown in Fig. 4(a) proves the presence of Cu, O and Ti in the catalyst, which is also confirmed by the electron dispersed spectroscopy (EDS) characterization shown in Supplementary Fig. S3. Cu 2p XPS spectrum is depicted in Fig. 4(b). Major peaks of Cu 2p3/2 at 932.5 eV and Cu 2p1/2 at 952.3 eV confirmed the existed of metallic copper20,21,36, demonstrated the successful photoreduction of CuCl2. Meanwhile, the peaks of Cu 2p3/2 at 934.6 eV and Cu 2p1/2 at 954.6 eV in combination with the satellite peaks at 941.5 eV and 944.3 eV are typical characteristics of CuO20,36,37, implying the uniformly surface oxidation of Cu nanoclusters exposed in air under ambient conditions. These results are consistent with the TEM characterization. O 1 s spectrum shown in Fig. 4(c) consists of two peaks. The major peak at 530.0 eV corresponds to O2− in CuO and TiO2. The secondary peak at 531.6 eV is attributed to the oxygen species adsorbed on the surface16,38. Ti 2p spectrum is shown in Fig. 4(d). The peaks of Ti 2p3/2 at 458.8 eV and Ti 2p1/2 at 464.5 eV are consistent with the typical TiO238. The result of XPS measurement further proved the existence of Cu/CuO composite on TiO2 substrate.
Catalytic Activity
Reduction of 4-NP to 4-aminophenol (AP) with NaBH4 was first carried out to evaluate the catalytic activity of Cu/CuO-TiO2. As shown in Supplementary Fig. S4, 4-NP aqueous solution exhibits a strong absorption peak at 316 nm, which remarkably shifted to 400 nm after the addition of NaBH4 due to the formation of 4-nitrophenolate ions under alkaline conditions in Fig. S4(a)26,39. This absorption peak remains unchanged in more than 30 minutes in Fig. S4(b) indicated that the reduction reaction did not proceed without catalyst. Figure S4(c) demonstrated that the reduction is still unable to proceed with only TiO2 NT substrate dipped into the solution. After Cu/CuO nanoclusters decoration, the Cu/CuO-TiO2 catalysts exhibit great catalytic activity towards 4-NP.
The time-dependent UV-vis absorption spectra for the reduction of 4-NP with C-0.02, C-0.1 and C-0.5 were compared in Fig. 5(a~c). The reduction started immediately after the immersion of the catalysts with no need of induction time and the absorption peak of 4-NP at 400 nm gradually decreased as the reaction proceeded. Meanwhile, new absorption peak of 4-AP at 300 nm appears and gradually increases. Isosbestic point between these two absorption peaks is also shown in the absorption spectra, indicating the clean conversion from 4-NP to 4-AP without any byproducts19. Catalyst C-0.1 exhibits the best catalytic activity for 4-NP, of which the reduction reaction is completed within 3.5 minutes as shown in Fig. 5(b). For catalyst C-0.02 and C-0.5, it takes more than 5 minutes to complete the reaction as shown in Fig. 5(a) and (c). The amount of NaBH4 in this system is excessive to ensure the reaction followed pseudo-first-order kinetics with respect to 4-NP only19. Therefore, the kinetics can be described as −kt = ln (Ct/C0), where k is the first-order rate constant, t is the reaction time, Ct and C0 stands for the 4-NP concentrations at time t and 015. The corresponding linear relationships of ln (Ct/C0) versus reaction time are shown in Fig. 5(d) and the rate constant, k, is calculated from the slopes of the fitted straight lines. The highest rate constant obtained from C-0.1 is 13.6 × 10−3 s−1. This excellent catalytic activity is attributed to the great amount of Cu/CuO nanoclusters evenly dispersed on TiO2 NT arrays, providing sufficient “clean” surfaces as active sites obtained from the green and surfactant-free photoreduction method. For catalyst C-0.02 and C-0.5, the k values are 5.4 × 10−3 s−1 and 3.0 × 10−3 s−1, respectively. The decrease of rate constant for C-0.02 is because the total amount of Cu/CuO nanoclusters decorated on TiO2 NT arrays is small, which provides limited active sites for 4-NP reduction compared with C-0.1. For C-0.5, although the amount of Cu/CuO is large enough to cover most of the TiO2 surface, the formation of Cu/CuO nanocrystal with large size reduces the surface area exposed to 4-NP and decrease the catalytic activity dramatically. Meanwhile, the turnover frequency (TOF) is another important parameter for catalysis and the TOF of C-0.1 is 115 h−1. The details of TOF calculation is provided in the end of Supplementary.
Different noble metal nanoparticles were decorated onto the TiO2 NT arrays using the same photoreduction method and their catalytic activities were compared with catalyst C-0.1. SEM characterizations shown in Supplementary Fig. S5(a–c) confirm the successful decoration of Au, Ag and Pd nanoparticles on TiO2, revealing that this surfactant-free method is suitable for various metals28. Figure 6(a–c) show the time-dependent UV-vis absorption spectra of 4-NP reduction with Au-TiO2, Ag-TiO2 and Pd-TiO2 as catalyst, respectively. The corresponding plots of ln (Ct/C0) versus reaction time shown in Fig. 6(d) indicates that the reduction of 4-NP by these noble metal catalysts also followed pseudo first-order kinetics. The calculated rate constants are 1.5 × 10−3 s−1 (Au-TiO2), 1.3 × 10−3 s−1 (Ag-TiO2) and 2.7 × 10−3 s−1 (Pd-TiO2) as listed in Supplementary Table S1. The rate constant of C-0.1 is higher than that of these noble metal nanoparticles fabricated in the same photoreduction method. Other metal nanostructures decorated bulk substrates as reusable catalysts for 4-NP reduction mentioned in references are also listed in Supplementary Table S18,10,26,36. As observed, the rate constant of C-0.1 in this article is also comparable to or higher than that of these catalysts, especially the noble metal catalysts. Considering the low-cost of Cu, this Cu/CuO-TiO2 catalyst shows certain superiority in practical applications.
Reusability and Universality
Reusability and stability are very important aspect of catalysts for their practical applications. To investigate the reusability of our catalyst, same C-0.1 catalyst was used repeatedly up to 7 times for the reduction of 4-NP. The plots of Ct/C0 versus reaction time are shown in Fig. 7(a). The catalytic activity of C-0.1 remains almost unchanged during the reusability test and the reduction reaction can still be completed within 5 minutes even for the seventh cycle. The corresponding conversion for each cycle after 5 minutes remains above 99% as shown in Fig. 7(b), which maintained very well compares with those in refs 15, 19, 25 and 26. This great stability of the catalytic in recycling is attributed to the firmly contacted between Cu/CuO nanoclusters and TiO2 NT arrays because the nanoclusters are in-situ photo-reduced and growth on TiO2 substrate instead of loading after formation, which prevent the aggregation or loss of the nanoclusters. It is worth noting that the recycle process of common powder catalysts by repeating centrifuging, washing and long-time drying in oven is very inefficient and costly for practical applications. However, the Cu/CuO-TiO2 catalyst we introduce in this work grows directly on a Ti foil, which makes the recycle of our catalyst very simple and convenient. All you need to do is tweezing the Ti foil out of the solution, rinsing with DI water and drying with N2. This whole recycling process only cost few minutes in total. All these properties indicate that this Cu/CuO-TiO2 catalyst is very stable and reusable in applications.
To examine the universality of Cu/CuO-TiO2 as catalyst for other dyes, MB, RhB and MO were also chosen as test targets to investigate the catalytic activity of C-0.115,40. Before the addition of catalyst C-0.1, the absorption peak of MB (665 nm), RhB (553 nm) and MO (464 nm) maintains unchanged or very slight decrease in the presence of NaBH4 and the corresponding time-dependent UV-vis absorption spectra are given in Supplementary Fig. S6(a–c). After the immersion of the catalyst C-0.1, the absorption intensity decreased very fast and all the reactions are completed in few minutes as shown in Fig. 8(a–c), which indicates that catalyst C-0.1 also exhibits great catalytic activity towards these organic dyes. The corresponding plots of ln (Ct/C0) versus reaction time of these dyes are shown in Fig. 8(d–f). The good linear correlation confirmed that these reactions still followed pseudo-first-order kinetics. The rate constant calculated from the slopes are 68.5 × 10−3 s−1 (MB), 31.5 × 10−3 s−1 (RhB) and 20.7 × 10−3 s−1 (MO), respectively.
Conclusion
In summary, a highly efficient and reusable Cu/CuO-TiO2 catalyst is fabricated using a green method of photoreduction. This non-noble metal catalyst exhibits excellent catalytic activity towards the reduction of 4-NP and other different organic dyes, which is attributed to the large amounts of exposed clean surface provided by the evenly dispersed Cu/CuO nanoclusters. The catalytic activity of this Cu/CuO-TiO2 catalyst is even higher than that of the noble metal-TiO2 catalyst prepared in the same method. The activity towards 4-NP remains almost unchanged for 7 cycles of the reduction because of the firm connection between the in-situ formed nanoclusters and TiO2 NT arrays. This low-cost Cu/CuO based catalyst is also very convenient for recycling compared with the complicated and costly recycle process for common powder catalysts. This novel Cu/CuO-TiO2 catalyst is expected to replace noble metals as a low-cost, highly efficient and easily reusable catalyst in catalytic applications.
Additional Information
How to cite this article: Jin, Z. et al. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 7, 39695; doi: 10.1038/srep39695 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Narayanan, K. B. & Sakthivel, N. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by silver-bionanocomposite using Cylindrocladium floridanum. Bioresour. Technol. 102, 10737–10740 (2011).
Pocurull, E., Marcé, R. M. & Borrull, F. Determination of phenolic compounds in natural waters by liquid chromatography with ultraviolet and electrochemical detection after on-line trace enrichment. J. Chromatogr. A 738, 1–9 (1996).
Feng, J. et al. CuFe2O4 magnetic nanoparticles: A simple and efficient catalyst for the reduction of nitrophenol. Chem. Eng. J. 221, 16–24 (2013).
Li, J. et al. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 201–202, 250–259 (2012).
Zhang, S. H. et al. In situ assembly of well-dispersed Ni nanoparticles on silica nanotubes and excellent catalytic activity in 4-nitrophenol reduction. Nanoscale 6, 11181–11188 (2014).
Li, A. Y., Luo, Q. J., Park, S. J. & Cooks, R. G. Synthesis and catalytic reactions of nanoparticles formed by electrospray ionization of coinage metals. Angew. Chem., Int. Ed. 53, 3147–3150 (2014).
Zeng, J., Zhang, Q., Chen, J. & Xia, Y. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 10, 30–35 (2010).
Prasad, M. D. & Krishna, M. G. Facile green chemistry-based synthesis and properties of free-standing Au- and Ag-PMMA films. ACS Sustainable Chem. Eng. 2, 1453–1460 (2014).
Damato, T. C., de Oliveira, C. C. S., Ando, R. A. & Camargo, P. H. C. A facile approach to TiO2 colloidal spheres decorated with Au nanoparticles displaying well-defined sizes and uniform dispersion. Langmuir 29, 1642–1649 (2013).
Oh, S. Y., Kim, J. & Kim, Y. Paper-based synthesis of Pd-dendrite supported porous gold. Mater. Lett. 154, 60–63 (2015).
Pozun, Z. D. et al. A systematic investigation of p-nitrophenol reduction by bimetallic dendrimer encapsulated nanoparticles. J. Phys. Chem. C 117, 7598–7604 (2013).
Chen, X. M., Cai, Z. X., Chen, X. & Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2, 5668–5674 (2014).
Tan, R. L. S. et al. Levelling the playing field: screening for synergistic effects in coalesced bimetallic nanoparticles. Nanoscale 8, 3447–3453 (2016).
Ma, A. et al. Interfacial nanodroplets guided construction of hierarchical Au, Au-Pt, and Au-Pd particles as excellent catalysts. Sci. Rep. 4, 4849, (2014).
Zhang, Y. et al. Hierarchical architectures of monodisperse porous Cu microspheres: synthesis, growth mechanism, high-efficiency and recyclable catalytic performance. J. Mater. Chem. A 2, 11966–11973 (2014).
Huang, C. J., Ye, W. Q., Liu, Q. W. & Qiu, X. Q. Dispersed Cu2O octahedrons on h-BN nanosheets for p-nitrophenol reduction. ACS Appl. Mater. Interfaces 6, 14469–14476 (2014).
Sarkar, C. & Dolui, S. K. Synthesis of copper oxide/reduced graphene oxide nanocomposite and its enhanced catalytic activity towards reduction of 4-nitrophenol. RSC Adv. 5, 60763–60769 (2015).
Sun, M., Liu, H., Liu, Y., Qu, J. & Li, J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 7, 1250–1269 (2015).
Zhang, P. H. et al. Facile fabrication of faceted copper nanocrystals with high catalytic activity for p-nitrophenol reduction. J. Mater. Chem. A 1, 1632–1638 (2013).
Ghosh, S., Das, R., Chowdhury, I. H., Bhanja, P. & Naskar, M. K. Rapid template-free synthesis of an air-stable hierarchical copper nanoassembly and its use as a reusable catalyst for 4-nitrophenol reduction. RSC Adv. 5, 101519–101524 (2015).
Niu, H. Y., Liu, S. L., Cai, Y. Q., Wu, F. C. & Zhao, X. L. MOF derived porous carbon supported Cu/Cu2O composite as high performance non-noble catalyst. Microporous Mesoporous Mater. 219, 48–53 (2016).
Prucek, R. et al. Polyacrylate-assisted synthesis of stable copper nanoparticles and copper(I) oxide nanocubes with high catalytic efficiency. J. Mater. Chem. 19, 8463–8469 (2009).
Mandlimath, T. R. & Gopal, B. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-anninophenol. J. Mol. Catal. A: Chem. 350, 9–15 (2011).
Jang, K. S. & Kim, J. D. In Situ Catalytic Activity of CuO Nanosheets Synthesized from a Surfactant-Lamellar Template. J. Nanosci. Nanotechnol. 11, 4496–4500 (2011).
Seh, Z. W., Liu, S. H., Zhang, S. Y., Shah, K. W. & Han, M. Y. Synthesis and multiple reuse of eccentric Au@TiO2 nanostructures as catalysts. Chem. Commun. 47, 6689–6691 (2011).
Yang, X. et al. Highly efficient reusable catalyst based on silicon nanowire arrays decorated with copper nanoparticles. J. Mater. Chem. A 2, 9040–9047 (2014).
Zhang, Z., Zhang, L., Hedhili, M. N., Zhang, H. & Wang, P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 13, 14–20 (2013).
Tanaka, A., Fuku, K., Nishi, T., Hashimoto, K. & Kominami, H. Functionalization of Au/TiO2 plasmonic photocatalysts with Pd by formation of a core-shell structure for effective dechlorination of chlorobenzene under irradiation of visible light. J. Phys. Chem. C 117, 16983–16989 (2013).
Zhang, Z. & Wang, P. Optimization of photoelectrochemical water splitting performance on hierarchical TiO2 nanotube arrays. Energy Environ. Sci. 5, 6506–6512 (2012).
Jin, Z., Wang, Q., Zheng, W. & Cui, X. Highly ordered periodic Au/TiO2 hetero-nanostructures for plasmon-induced enhancement of the activity and stability for ethanol electro-oxidation. ACS Appl. Mater. Interfaces 8, 5273–5279 (2016).
Leung, S.-F. et al. Progress and design concerns of nanostructured solar energy harvesting devices. Small 12, 2536–2548 (2016).
Wang, H., You, T., Shi, W., Li, J. & Guo, L. Au/TiO2/Au as a Plasmonic Coupling Photocatalyst. J. Phys. Chem. C 116, 6490–6494 (2012).
Zhang, G. et al. Visible-Light Induced Photocatalytic Activity of Electrospun-TiO2 in Arsenic(III) Oxidation. ACS Appl. Mater. Interfaces 7, 511–518 (2015).
Zhang, H., Lv, X., Li, Y., Wang, Y. & Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 4, 380–386 (2010).
Li, Y. et al. Anchoring CuO nanoparticles on nitrogen-doped reduced graphene oxide nanosheets as electrode material for supercapacitors. J. Electroanal. Chem. 727, 154–162 (2014).
Najdovski, I., Selvakannan, P. R. & O’Mullane, A. P. Electrochemical formation of Cu/Ag surfaces and their applicability as heterogeneous catalysts. RSC Adv. 4, 7207–7215 (2014).
Konsolakis, M. et al. Effect of preparation method on the solid state properties and the deN(2)O performance of CuO-CeO2 oxides. Catal. Sci. Technol. 5, 3714–3727 (2015).
Mi, Y. et al. Constructing a AZO/TiO2 core/shell nanocone array with uniformly dispersed Au NPs for enhancing photoelectrochemical water splitting. Adv. Energy Mater. 6, 1501496 (2016).
Yu, X., Cheng, G. & Zheng, S.-Y. Synthesis of self-assembled multifunctional nanocomposite catalysts with highly stabilized reactivity and magnetic recyclability. Sci. Rep. 6, 25459 (2016).
Ghosh, B. K., Hazra, S., Naik, B. & Ghosh, N. N. Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 269, 371–378 (2015).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21275064, 51571100), the Specialized Research Fund for the Doctoral Program of Higher Education (20130061110035), and the Program for New Century Excellent Talents in University (NCET-10-0433).
Author information
Authors and Affiliations
Contributions
X.Q.C. and Z.J. planned the experiments, collected and analyzed the data and wrote the paper. C.L. and K.Q. did the SEM and TEM characterization of the samples. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jin, Z., Liu, C., Qi, K. et al. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci Rep 7, 39695 (2017). https://doi.org/10.1038/srep39695
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39695
This article is cited by
-
Magnesium- and copper-substituted strontium–aluminium–hexaferrite (SrAl2Fe10O19): synthesis and scrutiny
Applied Physics A (2024)
-
Novel approaches to the degradation of nitrophenols using TiO2-biopolymer-ligand-metal complex as photocatalysts
Journal of Materials Science: Materials in Electronics (2024)
-
Correction to: TiCuN coating on brass faucets: from beautifully colored appearance to antibacterial properties
Chemical Papers (2023)
-
TiCuN coating on brass faucets: from beautifully colored appearance to antibacterial properties
Chemical Papers (2023)
-
Synthesis of CuO/α-Fe2O3 Nanocomposite by Q-Switched Pulsed Laser Ablation and its Catalytic Activity for Environmental Applications
Arabian Journal for Science and Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.