Abstract
Protein glycation involves formation of early (Amadori) and late advanced glycation endproducts (AGEs) together with free radicals via autoxidation of glucose and Amadori products. Glycation and increased free radical activity underlie the pathogenesis of diabetic complications. This study investigated whether aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro in a cell-free system. Proteins were glycated by incubation with sugars (glucose, methylglyoxal or ribose) ±5–15 mg/mL of aged and fresh garlic extracts. Advanced glycation endproducts were measured using SDS-PAGE gels and by ELISA whereas Amadori products were assessed by the fructosamine method. Colorimetric methods were used to assess antioxidant activity, free radical scavenging capacity, protein-bound carbonyl groups, thiol groups and metal chelation activities in addition to phenolic, total flavonoid and flavonol content of aged and fresh garlic extracts. Aged garlic inhibited AGEs by 56.4% compared to 33.5% for an equivalent concentration of fresh garlic extract. Similarly, aged garlic had a higher total phenolic content (129 ± 1.8 mg/g) compared to fresh garlic extract (56 ± 1.2 mg/g). Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract and is more suitable for use in future in vivo studies.
Similar content being viewed by others
Introduction
Chronic hyperglycaemia of diabetes mellitus underlies the development of long-term complications afflicting the eyes, nerves, blood vessels, kidneys and skin of affected individuals. Hyperglycaemia increases protein glycation, which is the non-enzymatic reaction between carbonyl groups from reducing sugars and protein amino groups forming an unstable Schiff base that rearranges to a more stable Amadori product. Once formed, the Amadori products undergo further reactions involving dicarbonyl intermediates such as 3-deoxyglucosones and ultimately forming fluorescent and cross-linked structures called advanced glycation endproducts (AGEs). Accumulation of tissue AGEs have been implicated in the development of diabetic complications1,2. Protein glycation is accompanied by free radical production from autoxidation of glucose (autoxidative glycation) and oxidation of glycated proteins (glycoxidation) to from AGEs2. Free radicals are capable of damaging biomolecules and this oxidative stress contributes towards the pathogenesis of diabetic complications2.
Despite advances in treatment, the long-term complications of diabetes are a major cause of concern. Thus, inhibition of AGE-mediated tissue damage and oxidative stress may offer therapeutic potential for preventing or delaying onset or progression of diabetic complications1. Various antiglycation compounds have been studied and include aminoguanidine, a drug found to be effective both in vitro and in vivo1. Other compounds include AGE cross-link breakers such as N-phenacylthiazolium bromide have been proposed as agents for reversing protein cross-linking caused by AGEs3. Furthermore, there is considerable interest in compounds capable of blocking interaction between AGEs and their receptor called receptor for AGEs (RAGE)4. Although many compounds have been studied, none has been approved for clinical use. Whilst the search for new synthetic antiglycation agents continues, little attention has been devoted to antiglycation agents from natural sources. Various studies have indicated that dietary supplementation with nutrients possessing both antiglycation and antioxidant properties may be a safe and simple complement to traditional therapies aimed at preventing diabetic complications5.
Garlic (Allium sativum) has been used as a flavouring agent, functional food and in folklore medicine. Indeed, its medicinal properties have been used to treat a wide variety of diseases since ancient times6. Garlic is a well-established prophylactic and therapeutic medicinal agent, which has been investigated for health benefits, resulting in more than 3,000 publications from all over the world7. Garlic is a rich source of water- and lipid-soluble organosulphur compounds, but the constituents responsible for the health benefits of garlic may vary in type and concentration, depending on different processing, preparations and soil conditions8. Garlic is available in different formulations; one of which is aged garlic extract and manufactured by soaking sliced raw garlic in 15–20% aqueous ethanol for up to 20-months at room temperature. The extract is then filtered and concentrated under reduced pressure at low temperature. During this ageing, the odorous and harsh irritating compounds in garlic are converted to odourless, non-irritating, safe sulphur compounds9. This process causes loss of allicin and increases activity of other water-soluble organosulphur compounds, such as S-allyl cysteine (SAC), S-allyl mercaptocysteine (SAMC), allixin and selenium, all of which are antioxidants8,10. Another antioxidant present in aged garlic extract is N-fructosyl arginine, which is not present in raw or heat-treated garlic preparations11. The aim of this study was to investigate and compare the abilities of aged and fresh garlic extracts to inhibit formation of AGEs. Furthermore, the antioxidant activity of aged garlic extract was also investigated and compared with that of fresh garlic extract.
Results
Effects of aged and fresh garlic extracts on cross-linked AGEs
Glycation of lysozyme by glucose produces crosslinked AGEs that present as dimers with an approximate molecular weight of 28 kDa. Glycated lysozyme (Fig. 1A, lane b) showed reduced electrophoretic mobility for the lysozyme monomer and the presence of dimers formed via AGE crosslinks compared to native lysozyme (Fig. 1A, lane a). Aged garlic (Fig. 1A, lanes c–e) and fresh garlic extracts (Fig. 1A, lanes f–h) inhibited AGE-induced dimerization of lysozyme in a dose-dependent manner. The mean values for aged garlic extract at 5, 10 and 15 mg/mL concentrations were 11.2%, 40.4% and 56.4%, compared to 5.9%, 24.9% and 33.5% for fresh garlic extract, respectively. Aged garlic extract showed a significantly (P < 0.01) greater inhibitory effect compared to fresh garlic extract at higher concentrations only (10–15 mg/mL) (Fig. 1B).
(A) SDS-PAGE gel showing the effects of aged and fresh garlic extracts on formation of crosslinked AGEs formed by glycation of lysozyme (10 mg/mL) by 0.5 M glucose in 0.1 M phosphate buffer, pH 7.4 for 4 weeks at 37 °C. Unmodified lysozyme (lane a), glycated lysozyme (lane b) or lysozyme glycated in the presence of 5 (lane c), 10 (lane d) or 15 mg/mL (lane e) of aged garlic extract or 5 (lane f), 10 (lane g) or 15 mg/mL (lane h) of fresh garlic extract. (B) Percentage inhibition of crosslinked AGEs. Each value represents the mean ± SD (n = 3). Statistical significance was defined as **P < 0.01 compared to aged garlic extract.
Amadorin activity of aged and fresh garlic extracts
Reincubation of dialysed Amadori-rich bovine serum albumin (BSA) following glycation with ribose results in the formation of AGEs as shown in Fig. 2. The kinetics of post-Amadori AGE formation was rapid over 2 days in the absence of any inhibitors. Both aged and fresh garlic extracts inhibited these post-Amadori reactions with aged garlic extract being more effective at all concentrations tested compared to glycated BSA. At low concentrations (5 mg/mL), aged garlic extract showed no significant inhibition of post-Amadori AGE formation compared to fresh garlic extract (Fig. 2A). From the data in Fig. 2B and C, it is apparent that aged garlic extract at higher concentrations (10–15 mg/mL) showed significant (P < 0.001) inhibition of post-Amadori AGE formation after 8 days compared to fresh garlic extract.
Effect of (A) 5 mg/ml, (B) 10 mg/ml and (C) 15 mg/ml of aged and fresh garlic extracts on post-Amadori AGE formation measured using ELISA at different time points. Results are presented as mean ± SD (n = 3). At each time interval, statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001 compared to fresh garlic extract.
Effect of aged and fresh garlic extracts on fructosamine formation
An increase in fructosamine occurs following incubation of BSA with glucose but not for BSA incubated alone (Fig. 3). Both extracts inhibited fructosamine production after 2 and 8 days of incubation with aged garlic being more effective compared to fresh garlic extract (Fig. 3). Compared to the glycated BSA, aged garlic extract showed significant (P < 0.001) inhibition in fructosamine formation after 2 and 8 days. Similarly, fresh garlic extract showed a significant (P < 0.01) inhibition of fructosamine formation. Fructosamine formation was inhibited more by aged garlic extract than by fresh garlic extract on day 8 (P < 0.01) but not on day 2 (Fig. 3).
Effect of aged and fresh garlic extracts on glycation-induced protein carbonyl and thiol group formation
Bovine serum albumin glycated for 4 days showed an increase in protein bound carbonyl groups compared to BSA alone as a negative control (Table 1). Both extracts inhibited formation of protein-bound carbonyl groups with aged garlic extract being more effective (Table 1). Compared to glycated BSA, aged garlic extract showed significant (P < 0.001) inhibition of carbonyl group formation. Similarly, fresh garlic extract showed significant (P < 0.01) inhibition of carbonyl group formation. Glycated BSA had a significantly (P < 0.001) lower thiol content compared to BSA alone. Aged garlic extract had a significant (P < 0.001) protective effect against oxidation of thiol groups and fresh garlic extract also showed a significant (P < 0.01) effect compared to glycated BSA. Aged garlic extract was significantly (P < 0.05) more effective than fresh garlic extract in the prevention of thiol group oxidation (Table 1).
Antioxidant activities of aged and fresh garlic extracts
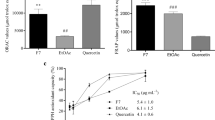
In the 2, 2-azino-bis 3–ethylbenzthiazoline–6–sulfonic acid or ABTS assay, both extracts displayed antioxidant activities, as they were able to scavenge the ABTS+. Aged garlic extract had a higher Trolox equivalent antioxidant capacity (TEAC) compared to fresh garlic extract (Table 2). There was no significant difference in TEAC between aged garlic extract and ascorbic acid. However, fresh garlic extract showed a significant (P < 0.001) difference in TEAC compared to ascorbic acid. Aged garlic extract had a protective effect and significantly (P < 0.001) reduced TEAC compared to fresh garlic extract. In the 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assay, ascorbic showed significant (P < 0.001) inhibition of DPPH radicals compared to aged and fresh garlic extracts. The effect of aged garlic extract was significantly (P < 0.001) different from fresh garlic extract. Indeed, aged garlic extract demonstrated a stronger DPPH scavenging ability than fresh garlic extract (Table 2).
Metal chelation activities of aged and fresh garlic extracts
Figure 4 showed the metal chelating effects of aged and fresh garlic extracts. Both extracts showed potent chelating abilities for ferrous ions. However, at all tested concentrations the fresh garlic had a stronger chelating effect compared to the aged garlic extract (Fig. 4). At higher concentrations (15–20 mg/mL), fresh garlic extracts showed significant (P < 0.05) chelating abilities for ferrous ions whereas aged garlic extract showed significant (P < 0.001) chelating effect compared to ethylenediaminetetraacetic acid (EDTA). However, EDTA showed significantly (P < 0.001) stronger Fe2+ -chelation activity even at minimal concentrations of 0.1 mg/mL compared to aged and fresh garlic extracts.
Phenolic, flavonoid and flavonol compounds in aged and fresh garlic extracts
Table 3 shows the concentration of total phenolic compounds was significantly (P < 0.001) higher in aged garlic compared to fresh garlic extract. Similarly, the total flavonoid and flavonol concentrations of aged garlic were significantly (P < 0.001) higher than fresh garlic extract (Table 3).
Discussion
There is considerable interest in antiglycation agents because of their therapeutic potential in reducing the morbidity and mortality associated with diabetes. Different strategies have been proposed to reverse the effects of AGEs, focusing on inhibition of AGE formation, removal of AGEs or interference with cellular effects of AGEs2. This study showed both aged and fresh garlic extracts inhibit the formation of AGEs and is in agreement with previous studies demonstrating aged garlic extract and SAC effectively inhibit AGE formation in vitro12. Indeed, aged garlic extract was shown to have more potent antiglycation properties compared to fresh garlic extract. Moreover, the addition of SAC to fresh garlic extract increased the inhibition of AGEs (results not shown). In addition to SAC, other compounds may be present in aged garlic extract contributing towards its antiglycation activity, although these compounds have not yet been determined. Fresh and aged garlic extracts inhibited early glycation products (fructosamine) formation possibly by modification of carbonyl or amino groups in the glycation reaction.
Protein oxidation has a key role in AGE formation and together with glycation generate protein-bound carbonyl groups, which have been detected in human tissues13. Furthermore, reaction with dinitrophenylhydrazine (DNPH) is a standard method for detecting carbonyl groups and can be used to follow changes in protein oxidation14. In this study, free carbonyl groups were generated by treating BSA with a high concentration of methylglyoxal. Aged garlic extract was more potent than fresh garlic in inhibiting protein-bound carbonyl groups with the results being comparable to the positive control, aminoguanidine and consistent with previous studies for aminoguanidine14.
Another protein modification that may potentially lead to structural changes is the loss of protein thiol groups. The oxidation of thiol groups was observed following glycation of BSA and this was inhibited by aged garlic extract and protects against oxidative protein damage. Therefore, the thiol assay provides mechanistic information concerning the effects of aged and fresh garlic extracts on AGEs.
Natural compounds with antioxidant properties can inhibit AGE formation. In this regard, compounds such as rutin15, garcinol16, loubuma tea17 and green tea18 have been shown to prevent AGE formation both in vitro and in vivo. Thus, the antiglycation activity of plant extracts could be attributed to their antioxidant properties. Indeed, several studies have demonstrated that the protective effects of garlic are due to their antioxidant and radical scavenging capacities19,20. The abilities of aged and fresh garlic extracts to scavenge ABTS˙ were compared with ascorbic acid, the latter being an excellent marker for determining the antioxidant capacity of hydrogen donating antioxidants. The higher scavenging activity of aged garlic compared to fresh garlic extract was deemed similar to the antioxidant capacity of ascorbic acid and may be related to the high polyphenolic content of aged garlic extract. Antioxidant compounds can also reduce the purple colour of DPPH˙ by providing hydrogen atoms or by electron donation and producing a colourless complex causing decreased absorbance, which is a measure of the extent of radical scavenging. The DPPH˙ scavenging activity of phenolic compounds in aged and fresh garlic extracts is mainly due to their redox properties and depends on the chemical structure of the compound, the number of hydroxyl groups, the substitution pattern of hydroxyl groups and on the method of analysis21.
In the metal chelation assay, ferrozine forms complexes with ferrous ions and, in the presence of ferrous chelating agents; the complex formation is disrupted, resulting in reduction of the red complex colour. Therefore, measurement of colour reduction reflects metal chelation activity of coexisting chelators22. Aged and fresh garlic extracts interfere with the formation of ferrous and ferrozine complexes, therefore are able to capture ferrous ions before formation of ferrozine and accordingly produce metal chelation activity. Unexpectedly, fresh garlic extract produced stronger ferrous chelation activity compared to aged garlic extract, although the latter had higher flavonoid content. The metal-chelating ability of aged and fresh garlic extracts depend on their phenolic structure and the number and position of hydroxyl groups. It may be that the flavonoids in aged garlic extract do not have the required structure, accounting for this result. In addition, it is believed that acidic and/or basic amino acids may play an important role in the chelation of metal ions.
Aged garlic extract, which contains the highest amount of phenolic, flavonoid and flavonol compounds, exhibited the greatest antioxidant activity. These results were in agreement with those of previous workers who reported that the total phenolic content of aged garlic extract was higher than that of fresh and steamed garlic extracts23. Flavonoids and flavonols are important natural phenolics, and are the most widespread group of natural compounds. The mechanism(s) of action of flavonoids and flavonols are through the scavenging or chelating process24. Findings of this study support the hypothesis that phenolic compound in certain plant extracts effectively inhibit the formation of AGEs. Indeed, compounds with phenolic, flavonoid and flavonols content effectively inhibited the formation of dicarbonyl compounds and AGEs25. Therefore, the difference in antioxidant and antiglycation activities between aged and fresh garlic extracts could be due to their composition of phenolic and/or other non-phenolic compounds.
The aging of garlic converts the harsh, unstable and irritating compounds found in raw garlic such as allicin to stable unique and beneficial compounds. Thus, the processing method of aging garlic may influence its antioxidant activities. The high antioxidant activities of aged garlic extract might be due to antioxidant compounds other than phenolic compounds. The antiglycation activity of aged garlic extract is mainly due to its organosulphur compounds. Aged garlic extract contains mostly stable water-soluble organosulphur compounds such as SAC and SAMC, which are powerful antioxidants and are largely responsible for aged garlic extract’s health benefits. The pharmacokinetics of SAC is well established in vivo, which has high bioavailability and is used for standardizing aged garlic extract26. Both phenolic and organosulphur compounds present in garlic possess radical scavenging properties27. However, the presence of other potential antioxidant compounds, such as vitamin C, vitamin E and β-carotene, may also play an important role21. This antioxidant property of aged garlic extract may at least in part, account for its antiglycation properties.
This study has several limitations, one of which is the use of high sugar concentrations to speed up the glycation reaction, which otherwise occurs slowly in vivo being dependent on degree and duration of glycaemia. However, such an approach is useful for mechanistic studies allowing them to be conducted in an acceptable timescale and reducing the possibility of microbial sample contamination. It is unclear how these extracts would be metabolized in vivo and whether the relevant active ingredients would be present in blood at appropriate concentrations or whether any synergistic effects would still be operational. However, the approach used does provide a simple screening process to assess antiglycation and antioxidant properties of interesting extracts.
Dietary supplementation with antioxidants may be a safe and simple way of complementing traditional therapies aimed at targeting and preventing diabetic complications. This study demonstrates that aged and fresh garlic extracts protect against protein glycation in vitro. Aged garlic extract inhibits the formation of AGEs more effectively than fresh garlic extract, and this finding together with previous data suggest that daily consumption of aged garlic extract might be beneficial for prevention of lifestyle-related diseases. It is important to identify all compounds in aged garlic extract and to clarify their precise role. The bioavailability of different garlic components and their ability to inhibit AGE formation require further investigation. Further research is needed to clarify whether aged garlic extract attenuates the development of diabetic vascular lesions in vivo.
Materials and Methods
Ethylenediaminetetraacetic acid was obtained from Fisher Scientific International, UK. Aged garlic extract was kindly provided by Wakunaga of America Company Ltd. Ribose, sodium dodecyl sulphate (SDS) and Tris (hydroxymethyl)-amino methane were obtained from BDH, UK. Acrylamide solution was obtained from Bio-Rad Laboratories (Hemel Hempstead, UK). Bromophenol blue was obtained from Serva, Germany. Ethanol, glacial acetic acid and methanol were obtained from Fisher Scientific International, UK. Dialysis tubing with a molecular weight cut off of 3.5 kDa and 67 kDa was obtained from Medical International Ltd Company (London, UK). Advanced glycation endproduct antibody (rabbit polyclonal to AGE antibody) and rabbit IgG secondary antibody (goat polyclonal to rabbit IgG) were purchased from Abcam, UK. All other reagents were of analytical reagent grade from Sigma-Aldrich Company (Poole, UK).
Protein glycation
Briefly, either BSA or lysozyme (10 mg/mL) were incubated in 0.5 M glucose or 0.1 M methylglyoxal with or without prospective inhibitors (aged and fresh garlic extracts) in 0.1 M sodium phosphate buffer containing 3 mM sodium azide, pH 7.4 at 37 °C in the dark for a defined period of time12. Control samples with or without prospective inhibitors were incubated under the same conditions. Aliquots were stored at −20 °C until analysis.
Preparation of fresh garlic extract
Fresh garlic was purchased from a retail food store (Manchester, UK). Garlic bulbs were identified by a botanist at the university. The bulbs (50 g) in good physical shape were peeled and weighed. Aqueous garlic extract was prepared according to an established method28. Briefly, 50 g of garlic was homogenized in 75 mL of cold 0.9% NaCl in the presence of some crushed ice. Homogenization was carried out in a blender at the highest speed with 1 minute bursts for a total of 12 minutes. The homogenized mixture was filtered through cheese cloth, centrifuged at 2000 × g for 10 minutes and the clear supernatant made up to 100 mL with saline. This aqueous garlic extract was stored in small aliquots at −20 °C until use. The dose of fresh garlic was calculated on the basis of the weight of fresh garlic in mg used to prepare a 1 mL extract (one millilitre of aqueous extract contained material from 500 mg of garlic). The aqueous garlic extract contains about 50% garlic material.
Interrupted post-Amadori assay
Ribose-glycated protein rich in Amadori adducts was prepared as described previously12. Briefly, BSA (10 mg/mL) was incubated in 0.5 M ribose in 0.1 M sodium phosphate buffer containing 3 mM sodium azide, pH 7.4 at 37 °C for 24-hours. Unbound sugar was removed by extensive dialysis against distilled water and this Amadori-rich protein reincubated with or without the prospective extract at 37 °C. Aliquots were taken at various intervals (0–8 days) and frozen for subsequent analysis before measurement of AGEs using the enzyme-linked immunosorbent assay (ELISA) below.
Measurement of fructosamine
Fructosamine content was quantified by a colorimetric method using nitroblue tetrazolium (NBT) as described previously29 and method calibrated using 1-deoxy-1-morpholino-D-fructose (DMF) standards.
Measurement of AGEs
Crosslinked AGEs
These were assessed by the percentage crosslinking of the protein following sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% gels as described previously12. These gels were stained with Coomassie blue, destained, photographed and analyzed using Gene snap programme from G Box Chem HR16 and Gene tool image analysis software from Bio Tools, Inc. (Edmonton, Alberta, Canada), respectively. Integrated density (I.D) was measured to analyze the one-dimensional electrophoretic gels and percentage inhibition of cross-linked AGEs was calculated using the formula:

AGEs by ELISA
Microtiter plates were coated with 100 μL of standards (1 ng/mL-1 mg/mL) and samples (1 μg/mL) diluted in 50 mM carbonate buffer, pH 9.7, overnight at 4 °C. After coating, the wells were washed with 300 μL saline solution containing 0.05% Tween-20. The wells were treated with 200 μL of blocking buffer (3–5% non-fat dry milk or 5% serum BSA) in sodium carbonate buffer or PBS for 2 hours at room temperature, followed by washing. A volume of 100 μL of AGE antibody (rabbit polyclonal to AGE antibodies) as primary antibody was added at a suitable titre (1:2500 to 1:20000) diluted in blocking buffer and incubated for 2 hours at 37 °C, followed by washing. Then, 100 μL of rabbit IgG antibody; goat polyclonal to rabbit IgG (horse radish peroxidase) as the secondary antibody was added at a titre of 1:4000 to 1:10000 diluted in blocking buffer immediately before use and incubated for 1 hour at 37 °C, followed by washing. ABTS diammonium salt substrate solution was added (100 μL/well) to the plates followed by an incubation at room temperature for up to 30 minutes. A 100 μL of stop solution (0.625 M oxalic acid) was added, mixed and absorbance determined at 450 nm.
Measurement of protein-bound carbonyl groups
Protein-bound carbonyl groups were measured using DNPH derivatisation as described previously30. The carbonyl content was calculated using a molar absorbance of 22,000 M−1cm−1 and the results expressed as the ratio of nmoles of DNPH reacted per mg of protein.
Measurement of thiol groups
Free thiol content was determined in BSA alone and in glycated BSA in the presence or absence of extracts according to an established method31 using 5,5′-dithiobisnitrobenzoic acid (DTNB) and L-cysteine hydrochloride as standards. Free thiol concentration was calculated using 14,150 M−1cm−1 as the molar extinction coefficient of DTNB. The results were expressed as the ratio of nmoles of DTNB reacted per mg of protein.
ABTS antioxidant assay
The standard ABTS assay32 was used for determination of TEAC of both aged and fresh garlic extracts. Trolox dissolved in ethanol was used as a reference standard (0–20 μM) whereas ascorbic acid was used as a positive control.
DPPH radical scavenging capacity assay
1, 1-Diphenyl-2-picryl-hydrazyl radical scavenging activity of extracts was measured according to a modified version of an existing method33. Trolox was used as the reference standard compound and ascorbic acid was used as positive control. Antioxidant activity of a sample was expressed in terms of micromole equivalents of trolox. The concentration causing 50% inhibition (IC50) of each extract was determined and compared with the corresponding TEAC.
Metal chelation assay
The ability of garlic extracts to chelate ferrous ions (Fe2+) was assessed according to a modified version of an established method34. EDTA was used as a positive control and diluted to 0.01–20 mg/mL for further use. The chelating activity of the extract for Fe2+ was calculated using the formula:

Measurement of total phenolic compounds
The amount of total phenolic compounds in garlic extracts was estimated according to a modified version of a previously reported method using Folin-Ciocalteu reagent35. The method was calibrated using 0.024–0.3 mg/mL ethanolic gallic acid solution (10 g/L) and results were expressed as milligrams per gram of extract of gallic acid equivalents.
Measurement of total flavonoids
The flavonoid content was determined according to a modified version of a previously described colorimetric procedure using quercetin as a reference compound36. Quercetin was used for standards and the flavonoid content was expressed in milligrams of quercetin equivalent per gram of extract.
Measurement of total flavonols
The content of flavonols was determined according to an established method35. Rutin standards were used to calibrate the method and flavonol content was expressed as milligrams of rutin equivalent per gram of extract.
Statistical analysis
Statistical analysis was performed using SPSS 19 software (SPSS Inc., Chicago, Illinois, USA). Data were presented as mean ± standard deviation (SD) from at least three independent experiments. The significant difference between test groups was analyzed using two-way analysis of variance (2-factor ANOVA), followed by Bonferroni Post Hoc tests for the comparisons between tested concentrations in aged and fresh garlic extracts. A Dunnett’s Post Hoc test was used to compare between aged and fresh garlic extracts against the control. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001.
Additional Information
How to cite this article: Elosta, A. et al. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 7, 39613; doi: 10.1038/srep39613 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmed, N. Advanced glycation endproducts: role in pathology of diabetic complications. Diabetes Res Clin Pract. 67, 3–21 (2005).
Elosta, A., Ghous, T. & Ahmed, N. Natural products as anti-glycation agents: Possible therapeutic potential for diabetic complications. Curr Diabetes Rev. 8, 92–108 (2012).
Vasan, S., Foiles, P. & Founds, H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 419, 89–96 (2003).
Alexiou, P., Chatzopoulou, M., Pegklidou, K. & Demopoulos, V. J. RAGE: a multi-ligand receptor unveiling novel insights in health and disease. Curr Med Chem. 17, 2232–2252 (2010).
Farsi, D. A. et al. Inhibition of non-enzymatic glycation by silk extracts from a Mexican land race and modern inbred lines of maize (Zea mays). Phytother Res. 22, 108–112 (2008).
Rahman, K. & Lowe, G. M. Garlic and cardiovascular disease: a critical review. J Nutr. 136, 736S–740S (2006).
Amagase, H., Petesch, B. L., Matsuura, H., Kasuga, S. & Itakura, Y. Intake of garlic and its bioactive components. J Nutr. 131, 955S–962S (2001).
Borek, C. Antioxidant health effects of aged garlic extract. J Nutr. 131, 1010S–1015S (2001).
Weiss, N. et al. Aged garlic extract improves homocysteine-induced endothelial dysfunction in macro- and microcirculation. J Nutr. 136, 750S–754S (2006).
Dillon, S. A., Burmi, R. S., Lowe, G. M., Billington, D. & Rahman, K. Antioxidant properties of aged garlic extract: an in vitro study incorporating human low-density lipoprotein. Life Sci. 72, 1583–1594 (2003).
Ryu, K., Ide, N., Matsuura, H. & Itakura, Y. (N alpha-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound identified in aged garlic extract. J Nutr. 131, 972S–976S (2001).
Ahmad, M. S., Pischetsrieder, M. & Ahmed, N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 561, 32–38 (2007).
Stadtman, E. R. Protein oxidation and aging. Science. 257, 1220–1224 (1992).
Liggins, J. & Furth, A. J. Role of protein-bound carbonyl groups in the formation of advanced glycation endproducts. Biochim Biophys Acta. 1361, 123–130 (1997).
Kiho, T., Usui, S., Hirano, K., Aizawa, K. & Inakuma, T. Tomato paste fraction inhibiting the formation of advanced glycation endproducts. Biosci Biotechnol Biochem. 68, 200–205 (2004).
Yamaguchi, F., Ariga, T., Yoshimura, Y. & Nakazawa, H. Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 48, 180–185 (2000).
Yokozawa, T. & Nakagawa, T. Inhibitory effects of Luobuma tea and its components against glucose-mediated protein damage. Food Chem Toxicol. 42, 975–981 (2004).
Rutter, K. et al. Green tea extract suppresses the age-related increase in collagen crosslinking and fluorescent products in C57BL/6 mice. Int J Vitam Nutr Res. 73, 453–460 (2003).
Dhawan, V. & Jain, S. Garlic supplementation prevents oxidative DNA damage in essential hypertension. Mol Cell Biochem. 275, 85–94 (2005).
Jastrzebski, Z. et al. The bioactivity of processed garlic (Allium sativum L.) as shown in vitro and in vivo studies on rats. Food Chem Toxicol. 45, 1626–1633 (2007).
Molay Kumar, R., Makiko, T. & Seiichiro, I. Thermal processing enhances anti-radical activity and reduces pro-oxidant activity in water-soluble fraction of selected vegetables. J Sci Food Agric. 87, 2259–2265 (2007).
Zhao, G.-R., Xiang, Z.-J., Ye, T.-X., Yuan, Y.-J. & Guo, Z.-X. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng . Food Chemistry. 99, 767–774 (2006).
Choi, D. J. et al. Physicochemical characteristics of black garlic (Allium sativum L.). J Korean Soc Food Sci Nutr. 37, 465–471 (2008).
Kessler, M., Ubeaud, G. & Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Sci. 55, 131–42 (2003).
Wu, J.-W., Hsieh, C.-L., Wang, H.-Y. & Chen, H.-Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chemistry. 113, 78–84 (2009).
Nagae, S. et al. Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med. 60, 214–217 (1994).
Queiroz, Y. S., Ishimoto, E. Y., Bastos, D. H. M., Sampaio, G. R. & Torres, E. A. F. S. Garlic (Allium sativum L.) and ready-to-eat garlic products: In vitro antioxidant activity. Food Chemistry. 115, 371–374 (2009).
Ali, M. & Mohammed, S. Y. Selective suppression of platelet thromboxane formation with sparing of vascular prostacyclin synthesis by aqueous extract of garlic in rabbits. Prostaglandins Leukot Med. 25, 139–146 (1986).
Johnson, R. N., Metcalf, P. A. & Baker, J. R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta. 127, 87–95 (1983).
Uchida, K. et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci. 95, 4882–4887 (1998).
Ellman, G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 82, 70–77 (1959).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26, 1231–1237 (1999).
Tang, S. Y. et al. Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional Chinese medicines. Free Radic Biol Med. 36, 1575–1587 (2004).
Wang, H., Gao, X. D., Zhou, G. C., Cai, L. & Yao, W. B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chemistry. 106, 888–895 (2008).
Miliauskas, G., Venskutonis, P. R. & van Beek, T. A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 85, 231–237 (2004).
Woisky, R. G. & Salatino, A. Analysis of propolis: some parameters and procedures for chemical quality. J. Apic. Res. 37, 99–105 (1998).
Acknowledgements
We are grateful to Wakunaga of America Company Ltd for providing us with the aged garlic extract used in this study. We are also grateful to the Ministry of Higher Education, Libya for providing a PhD Scholarship to Dr Abdulhakim Elosta. We would like to acknowledge Tor Yip for his assistance with the illustrations.
Author information
Authors and Affiliations
Contributions
A.E., M.S., K.R. and N.A. designed the research. A.E. conducted the research; A.E., M.S., K.R. and N.A. analysed the data and N.A. and A.E. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Elosta, A., Slevin, M., Rahman, K. et al. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci Rep 7, 39613 (2017). https://doi.org/10.1038/srep39613
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39613
This article is cited by
-
Anti-glycation properties of Illicium verum Hook. f. fruit in-vitro and in a diabetic rat model
BMC Complementary Medicine and Therapies (2022)
-
Black garlic fermentation with green tea extract reduced HMF and improved bioactive properties: optimization study with response surface methodology
Journal of Food Measurement and Characterization (2022)
-
S-1-propenylcysteine improves TNF-α-induced vascular endothelial barrier dysfunction by suppressing the GEF-H1/RhoA/Rac pathway
Cell Communication and Signaling (2021)
-
Attenuation of glycation and biochemical aberrations in fructose‐loaded rats by polyphenol‐rich ethyl acetate fraction of Parkia biglobosa (jacq.) Benth. (Mimosaceae) leaves
Clinical Phytoscience (2021)
-
B procyanidins of Annona crassiflora fruit peel inhibited glycation, lipid peroxidation and protein-bound carbonyls, with protective effects on glycated catalase
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.