Abstract
Dredging and natural sediment resuspension events can cause high levels of turbidity, reducing the amount of light available for photosynthetic benthic biota. To determine how marine sponges respond to light attenuation, five species were experimentally exposed to a range of light treatments. Tolerance thresholds and capacity for recovery varied markedly amongst species. Whilst light attenuation had no effect on the heterotrophic species Stylissa flabelliformis and Ianthella basta, the phototrophic species Cliona orientalis and Carteriospongia foliascens discoloured (bleached) over a 28 day exposure period to very low light (<0.8 mol photons m−2 d−1). In darkness, both species discoloured within a few days, concomitant with reduced fluorescence yields, chlorophyll concentrations and shifts in their associated microbiomes. The phototrophic species Cymbastela coralliophila was less impacted by light reduction. C. orientalis and C. coralliophila exhibited full recovery under normal light conditions, whilst C. foliascens did not recover and showed high levels of mortality. The light treatments used in the study are directly relevant to conditions that can occur in situ during dredging projects, indicating that light attenuation poses a risk to photosynthetic marine sponges. Examining benthic light levels over temporal scales would enable dredging proponents to be aware of conditions that could impact on sponge physiology.
Similar content being viewed by others
Introduction
Sponges are filter-feeding organisms with important ecosystem roles including habitat provision, seawater filtration, primary production and binding and/or erosion of the carbonate reef structure1. This diversity of functional roles means that changes to the abundance or composition of sponge assemblages is likely to significantly impact other reef organisms and overall ecosystem functioning2. Importantly, sponges host dense and diverse microbial symbionts that are intimately linked to their health, fitness and nutrition3,4. Many of these sponge symbionts are photosynthetic and in some cases they produce >50% of the energy requirements of the host5,6. In these mutually beneficial partnerships, the photosymbionts obtain energy from sunlight and provide the host with glucose, glycerol and amino acids and, in return, are provided with a protected environment and many of the compounds required for photosynthesis7,8,9,10. These sponges are described as ‘phototrophic’ or ‘mixotrophic’ depending on their degree of nutritional dependence from the symbiont primary production6,11.
Phototrophic sponges are common in shallow tropical reef environments world-wide, including the Mediterranean, Red Sea and the Caribbean3,6 and can comprise up to 80% of individuals and biomass in some coral reefs in the Indo-West Pacific and the Great Barrier Reef (GBR)3,12. In these regions phototrophic sponges have important ecological roles as net primary producers as well as in recycling organic carbon through shedding of cellular material which is rapidly consumed by detritivores3,13.
Due to their nutritional dependence on the products of photosynthesis, phototrophic sponges may be particularly sensitive to turbidity (water cloudiness) and light reduction caused by increased suspended sediments in the water column14,15,16,17. One cause of high turbidity is dredging of the sea bed and the subsequent disposal of the sediment at offshore dredge material placement sites18. Recent analyses of the effects of plumes from dredging have shown the marked effects the suspended sediments can have on benthic light levels19,20,21. Close to working dredges all benthic light can be extinguished during the daytime; however, a more common feature is very low ‘caliginous’ or daytime twilight periods, which can occur over extended periods (i.e., days to weeks)19. The high suspended sediment concentrations (SSCs), and the resulting light attenuation, decreases with increasing distance from dredging and understanding the impact of light reduction at different degrees is important for environmental impact assessments and managing dredging programs when underway.
The impacts of light reduction are likely to vary between sponge species, and this is likely to depend on the flexibility of their feeding strategy. For example, whilst the nutrition of some phototrophic sponges can be adversely affected by light reduction and a reduction of photosymbiont derived carbon, other species may have the ability to increase heterotrophic feeding rates to compensate for the decrease in photosynthetic yields6,7. Although cyanobacteria are the dominant photosymbiont taxa in most sponges10,22, dinoflagellates (i.e., Symbiodinium spp.) are also found in association with clionaid sponges23,24; and rhodophytes, chlorophytes and diatoms have been reported from a diverse range of sponges22,25,26. Therefore, responses to light attenuation may also differ depending on the symbiont composition within the host. Furthermore, highly specialised, host-specific symbionts often provide a greater benefit to their hosts than generalist symbionts14, and thus any negative effects from light attenuation will have a greater relative impact on those sponge species that host obligate photosymbionts.
Close to dredging, sponges will be exposed to low light conditions in combination with high SSCs and sedimentation20. This makes it difficult to identify cause-effect pathways, to identify potential bio-indicators and to establish the dose-response relationships needed for impact-prediction purposes. The effects of sedimentation on different sponge species, with a focus on sponge morphology, have been described previously27. In the current study, we examined the effects of light reduction alone on five abundant and geographically widespread sponge species1,28, spanning heterotrophic and phototrophic nutritional modes. In future studies, the effects of elevated SSC alone, sediment smothering alone and a combination of all dredging related factors will be described.
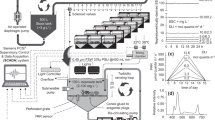
Using environmentally relevant exposure scenarios is essential for risk assessment purposes, and we used light reduction scenarios recently reported in several large-scale capital dredging projects on reefs in Western Australia19,21. The Daily Light Integral (DLI), which is the total photosynthetically active radiation (PAR) received each day as a function of light intensity and duration (mol photons m−2 d−1), was calculated over different running mean time periods from 1–30 days. For several shallow water sites (~6–9 m depth) located <1 Km from the dredge, the 5th percentile (P5) of DLIs ranged from 1.3–2.1 mol photons m−2 d−1 before dredging, but was 0.4–0.7 mol photons m−2 d−1 during the dredging phase. The number of consecutive days of very low light (<0.8 mol photons m−2 d−1) ranged from 0–10 d before dredging, but from 11–25 d during the dredging phase. For near complete loss of all light, the worst case scenario during the baseline phase was 2 consecutive days increasing to 5 consecutive days during the dredging phase19.
In this study, a suite of different response variables were used to examine the effects of environmentally realistic light reduction over an extended period (30 d), with a particular focus on changes in sponge symbiosis.
Results
Host response (health)
All sponges survived the 28 d experimental treatments, with no observable partial mortality (tissue necrosis). Changes in colour were observed in two of the three phototrophic species. Cliona orientalis discoloured after 72 h in the 0 mol photons m−2 d−1 treatment, and milder discolouration occurred in some individuals in the 0.8 mol photons m−2 d−1 treatment (see Supplementary Fig. S1). Despite this rapid response, the colouration returned to normal when returned to natural light for the 14 d observational period. Carteriospongia foliascens also discoloured in the 0 mol photons m−2 d−1 treatment after 7 d, and whilst there was no partial mortality at the end of the experimental period (Supplementary Fig. S1), all discoloured individuals died during the observational period. The third phototrophic sponge species, Cymbastela coralliophila, and the heterotrophic species Stylissa flabelliformis and Ianthella basta, showed no signs of discolouration throughout the experimental or recovery periods (Supplementary Fig. S1).
Host response (growth)
S. flabelliformis, I. basta, C. coralliophila and C. foliascens grew, or gained weight, over the experimental period and 14 d observational period in almost all treatments (Fig. 1a). Some individuals of C. foliascens from the 0 and 0.8 mol photons m−2 d−1 treatments lost weight during the recovery phase. The buoyant weight of the bioeroding, encrusting sponge C. orientalis decreased across all treatments (Fig. 1a), reflecting active bioerosion of the coral substrate. The percentage change in buoyant weight from the start of the experimental period to the end of the observational period was not significantly different between treatments for any species except C. orientalis (Table 1A). C. orientalis exposed to the 0 and 0.8 mol photons m−2 d−1 treatments lost significantly less weight than individuals in the 8.1 mol photons m−2 d−1 treatment. Accordingly, the mass of excavated substrate per unit area (i.e., surface area) in C. orientalis was the lowest under the 0 mol photons m−2 d−1 treatment, although this was not statistically significant (ANOVA: P = 0.870).
Physiological responses of sponges to light conditions.
(a) Percentage buoyant weight change (note: y-axis scales differ between species), and (b) Chlorophyll a concentrations, for the five species, after the 28 d experimental period and 14 d observational period (mean ± SE). Asterisks show statistically significant differences between the experiment and recovery phase (T-tests: P < 0.05). Light treatments include 0, 0.8, 3.2, 8.1 mol photons m−2 d−1 and natural light (NL, 3.2–6.5 mol photons m−2 d−1).
No change in sponge surface area between treatments could be detected at the end of the experimental period for any species and, with the exception of C. foliascens, no significant differences in surface area were detected between treatments from the start of the experimental period until the end of the observational period (Table 1B). A significant reduction in surface area was observed for C. foliascens at the end of the observational period (consistent with partial mortality in some individuals exposed to the 0 mol photons m−2 d−1 treatment, see Supplementary Fig. S2). Surface area could not be measured in C. orientalis which grows deeper into the coral substrate whilst still maintaining a constant surface area due to its bioeroding nature.
Microbial community response (pigment analyses)
Chlorophyll a concentrations of the phototrophic species C. coralliophila, C. foliascens and C. orientalis were typically higher than ~100 μg Chl a/g sponge, and concentrations in the heterotrophic species S. flabelliformis and I. basta were negligible (overall, < 10 μg Chl a/g sponge) (Fig. 1b). The only exception to this occurred in the 3.2 and 8.1 mol photons m−2 s−1 treatments for I. basta during the observational period, in which an algal bloom corresponded with the time of sampling (Fig. 1b). In both heterotrophic species, no significant difference in Chl a was detected between light treatments after the experimental or observational period (T-tests: P > 0.1) (Table 1C). The Chl a concentration of the phototrophic species under natural light was similar to the freshly collected field samples and t = 0 controls (ANOVA: P > 0.05). There were significant differences between the light treatments in the phototrophic species, and the highest Chl a concentration was observed in the natural light controls, followed by the 8.1, 3.2, 0.8 and 0 mol photons m−2 d−1 treatments (Fig. 1b, Table 1C). In the lower light treatments (0 and 0.8 mol photons m−2 d−1) there was an overall increase in Chl a concentration after the observational period in natural light (Fig. 1b).
Total chlorophyll was highly correlated to Chl a in the three phototrophic species (R2 = 0.834, 0.992, 0.994 for C. coralliophila, C. foliascens and C. orientalis, respectively, P < 0.001). However, total chlorophyll was also marginally correlated to Chl d in C. coralliophila (R2 = 0.305, P = 0.07), and highly correlated to Chl d in C. foliascens (R2 = 0.847, P < 0.001), and to Chl c in C. orientalis (R2 = 0.906, P < 0.001), consistent with the abundance of cyanobacteria in C. foliascens and C. coralliophila and Symbiodinium sp. in C. orientalis23,24. Accordingly, significantly lower values of Chl d and c were retrieved from C. foliascens and C. orientalis, respectively, in the 0 mol photons m−2 d−1 treatment (ANOVA: P < 0.001), while no signficant differences in Chl d were observed in C. coralliophila (ANOVA: P < 0.681).
Ultra Performance Liquid Chromatography (UPLC) analysis detected Chl a, Chl b, Chl c, Pheophytin a and six Carotenoids (i.e., Peridinin, Neoxanthin, Violaxanthin, Diadinoxanthin, Dinoxanthin and Zeaxanthin) with distinct, species-specific pigment profiles. Non-metric Multi-Dimensional Scaling (nMDS) analysis of the normalized spectrophotometry data and UPLC data showed that both approaches resulted in comparable sample groupings (see Supplementary Fig. S3) and were significantly correlated (R2 ≥ 0.8 and P < 0.001 in all cases). C. foliascens and C. coralliophila samples showed greater similarity in pigment profiles than C. orientalis, consistent with the presence of cyanobacterial photosymbionts in the former species and Symbiodinium sp. in the latter (Supplementary Fig. S3).
Non-metric MDS ordination analysis revealed that most sponge samples in the zero light treatment grouped closely together irrespective of species (Supplementary Fig. S3). PERMANOVA confirmed significant differences between species and treatments for both methods (Table 2). Subsequent pair-wise tests showed significant differences between samples in the dark and the natural light controls in all cases (Table 2) and C. foliascens samples in the 0.8 mol photons m−2 d−1 treatment differed from samples in the control and dark treatments.
Zeaxanthin is a pigment commonly related to the presence of Cyanobacteria29. Accordingly, it was significantly higher in C. coralliophila and C. foliascens than in C. orientalis (ANOVA: P = 0.002), while no significant differences between C. coralliophila and C. foliascens were detected (ANOVA: P > 0.05). This pigment was lowest in samples of C. coralliophila and C. foliascens in the zero light treatment (ANOVA: P = 0.103, < 0.005, respectively), followed by samples in the 0.8 mol photons m−2 s−1 and the natural light treatments. While Zeaxanthin concentration in C. orientalis was not significantly different between treatments (ANOVA: P = 0.469), concentrations dropped to zero in samples in the 0 mol photons m−2 d−1 treatment. On the other hand, Peridinin, which is an additional light harvesting pigment common in Symbiodinium sp., was exclusively present in C. orientalis. Whilst Peridinin was lowest in samples exposed to zero light, differences between treatments were not significant (ANOVA: P = 0.085).
Additional correlations were performed to determine which pigments best explained trends in sponge growth (i.e., based in buoyant weights at the end of the experimental period) within each phototrophic species. No significant correlations between any pigment and buoyant weight were detected in C. coralliophila and C. orientalis. However, growth rates were significantly correlated to all pigments in C. foliascens (i.e., Chl a, Chl d, Pheophytin, Zeaxanthin, Carotenoids and Total Chl; R2 > 0.7, P < 0.05), corroborating the higher degree of C. foliascens dependence on phototrophic nutrition.
Microbial community response (chlorophyll fluorescence)
Chlorophyll fluorescence was not significantly different between treatments at the start of the experiment for any species (Fig. 2). After 7 d, maximum quantum yields varied significantly among light intensities for all of the phototrophic species. Overall, individuals exposed to the 0.8 and 3.2 mol photons m−2 d−1 treatments exhibited increased photosynthetic efficiency during the first weeks (Fig. 2). Subsequently, both C. orientalis and C. foliascens showed a significant decrease in chlorophyll fluorescence in the 0 mol photons m−2 d−1 treatment. With the exception of C. foliascens in the zero light treatment, which suffered 100% mortality, fluorescence values of all other samples returned to normal during the observational phase under natural light conditions.
Temporal evolution of fluorescence levels.
Mean values (±SE) of fluorescence (maximum quantum yield) for the three phototrophic species per light treatment 0, 0.8, 3.2, 8.1 mol photons m−2 d−1 and natural light (NL, 3.2–6.5 mol photons m−2 d−1). Measurements were taken at the start of the experiment and then weekly until the end of the experimental and observational (O) periods. Asterisks show statistically significant differences among treatments (ANOVA: P < 0.05).
Microbial community response (microbiome)
A total of 13,240,437 high quality 16 S rRNA gene amplicon sequences were recovered from the 5 sponge species (n = 168 individual samples) and 5 seawater samples (environmental controls). Following quality filtering, 25 samples with low (<4906) read numbers were eliminated from the dataset which included 6 from S. flabelliformis, 10 from I. basta, 8 from C. coralliophila, and 1 seawater environmental control. The remaining samples were sub sampled down to the lowest read number (6587). Each sponge species maintained a unique microbial community (Table 3A, Fig. 3, see also Supplementary Fig. S4) that was distinct from the seawater microbiome (Table 3B, Fig. 3, Supplementary Fig. S4). Aquarium acclimation (for ~6 weeks) did not affect the sponge-associated microbial community of any species, as evidenced by the microbiome similarity of the field controls and the t = 0 experimental samples (Table 3A). Overall, the microbial community of the heterotrophic species S. flabelliformis and I. basta, and the phototrophic species C. coralliophila, was consistent amongst all sampling times (Table 3A). Highly significant differences in microbial community composition were observed in the phototrophic species C. foliascens and C. orientalis over time (i.e., from t = 0 until the end of the experimental period) (Table 3A).
Phylum-level bar chart.
Average relative abundance of each bacteria phylum (and class for Proteobacteria) for each species and light treatment (0, 0.8, 3.2, 8.1 mol photons m−2 d−1 and natural light: NL, 3.2–6.5 mol photons m−2 d−1). Only OTUs representing greater than 1% of the overall community were included. When significant differences in time were detected, samples from experimental (E) and observational (O) periods were plotted separately (i.e., 0 mol photons m−2 d−1 in C. orientalis, Table 3).
Microbial community composition did not differ between light treatments for either heterotrophic species or the phototrophic C. coralliophila, but differed between treatments in the phototrophic C. foliascens and C. orientalis (Table 3C, Fig. 3). Whilst no significant microbial shifts were detected in C. orientalis under different light treatments when all sample times were compared together (experimental and observational periods), differences were evident within sampling times when analysed independently (particularly in the zero light treatment) (Table 3C, Figs 3 and 4).
PCO analyses of C. foliascens and C. orientalis.
Principal coordinate analysis plots for C. foliascens and C. orientalis which showed significant differences based on light treatment or time according to PERMANOVA analysis (Table 3C). Square symbols represent samples at the end of 28 d experimental period, circles are samples at the end of the observational period and colours indicate the 5 light treatments (0, 0.8, 3.2, 8.1 mol photons m−2 d−1 and natural light: NL, 3.2–6.5 mol photons m−2 d−1).
At the Phylum level, the microbiome of C. coralliophila was primarily comprised of Alpha and Gammaproteobacteria, Acidobacteria and Cyanobacteria with only a small decrease in the relative abundance of Cyanobacteria occurring in C. coralliophila in the absence of light (Fig. 3). In contrast, the microbial community of C. foliascens in the zero light treatment showed a complete loss of Cyanobacteria and no recovery after the observational period (Table 3C, Figs 3 and 4). The prokaryotic microbiome of C. orientalis was primarily comprised of Alpha and Gammaproteobacteria and while minor representation of Cyanobacteria was evident in C. orientalis from most light treatments, this taxa was absent in the zero light treatment (Figs 3, 5 and Table S1). However, C. orientalis was able to re-establish its native microbiome after recovery under natural light (Fig. 4).
Cytoscape networks of C. foliascens and C. orientalis microbiome.
Networks of the microbiome community on 3 light treatments (0, 0.8 and natural light (3.2−6.5 mol photons m−2 d−1)) for the phototrophic species C. foliascens and C. orientalis. Circles correspond to different OTUs (with OTU numbers) and colours relate to their Phylum or Class level in the case of Proteobacteria. Only samples at the end of the 28 d experimental period were used (n = 3 per treatment).
Network analyses of the 100 most discriminatory operational taxonomic units (OTUs) were performed on C. foliascens and C. orientalis, for the 0, 0.8 mol photons m−2 d−1 and natural light treatments. The microbial community in C. foliascens was comprised of a large core microbiome (i.e., the component shared between samples in all treatments) that was unaffected by light treatment (Fig. 5). However, the three dominant Cyanobacteria OTUs (836, 5342 and 7813) were highly sensitive to light attenuation, being exclusively present in samples from the natural light treatment only. In contrast, Cyanobacteria OTUs 6 and 173 could tolerate the 0.8 mol photons m−2 d−1 treatment, but disappeared in the absence of light. This species also appears to shift its photosymbiont community under reduced light, with samples at the 0.8 mol photons m−2 d−1 acquiring novel Cyanobacteria OTUs 568 and 2339 which were exclusively present in C. foliascens from this treatment. Network analysis of the C. orientalis microbiome revealed a much smaller core community, and a large number of OTUs that were highly specific to samples in each light treatment (Fig. 5). As in C. foliascens, some Cyanobacteria OTUs (6 and 110) persisted under the natural light and 0.8 mol photons m−2 d−1 treatments, but disappeared in the zero light treatment, while Cyanobacteria OTU 61 was highly light sensitive and persisted in C. orientalis only under natural light conditions. Apart from the Cyanobacteria, the light sensitive community (i.e., taxa which were exclusively present in samples under natural light) included representatives of the Phyla Proteobacteria, Bacteroidetes, Chlamydiae, Verrucomicrobia and Acidobacteria as well as the candidate phylum SBR1093 and the Archaea. In contrast, in the zero light treatment, C. orientalis was colonised by novel OTUs within the Actinobacteria, Planctomycetes, Spirochaetes, Chloroflexi and additional OTUs affiliated to the Proteobacteria and Acidobacteria.
Discussion
Prolonged periods of low light caused by natural sediment resuspension events or dredging activities have the potential to adversely affect phototrophic sponges, although tolerance thresholds and capacity for recovery varied markedly in this study amongst sponge species. In contrast, heterotrophic sponge species appear unaffected by light attenuation as indicated by highly stable microbiomes, similar growth rates across all treatments and no visible signs of stress, including pigmentation changes or partial mortality.
The photosynthetic bioeroding sponge C. orientalis discoloured within as little as 7 d in darkness and exhibited minor discolouration after 28 d in the highly light attenuated treatment (0.8 mol photons m−2 d−1). These visible effects were accompanied by a significant decrease in chlorophyll a concentrations and changes in maximum quantum yields. This discolouration was ‘sponge bleaching’30, caused by loss of all Cyanobacteria and a marked reduction in the concentration of Chlorophyll a-containing Symbiodinium sp. Although bleaching occurred rapidly, C. orientalis still maintained quite high fluorescence values and also showed a rapid recovery of pigmentation on return to natural light conditions. Cliona varians, a congeneric species from the Caribbean, can also rapidly recover its native symbionts (within 2 weeks) following 8 months of exposure to total darkness31. Histological analysis of the C. orientalis tissues revealed the presence of some Symbiodinium deep in the tissue even after 28 d in darkness. Bioeroding Cliona sponges appear to be able to cope with extended periods of light reduction by retracting tissue and symbionts deep into the host coral skeleton32. This behavioural response could also have contributed to at least some of the discolouration, similar to effects observed with scleractinian corals where extreme retraction of the Symbiodinium spp. containing tissues during sub-aerial exposure can cause them to pale. This bleaching in corals is rapidly reversible (within hours) on return to more benign conditions.
It is not clear how the Symbiodinium sp. in C. orientalis could survive such periods of complete darkness. In culture, Symbiodinium spp. have been reported to undertake heterotrophic feeding33, although it remains to be determined whether this can occur in hospite. Nevertheless, the remnant symbiont population is significant, as the rapid division of this population can mean a relatively quick recovery from bleaching on return to the more favourable conditions.
C. foliascens survived the 28 d exposure to complete darkness, but underwent a loss of photosymbionts, a large shift in the microbiome and subsequently died in the observational period on return to natural light conditions. In contrast, the phototrophic C. coralliophila showed a high tolerance to reduced light as evidenced by only minor bleaching, slight reductions in chlorophyll a, minor changes in quantum yield values and a decrease in the relative abundance of Cyanobacteria in darkness. No significant changes were observed in the overall microbiome and complete recovery was observed within two weeks under natural light conditions.
Light is a critical factor controlling the growth and population demographics of phototrophic sponges as photosynthetic productivity from symbiotic microbes can provide between 48–80% of a sponge’s energy requirements3. Reduced light availability is therefore linked to energy depletion and associated mass reduction, as observed here for C. foliascens and as previously reported for other sponge species12,14. Light is also considered an important factor influencing excavation rates by bioeroding species1,24, and although some Symbiodinium sp. remained deep in the tissue even after four weeks without light, C. orientalis exposed to low light intensity also appeared to have reduced bioerosion capability, as inferred by a significantly smaller reduction in buoyant weight compared to control samples.
Although most phototrophic sponge species rely primarily on symbiotic relationships for their energy needs6,12, some have the ability to activate their heterotrophic metabolism under certain conditions. This switch to heterotrophy would enable sponges to survive under reduced light conditions, as has been reported in Petrosia ficiformis (Poiret, 1789), Aplysina fulva (Pallas, 1766) and Neopetrosia subtriangularis (Duchassaing, 1850)6,7. The same switching has also been observed in photosymbiotic corals during low light associated with natural turbidity events34.
The different responses of the three phototrophic sponges under low light conditions may be related to different degrees of dependency on the photosynthetic symbionts. For instance, Phyllospongia lamellosa (Esper, 1794), a foliaceous phototrophic sponge closely related to C. foliascens35, relies on photosynthesis for the majority of its respiratory carbon requirements12. In C. foliascens the symbiosis is highly specialized and largely driven by a Synechococcus sp. (OTU 6)36. The strong correlation between photopigments and growth rates in C. foliascens provides further support for a high degree of nutritional dependence on photosymbionts. In contrast, C. coralliophila tolerated the 4-week light attenuation and its growth rate showed no correlation with any pigment, suggesting that it may have the ability to increase its heterotrophic feeding rates when ambient light conditions change and photosynthetic activity of symbionts is reduced. However, longer-term experiments are still required as a 90 day light attenuation study on a congeneric species, Cymbastela concentrica (Lendenfeld, 1887), resulted in bleaching, necrosis, and significant reductions in growth, reproductive status and concentration of chlorophyll a15.
While many sponge species host highly stable microbiomes irrespective of environment37,38,39,40, recent research has also indicated that shifts in the sponge-associated microbial community may confer a mechanism for local host acclimation to different environmental conditions41. The large shifts in the microbiome of C. foliascens and C. orientalis under low light conditions, including the appearance of novel photosynthetic microorganisms under highly light attenuated conditions (DLI of 0.8 mol photons m−2 d−1), may indicate an attempt at symbiont shuffling to a community better able to function under reduced irradiance. The rapid microbiome recovery of both C. orientalis and C. coralliophila under natural light further indicates significant flexibility in symbiosis in these species. However, while some species may have the ability to successfully alter the composition of their microbial community under different environmental conditions42, an extensive body of literature also highlights that species with intimate and/or potentially obligate symbioses can be adversely impacted by disruption of the microbiome43,44 or loss of symbiotic function45. The rapid deterioration in health of C. foliascens following the microbiome shift is consistent with its intimate reliance on a highly specialised microbial community.
The levels of light reduction attained close to dredging activities were recently described in a large-scale capital project on reefs in Western Australia19,21. The light treatments used in the present study are clearly relevant to conditions that can occur during dredging, showing that light attenuation is not only a hazard to photosynthetic sponges but also a risk in close proximity to dredging operations46. This study examined the specific effects of light reduction on sponges, with the combined effects of elevated SSCs and light attenuation being investigated in subsequent experiments. Nevertheless, based on light reduction alone, the rapid bleaching response observed in C. foliascens (irreversible) and C. orientalis (reversible) suggests that discolouration of sponges would be an effective bioindicator during dredging operations, as long as natural variations in colour are taken into consideration. The results of this study could be used in conjunction with water quality monitoring programs to alert dredging proponents to levels of light reduction that, if continued, could result in harm to phototrophic sponges. In combination with sediment plume models and estimates of the light attenuation by the suspended sediment, these results could be used to make predictions of the likely effects of dredging (i.e., at the EIA stage). By modelling different dredging scenarios (i.e., volume of material dredged, tidal phase, over flow options, etc.) the information could also be used to identify optimal dredging scenarios that minimise the likelihood and extent of impact. Benthic light availability should be considered over multiple different time periods (from days to weeks) allowing dredging proponents to be aware of, or alerted to, conditions that could potentially impact sponge physiology.
Methods
Sample collection
To facilitate a response comparison across nutritional modes, the study was conducted with the phototrophic sponges Cymbastela coralliophila (Hooper & Berquist 1992), Carteriospongia foliascens (Pallas, 1766) and Cliona orientalis (Thiele, 1900), and the heterotrophic sponges Stylissa flabelliformis (Hentschel, 1912) and Ianthella basta (Pallas, 1976)12,23,27,47,48. All sponges were collected from 3–15 m depth from either the Palm Islands or Davies Reef, Great Barrier Reef (GBR) (see Supplementary Table S2). C. orientalis is an encrusting sponge that bioerodes coral, hence cores of sponge on top of dead coral substrate where chiselled from Porites sp. colonies. Sponges were cut into similar sized explants (~10 × 10 cm), and placed in natural light acclimation tanks with flow-through seawater for up to 6 weeks until they had completely healed.
Experimental Set up
All experiments were performed in the National Sea Simulator (SeaSim) at the Australian Institute of Marine Science (AIMS, Townsville). Tests were conducted in 50 L acrylic tanks supplied with a continous inflow of 5 μm filtered seawater at a rate of 833 mL min−1. The high inflow rates ensured one complete turnover of the tanks per hour of partially filtered seawater, which ensured that sponges received sufficient particulate food including microoganisms. Experiments were conducted in a constant environment room with water temperature set to 29 °C, representing the temperature at the time of sponge collection. Sponges were exposed to 5 different light treatments selected to represent different levels of light attenuation in a dredging plume19,49. Tanks were illuminated on a 12:12 h L:D cycle, with a light regime designed to simulate daily conditions on the reef. Each day there was a 3 hour morning period (from 06:00–09:00) of gradually increasing light from darkness to a maximum instantaneous light level of 25, 100 and 250 μmol photons m−2 s−1 for each of the 3 treatments, followed by a 3 h afternoon period (from 15:00–18:00) of gradually decreasing light until full darkness. A natural light control (~150–300 μmol photons m−2 s−1 at noon) was also used, and the last treatment was complete darkness. Factoring in the increase and decrease in light levels and maximum instantaneous light level during the middle of the day, the daily light integrals (DLIs) were 0, 0.8, 3.2 and 8.1 mol photons m−2 d−1 respectively, and 3.2–6.5 mol photons m−2 d−1 for the natural light control, depending on weather conditions. The light attenuation experiment was conducted for a 28 d ‘experimental’ period, followed by a 14 d ‘observational’ (i.e., recovery) period under natural light. There were 3 tanks per treatment, each with 2 sponge replicates per species (i.e., 6 replicates of each species per treatment).
Studied parameters
The effect of the different light treatments on the sponge holobiont was determined using a suite of response variables, with a particular focus on changes in sponge photosymbionts and composition of the sponge microbial community. For each species, 3 extra individuals were collected as field controls, and immediately processed in situ (see below) in order to determine any potential effects of the handling and experimental enclosures. At the start of the experiment, 3 additional individuals per species were sampled (t = 0 controls) to obtain baseline data on sponge health. Unless otherwise stated, statistical analyses were performed using the software SigmaPlot v.11.0 (Systat Software Inc.).
Sponge growth
To obtain a proxy for sponge growth, initial and final weights were measured for all individuals using a buoyant weight scale (±0.001 g) at a constant temperature of 29 °C. Among a selection of methods to measure growth, buoyant weights were used to prevent potential blockages from air exposure, to allow subsequent tissue sampling and because they are good estimators of sponge growth across environmental gradients50. In the encrusting C. orientalis, buoyant weights indicate the net change between the weight of tissue (i.e., mostly spicules) and changes in the CaCO3 substrate caused by its bioeroding activity. Hence, weights in C. orientalis are not comparable with other sponge species. The mass of excavated substrate per unit area was also calculated in C. orientalis using surface area based on images (see below). However, due to the irregularity in shape of some of the replicates, these values are an estimation rather than exact size values. The percentage weight change between day 0 and the end of the recovery phase was assessed for each species separately using one-way analysis of variance (ANOVA) with treatment as the fixed factor. The mass of excavated substrate per unit area between day 0 and the end of the experimental phase was also assessed for C. orientalis using one-way ANOVA and treatment as fixed factor.
A 2-dimensional proxy for partial mortality (tissue necrosis), size change (i.e., surface area), and colour was recorded weekly using a digital camera with underwater housing (Canon PowerShot SX50-HS) and image analysis software (Image J51). Images were always taken under maximum instantaneous light levels (9.00–15.00 h) to eliminate any possible diurnal variation in colour. Changes in percent surface area after the experimental and observational periods were studied for each species separately using one-way ANOVA with treatment as the fixed factor. In any instances where homogeneity of variances and normality were not evident, a Kruskal-Wallis one-way ANOVA on ranks was performed.
Pigment analyses
Pigment analyses were performed spectrophotometrically on the field controls, on the sponges collected at t = 0 and at the end of the experimental and observational periods. Two 1 × 0.5 × 0.5 cm pieces of tissue incorporating pinacoderm and mesohyl regions were excised per individual, placed immediately in liquid nitrogen and stored at −80 °C until pigment and symbiont analysis. Pigments were extracted from a weighed piece of sponge using 95% ethanol and a Bead Beater (Bio Spec Products Inc., Bartlesville, USA)27. Absorbance at 470, 632, 649, 665, 696 and 750 nm was measured using a Power Wave Microplate Scanning Spectrophotometer (BIO-TEK® Instruments Inc., Vermont USA). Chlorophyll a, b, c and d, and total carotenoids were calculated using the equations provided in refs 52,53 and standardized to sponge wet weight.
The concentration of chlorophyll a was used as a proxy for changes in photosymbiont health/activity (i.e., bleaching) due to light attenuation3. Changes in chlorophyll a concentration during the experimental period were assessed for each species separately using a one-way ANOVA with treatment as the fixed factor. In any instances where homogeneity of variances and normality were not evident, a Kruskal-Wallis one-way ANOVA on ranks was performed. Differences between chlorophyll a at the end of the experimental and observational periods were assessed with a t-test for each treatment and species, separately. Photopigments were additionally measured in a selection of samples (phototrophic species from the 0 and 0.8 mol photons m−2 d−1 and natural light treatments, n = 27) by Ultra Performance Liquid Chromatography (UPLC) to determine the type and quantity of pigments present54.
All pigments retrieved by spectrophotometry and UPLC were used to build resemblance matrices based on normalized data. Non-metric Multi-Dimensional Scaling (nMDS) plots were created for each method separately using Euclidean distances. Two factors were determined (species, light treatment) and examined by PERMANOVA (Permutational multivariate ANOVA based on distances). Analyses were performed using PRIMER 6 (Primer-E Ltd, UK).
Chlorophyll fluorescence
Changes in photosynthetic capacity (maximum quantum yield [Fv/Fm]) of the sponge’s phototrophic symbionts were measured with a Diving-PAM (pulse amplitude modulation) chlorophyll fluorometer (Heinz Walz GmbH, Effeltrich, Germany). Chlorophyll fluorescence measurements were obtained 10 mm from the sponge tissue by placing a 6 mm fibre-optic probe perpendicular to the surface. Initial fluorescence was determined using a pulse-modulated red measuring light (655 nm, 0.15 μmol photons m−2 s−1 at 0.6 kHz). Three measures (maximum quantum yield) were obtained weekly on dark adapted samples (30 min) for the three phototrophic species (i.e., C. coralliophila, C. foliascens and C. orientalis) throughout the experimental and observational periods. One-way ANOVAs were performed to test whether treatment had any effect on chlorophyll fluorescence of the sponges at any given time. The effect of time within every treatment and species was also assessed with a one-way ANOVA.
Microbial community analysis
The composition of the sponges microbial community was assessed using Illumina amplicon sequencing of the 16 S rRNA gene. Samples were collected at the end of the experimental and observation period, immediately frozen in liquid nitrogen and stored at −80 °C. Water samples were simultaneously collected from each tank to facilitate a direct comparison with microbes present in the surrounding environment. DNA was extracted from ~0.25 g of sponge tissue using the PowerSoil®-htp 96 Well Soil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA) according to standard protocols (http://press.igsb.anl.gov/earthmicrobiome/emp-standard-protocols/dna-extraction-protocol/). Microbial communities in seawater were collected by passing 1 L of seawater through 0.2 μm Sterivex filters and DNA was extracted from the filters as previously described55. Aliquots of the extracted DNA were shipped to the University of Colorado, (Boulder, Colorado, USA) for sequencing using standard Earth Microbiome Project (http://www.earthmicrobiome.org/emp-standard-protocols/16s/) protocols. Briefly, the V4 region of the 16 S rRNA gene was amplified using the primer 515 f – 806r and sequenced using the HiSeq2500 platform (Illumina). Processed sequences and meta-data are available via the following portal (http://qiita.microbio.me/) under study number 10533.
Amplicon sequence data was processed in Mothur v.1.35.156. Firstly, quality-filtered, demultiplexed fastq sequences were trimmed according to quality and processed as per57. Only sequences that aligned to the expected position were kept. Aligned reads were reduced to non-redundant sequences and chimeric sequences were detected using Uchime58. Aligned sequences were phylogenetically classified based on the Ribosomal Database Project (RDP) reference file v.1459, and all undersigned sequences removed (taxon = Chloroplast-Mitochondria-unkown-Eukaryota). Pairwise distances between aligned sequences were calculated and used for clustering. Operational taxonomic units (OTUs) were retrieved based on the distance among the clustered sequences and were further classified based on the Greengenes taxonomy60.
Pivot tables were used to condense tables by phylum and class for graphical interpretation. OTU data was normalised to account for sampling depth and then square-root transformed to reduce the effect of abundant OTUs. Bray-Curtis distance matrices were constructed to examine additional patterns of community structure and visualised using non-metric multidimensional plots (nMDS) and principal coordinate analyses (PCO). Permutational analysis of variance (PERMANOVA, using 9,999 permutations) was used to determine significant differences in microbial communities based on source (sponge versus environmental control), time of sampling (field controls, t = 0, experimental and observational period), and all five light treatments. All multidimensional statistical analyses were performed in PRIMER 6. Similarity Percentage (SIMPER) analysis was used to determine the OTUs that contribute to the differences between the natural light, 0.8 and 0 mol photons m−2 d−1 treatments) for each phototrphic species, separetely. The 100 OTUs with the most discriminating power from the SIMPER analysis were used to create networks on Cytoscape 3.2.0 (www.cytoscape.org)61.
Additional Information
How to cite this article: Pineda, M.-C. et al. Effects of light attenuation on the sponge holobiont- implications for dredging management. Sci. Rep. 6, 39038; doi: 10.1038/srep39038 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bell, J. J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 79, 341–353 (2008).
Peterson, B., Chester, C., Jochem, F. & Fourqurean, J. Potential role of sponge communities in controlling phytoplankton blooms in Florida Bay. Mar. Ecol. Prog. Ser. 328, 93–103 (2006).
Wilkinson, C. R. Net primary productivity in coral reef sponges. Science. 219, 410–412 (1983).
Webster, N. S. & Taylor, M. W. Marine sponges and their microbial symbionts: love and other relationships. Environ. Microbiol. 14, 335–46 (2012).
Wilkinson, C. R. Interocean differences in size and nutrition of coral reef sponge populations. Science. 236, 1654–7 (1987).
Erwin, P. & Thacker, R. Phototrophic nutrition and symbiont diversity of two Caribbean sponge–cyanobacteria symbioses. Mar. Ecol. Prog. Ser. 362, 139–147 (2008).
Arillo, A., Bavestrello, G., Burlando, B. & Sara, M. Metabolic integration between symbiotic cyanobacteria and sponges: a possible mechanism. Mar. Biol. 117, 159–162 (1993).
Freeman, C. J. & Thacker, R. W. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 56, 1577–1586 (2011).
Thacker, R. W. & Freeman, C. J. In Adv. Mar. Biol. ( Becerro, M. A., Uriz, M. J., Maldonado, M. & Turon, X. ) 62, 57–111 (Elsevier Ltd, 2012).
Usher, K. M. The ecology and phylogeny of cyanobacterial symbionts in sponges. Mar. Ecol. 29, 178–192 (2008).
Wilkinson, C. & Trott, L. Light as a factor determining the distribution of sponges across the central Great Barrier Reef. In Proc. 5th int Coral Reef Symp., Tahiti 5, 125–130 (1985).
Cheshire, A. C. A., Wilkinson, C. R. C., Seddon, S. & Westphalen, G. Bathymetric and seasonal changes in photosynthesis and respiration of the phototrophic sponge Phyllospongia lamellosa in comparison with respiration by the heterotrophic sponge Ianthella basta on Davies Reef, Great Barrier Reef. Mar. Freshw. Res. 48, 589–599 (1997).
de Goeij, J. M. et al. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342, 108–10 (2013).
Thacker, R. W. Impacts of shading on sponge-cyanobacteria symbioses: A comparison between host-specific and generalist associations. Integr. Comp. Biol. 45, 369–376 (2005).
Roberts, D., Davis, A. & Cummins, S. Experimental manipulation of shade, silt, nutrients and salinity on the temperate reef sponge Cymbastela concentrica. Mar. Ecol. Prog. Ser. 307, 143–154 (2006).
Bell, J. J. et al. Sediment impacts on marine sponges. Mar. Pollut. Bull. 94, 5–13 (2015).
Stubler, A. D., Duckworth, A. R. & Peterson, B. J. The effects of coastal development on sponge abundance, diversity, and community composition on Jamaican coral reefs. Mar. Pollut. Bull. 93, 261–270 (2015).
Morton, W. Ecological effects of dredging and dredge spoil disposal: A literature review. United States Department of the Interior. Fish and Wildlife service (1977).
Jones, R., Fisher, R., Stark, C. & Ridd, P. Temporal patterns in seawater quality from dredging in tropical environments. PLoS One 10, e0137112 (2015).
Jones, R., Bessell-Browne, P., Fisher, R., Klonowski, W. & Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 102, 9–29 (2016).
Fisher, R., Stark, C., Ridd, P. & Jones, R. Spatial patterns in water quality changes during dredging in tropical environments. PLoS One 10, e0143309 (2015).
Rützler, K. In New Perspect. sponge Biol. ( Rützler, K. ) 455–466 (Smithsonian Institute Press, 1990).
Schönberg, C. H. L. & Loh, W. K. W. Molecular identity of the unique symbiotic dinoflagellates found in the bioeroding demosponge Cliona orientalis. Mar. Ecol. Prog. Ser. 299, 157–166 (2005).
Hill, M., Allenby, A., Ramsby, B., Schönberg, C. & Hill, A. Symbiodinium diversity among host clionaid sponges from Caribbean and Pacific reefs: Evidence of heteroplasmy and putative host-specific symbiont lineages. Mol. Phylogenet. Evol. 59, 81–8 (2011).
Taylor, M. W., Radax, R., Steger, D. & Wagner, M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 71, 295–347 (2007).
Lemloh, M.-L., Fromont, J., Brümmer, F. & Usher, K. M. Diversity and abundance of photosynthetic sponges in temperate Western Australia. BMC Ecol. 9, 4 (2009).
Pineda, M. C., Duckworth, A. & Webster, N. Appearance matters: sedimentation effects on different sponge morphologies. J. Mar. Biol. Assoc. United Kingdom 96, 481–492 (2016).
Fromont, J. Porifera (sponges) of the Dampier Archipelago, Western Australia: Rec. West. Aust. Museum 100, 69–100 (2004).
Rowan, K. S. Photosynthetic pigments of algae. (Cambridge University Press, 1989).
Fromont, J. & Garson, M. Sponge bleaching on the West and East coasts of Australia. Coral Reefs 18, 340 (1999).
Riesgo, A. et al. Transcriptomic analysis of differential host gene expression upon uptake of symbionts: a case study with Symbiodinium and the major bioeroding sponge Cliona varians. BMC Genomics 15, 376 (2014).
Schönberg, C. H. L. & Suwa, R. In Porifera Res. Biodiversity, Innov. Sustain. ( Custódio, M., Hajdu, E., Lôbo-Hajdu, G. & Muricy, G. ) 569–580 (Publ Museu Nac Rio de Janeiro, 2007).
Jeong, H. J. et al. Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium. Proc. Natl. Acad. Sci. USA 109, 12604–9 (2012).
Bessell-Browne, P., Stat, M., Thomson, D. & Clode, P. L. Coscinaraea marshae corals that have survived prolonged bleaching exhibit signs of increased heterotrophic feeding. Coral Reefs 33, 795–804 (2014).
Abdul Wahab, M. A., Fromont, J., Whalan, S., Webster, N. & Andreakis, N. Combining morphometrics with molecular taxonomy: How different are similar foliose keratose sponges from the Australian tropics? Mol. Phylogenet. Evol. 73, 23–39 (2014).
Webster, N. S. et al. Same, same but different: symbiotic bacterial associations in GBR sponges. Front. Microbiol. 3, 444 (2013).
Erwin, P. M., Pita, L., López-Legentil, S. & Turon, X. Stability of sponge-associated bacteria over large seasonal shifts in temperature and irradiance. Appl. Environ. Microbiol. 78, 7358–68 (2012).
Webster, N. S., Botté, E. S., Soo, R. M. & Whalan, S. The larval sponge holobiont exhibits high thermal tolerance. Environ. Microbiol. Rep. 3, 756–762 (2011).
Luter, H. M., Whalan, S. & Webster, N. S. Thermal and sedimentation stress are unlikely causes of brown spot syndrome in the Coral Reef sponge, Ianthella basta. PLoS One 7, 1–9 (2012).
Simister, R., Taylor, M. W., Tsai, P. & Webster, N. Sponge-microbe associations survive high nutrients and temperatures. PLoS One 7, e52220 (2012).
Morrow, K. M. et al. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J. 9, 894–908 (2015).
Webster, N. S. et al. Bacterial community dynamics in the marine sponge Rhopaloeides odorabile under in situ and ex situ cultivation. Mar. Biotechnol. (NY). 13, 296–304 (2011).
Simister, R. et al. Thermal stress responses in the bacterial biosphere of the Great Barrier Reef sponge, Rhopaloeides odorabile. Environ. Microbiol. 14, 3232–46 (2012).
Webster, N. S., Cobb, R. E. & Negri, A. P. Temperature thresholds for bacterial symbiosis with a sponge. ISME J. 2, 830–42 (2008).
Fan, L., Liu, M., Simister, R., Webster, N. S. & Thomas, T. Marine microbial symbiosis heats up: the phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J. 7, 991–1002 (2013).
Harris, C. A. et al. Principles of sound ecotoxicology. Environ. Sci. Technol. 48, 3100–3111 (2014).
Cheshire, A. et al. Preliminary study of the distribution and photophysiology of the temperate phototrophic sponge Cymbastela sp. from South Australia. Mar. Freshw. Res. 46, 1211–1216 (1995).
Ridley, C. P., Faulkner, D. & Haygood, M. G. Investigation of Oscillatoria spongeliae-dominated bacterial communities in four dictyoceratid sponges. Appl. Environ. Microbiol. 71, 7366–75 (2005).
Anthony, K. R. N., Ridd, P. V., Orpin, A. R., Larcombe, P. & Lough, J. Temporal variation of light availability in coastal benthic habitats: Effects of clouds, turbidity and tides. Limnol. Oceanogr. 49, 2201–2211 (2004).
Trussell, G. C., Lesser, M. P., Patterson, M. R. & Genovese, S. J. Depth-specific differences in growth of the reef sponge. Callyspongia vaginalis : role of bottom-up effects. 323, 149–158 (2006).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Lichtenthaler, H. Chlorophylls and Carotenoids -Pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987).
Ritchie, R. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46, 115–126 (2008).
Uthicke, S., Vogel, N., Doyle, J., Schmidt, C. & Humphrey, C. Interactive effects of climate change and eutrophication on the dinoflagellate-bearing benthic foraminifer Marginopora vertebralis. Coral Reefs 31, 401–414 (2012).
Webster, N. S. et al. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ. Microbiol. 12, 2070–82 (2010).
Schloss, P. D. et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Luter, H. M. et al. Biogeographic variation in the microbiome of the ecologically important sponge, Carteriospongia foliascens. PeerJ 3, e1435 (2015).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Cole, J. R. et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642 (2014).
DeSantis, T. Z. et al. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006).
Shannon, P. et al. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
This research was funded by the Western Australian Marine Science Institution (WAMSI) as part of the WAMSI Dredging Science Node, and made possible through investment from Chevron Australia, Woodside Energy Limited, BHP Billiton as environmental offsets and by co-investment from the WAMSI Joint Venture partners. The commercial entities had no role in data analysis, decision to publish, or preparation of the manuscript. NSW was funded through an Australian Research Council Future Fellowship FT120100480. The views expressed herein are those of the authors and not necessarily those of WAMSI. All collections were performed under Great Barrier Reef Marine Park Regulations 1983 (Commonwealth) and Marine Parks regulations 2006 (Queensland) Permit G12/35236.1 and Permit G13/35758.1. We are thankful to staff at the AIMS National Sea Simulator for their help during experiment set up. Dr. C. Schönberg and Dr. J. Fromont contributed to the species identification. Dr. M. Abdul Wahab commented on a previous version of the manuscript. Sequence data was obtained through support from the Earth Microbiome Project and Dr. H. Luter, Dr. L. Moitinho-Silva, Dr. P. Laffy, C. Astudillo and G. Millar provided useful advice on the microbial community data analyses and use of the HPC.
Author information
Authors and Affiliations
Contributions
M.C.P., B.S., A.D., R.J. and N.S.W. designed the experiment. M.C.P. and B.S. undertook the experiment. M.C.P., B.S. and J.D. undertook laboratory analyses. M.C.P. and B.S. analysed the data. M.C.P., B.S., A.D., R.J. and N.S.W. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pineda, MC., Strehlow, B., Duckworth, A. et al. Effects of light attenuation on the sponge holobiont- implications for dredging management. Sci Rep 6, 39038 (2016). https://doi.org/10.1038/srep39038
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39038
This article is cited by
-
Harnessing solar power: photoautotrophy supplements the diet of a low-light dwelling sponge
The ISME Journal (2022)
-
Bacterial diversity associated with a newly described bioeroding sponge, Cliona thomasi, from the coral reefs on the West Coast of India
Folia Microbiologica (2021)
-
Microbial diversity of the glass sponge Vazella pourtalesii in response to anthropogenic activities
Conservation Genetics (2020)
-
Microbiome structure of ecologically important bioeroding sponges (family Clionaidae): the role of host phylogeny and environmental plasticity
Coral Reefs (2020)
-
Exploring the diversity-stability paradigm using sponge microbial communities
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.