Abstract
The cardiothoracic ratio (CTR) and peripheral arterial occlusive disease (PAOD) are related to mortality in hemodialysis patients. However, data on the association between PAOD and CTR are limited. In this study, we aim to elucidate this relationship in patients on chronic hemodialysis. Using a retrospective cross-sectional study of 622 Taiwanese patients, we investigated the association of PAOD and CTR. PAOD was significantly associated with CTR in the crude analysis. The odds ratio (OR) for CTR >0.5 was 1.77 [95% confidence interval (CI), 1.32–2.37], and the odds ratio for CTR >0.6 was 2.18 [95% CI, 1.44–3.30]. After adjusting for confounding variables, this difference continued to exhibit significant predictive power for CTR >0.6 (OR, 1.88; 95% CI, 1.14–3.11), but the predictive power for CTR >0.5 was attenuated (OR, 1.41; 95% CI, 0.98–2.03). In the subgroup analysis, PAOD was an independent factor for CTR >0.6, particularly in elderly and female patients or patients with hemoglobin >10 mg/dl and with no history of cardiovascular disease. In this research, we showed that the detection of PAOD was independently associated with CTR >0.6 in patients on chronic hemodialysis.
Similar content being viewed by others
Introduction
The cardiothoracic ratio (CTR) represents the left ventricular size and is estimated from chest X-rays as a proportion of the thoracic diameter1; furthermore, CTR is negatively associated with cardiac systolic dysfunction2. In general, the higher the CTR value, the larger the size of the heart. A CTR >50% is thought to represent cardiomegaly and is a prognostic factor in elderly populations3, patients with congestive heart failure4, and patients on dialysis5,6. Moreover, an enlarged heart increases the risk of fatal arrhythmia7. Therefore, CTR provides an easier representation of the status of cardiac remodeling and an easy method to assess the heart condition of chronic kidney disease (CKD) patients.
The ankle–brachial index (ABI), which is a ratio of ankle to brachial systolic blood pressure (BP), is a simple, non-invasive and reliable tool to diagnose peripheral arterial occlusive disease (PAOD)8, an atherosclerotic disorder that refers to varying degrees of occlusion of the lower limb arteries and is frequently observed in CKD9,10 and chronic hemodialysis (CHD) patients11,12. Furthermore, the ABI is a predictor of all-cause or cardiovascular mortality in CHD patients12,13,14,15,16. A lower (<1.0) or higher (>1.4) ABI value in CKD patients induces a higher event rate of acute myocardial infarction or cardiovascular disease (CVD)17.
Both cardiomegaly and PAOD contribute to CVD development in CHD patients. However, little evidence is available to evaluate the association of PAOD with CTR in patients with CHD. Akasawa et al. reported that PAOD had no significant association with CTR >0.518. However, their PAOD diagnosis was assessed based on medical history alone and may have underestimated PAOD prevalence. We postulated that PAOD would complicate cardiac enlargement, which indicates cardiac remodeling in patients with CHD. This study investigates the association between PAOD and CTR in patients with CHD.
Methods and Materials
Study design and patients
This retrospective cross-sectional study was conducted at a single medical center. To be eligible for this study, patients must have undergone regular hemodialysis (HD) for at least 3 months before inclusion. Patients were required to have been clinically stable for 3 months prior to the study; specifically, patients with acute cardiovascular event cerebrovascular disease, infection, or other active diseases were excluded. Finally, a total of 622 patients on regular HD in the dialysis unit of Shin Kong Wu Ho-Su Memorial Hospital from December 2009 to December 2012 were included in the study (Fig. 1). This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of Shin-Kong Wu Ho-Su Memorial Hospital. Informed consent was waived because our study was based on medical chart review. Patient information was anonymized and de-identified prior to analysis.
Medical and laboratory data
Demographic and medical data were obtained from the patients’ medical records upon entry into the study and included age; gender; smoking history (never vs. ever); blood pressure (BP); history of diabetes mellitus (DM), hypertension, coronary artery disease, or cerebrovascular disease; body mass index (BMI; weight/height2); duration on HD, CTR, and ABI; co-morbid conditions; intake of renin-angiotensin system (RAS) blockers, statins, beta-blockers, and anti-platelet agents; serum levels of blood urea nitrogen (BUN), creatinine (Cr), albumin, uric acid, total cholesterol (TC), triglyceride (TG), iron profile, hemoglobin (Hb), intact parathyroid hormone (iPTH), ionized calcium (iCa), and phosphate (P); and urea kinetics (Kt/V), determined according to the procedure described by Shinzato et al.19 CVD was diagnosed according to documented histories of coronary artery or cerebrovascular disease. Blood samples were collected after at least 8 hours of fasting and before the dialysis session.
Cardiothoracic ratio measurement
At the end of the year at our medical center, posterior-anterior chest radiographs were routinely obtained after HD sessions in patients with CHD to measure the CTR. Computer assistance was employed to ensure accurate measurement. A reference vertical line was drawn on the radiograph through the midpoint of the spine from the sternum to the diaphragm. The maximum transverse diameter of the heart was obtained by adding the widest distance at the midline from the right to the left heart borders. Thoracic width was measured as the distance between the inner aspects of the widest points of the rib cage. The CTR was determined by dividing the maximal horizontal width of the heart by the horizontal inner width of the rib cage. Therefore, a CTR >50% was defined as cardiomegaly; a higher CTR indicated increased severity of cardiomegaly.
Ankle brachial index
At our medical center, a PAOD survey for ABI measurement was performed in patients with CHD from 2009 and 2012. ABI was measured using a sphygmomanometer and a sphygmograph device during the HD session. BP was measured in all patients after a minimum rest of 5 minutes: cuffs with pressure sensors were wrapped around the arm without vascular access and around both ankles. The systolic BP of the brachial pulses was recorded for the upper limb. To measure the systolic BP of the dorsalis pedis and posterior tibial arteries in the lower limbs, the BP cuff was applied proximal to the ankle, inflated rapidly and deflated gradually. The mean of these two readings was used as the ankle systolic BP. The ABI was calculated by dividing the ankle systolic BP by the brachial artery systolic BP. The systolic BP of the arm without dialysis access and the bilateral ankle pressure were used separately for the calculation. Patients with an ABI of <0.9 in either leg were considered to have varying degrees of PAOD in their lower extremities.
Statistical analyses
Data were expressed as the mean ± standard deviation (SD) or median (25th, 75th percentile), as appropriate for continuous or categorical variables. Independent t-tests were used to compare the means of continuous variables, and the chi-square test was used for categorical variables. Moreover, a generalized mixed linear model was used to determine the risks of CTR >0.5 and CTR >0.6 with a link function of logit. We modeled the variance-covariance of residuals for repeated measurements as first order autoregression [AR (1)]. Using a modified stepwise procedure with five modeling steps, a separate regression model was used for dichotomous CTR as a function of PAOD. We also performed subgroup analysis including factors such as DM, age (≤65 years and >65 years), gender, previous CVD and hemoglobin level (≤10 g/dL and >10 g/dL). A P value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SAS for Windows version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
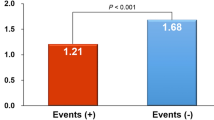
The 622 CHD patients had a mean age of 62.7 ± 13.5 years and had been on HD for a mean duration of 6.9 ± 5.3 years. Among them, 40.7% were diabetic, and 49.7% were men. CTR was >0.5 in 308 (49.8%) patients and >0.6 in 74 (11.9%) patients. The mean CTR was 51 ± 7.4% (25th–75th percentile, 46–56%), as shown in Fig. 2. Moreover, a significant linear association was observed between CTR and ABI on the right side and the left side (Fig. 3). In total, 177 (28.4%) patients were diagnosed with PAOD based on an ABI of <0.9 in either leg. The other clinical characteristics of the participants are presented in Table 1.
PAOD and non-PAOD
Table 1 also lists the demographic and clinical data of the participants stratified by the presence of PAOD. Patients with PAOD had significantly older age and increased Kt/V and CTRs, in either dichotomous or continuous form; reduced albumin, P and BP; and a reduced incidence of previous CVD and DM (all P values, <0.05). No significant difference was noted regarding gender, HD duration, smoking status, medication use, BMI, iron profile, lipid profile, Hb, iPTH and iCa among the subgroups dichotomized by PAOD (all P values, <0.05).
Determinants of CTR >0.5 and CTR >0.6
Factors associated with CTR >0.5 and >0.6 were assessed by a generalized mixed model with link function of logit. Table 2 shows that in the crude analysis, age, gender, DM, previous CVD, smoking status, diastolic BP, BMI, albumin, Hb, transferrin saturation, P and PAOD were significantly associated with CTR >0.5 (all P values < 0.05). After adjusting for multiple variables, only age [odds ratio (OR), 1.04; 95% confidence interval (CI), 1.02–1.06], gender (OR, 0.59; 95% CI, 0.39–0.89), previous CVD (OR, 1.75; 95% CI, 1.22–2.50), BMI (OR, 1.06; 95% CI, 1.01–1.10), TG (OR, 0.99; 95% CI, 0.99–1.00), Kt/V (OR, 0.33; 95% CI, 0.14–0.76), Hb (OR, 0.81; 95% CI, 0.70–0.94) and transferrin saturation (OR, 0.97; 95% CI, 0.95–0.98) exhibited significant associations with CTR >0.5.
In the crude analysis, age, gender, previous CVD and DM, smoking status, albumin, Hb, transferrin saturation, and PAOD were significantly associated with CTR >0.6 (Table 3). After adjusting for multiple variables, only age (OR, 1.05; 95% CI, 1.02–1.07), previous CVD (OR, 1.68; 95% CI, 1.02–2.77), smoking (OR, 0.25; 95% CI, 0.11–0.62), transferrin saturation (OR, 0.96; 95% CI, 0.94–0.98) and PAOD (OR, 1.88; 95% CI, 1.14–3.11) remained significantly associated with CTR >0.6.
Association between PAOD and CTR
On further crude analysis, PAOD was a significant risk factor for CTR 0.5–0.6 (OR, 1.52; 95% CI, 1.10–2.08) and CTR >0.6 (OR, 2.18; 95% CI, 1.44–3.30). However, after adjusting for the multiple variables age, gender, HD duration, DM, previous CVD, smoking status, BP, albumin, TG, TC, BMI, Hb, iPTH, ferritin, TSAT, KT/V, iCa, P and intake of anti-platelet, RAS blockers, beta blockers and statins, the ability of PAOD to predict a CTR of 0.5 to 0.6 was attenuated (OR, 1.24; 95% CI, 0.85–1.81) but remained significant for CTR >0.6 (OR, 1.88; 95% CI, 1.14–3.11) (Table 4).
Subgroup analysis of CTR >0.6
We investigated the association between PAOD and CTR >0.6 in analyses stratified by covariates, including history of DM and previous CVD, Hb (>10 g/dl and ≤10 g/dl), age (<60 years and ≥60 years) and gender. Figure 4 shows that after multivariate adjustment for demographic characteristics and dialysis-related chemistry data and medications, PAOD had a significant predictive power for CTR >0.6 in older patients (OR, 2.80; 95% CI, 1.37–5.71), female patients (OR, 2.96; 95% CI, 1.09–3.89), patients without CVD history (OR, 3.93; 95% CI, 1.92–8.08) and patients with Hb > 10 mg/dl (OR, 2.12; 95% CI, 1.09–4.09).
Subgroup analysis of the effect of PAOD on cardiothoracic ratio >0.6.
The full model comprised adjusted variables, including age, gender, hemodialysis duration, diabetes mellitus, previous cardiovascular disease, smoking status, blood pressure, albumin, triglyceride, total cholesterol, body mass index, hemoglobin, intact parathyroid hormone, ferritin, transferrin saturation, urea kinetics, ionized calcium, phosphate and medications.
Discussion
In this cross-sectional study of 622 patients with CHD, PAOD was independently correlated with CTR, especially with CTR >0.6. The correlation between PAOD and CTR >0.6 was independent of traditional anemia risk factors and dialysis quality. Moreover, in the subgroup analysis, this association was significant in the elderly, women and patients without CVD history and with Hb > 10 mg/dl. The results of this study could provide physicians with evidence to manage PAOD and prevent further cardiac enlargement during the dialysis of end-stage renal disease (ESRD) patients.
After adjusting for multiple variables in our CHD patients, we hypothesized that PAOD was significantly associated with severe cardiomegaly (CTR >0.6) but not with CTR >0.5. There are several co-morbidities in dialysis patients, such as DM, hypertension, anemia, malnutrition and chronic inflammation; fluid status may also contribute to cardiomegaly20,21,22. Moreover, reduced ABI is associated with PAOD23,24 and secondary to DM, smoking, hypertension, advanced age and hyperlipidemia25. These results were comparable with our data. Therefore, mild cardiomegaly and PAOD may develop concurrently due to similar risk factors. However, peripheral arterial occlusion may further induce a pumping load to the heart, which may eventually contribute to cardiomegaly progression. Therefore, it is reasonable to think that PAOD might play a major role in the exacerbation but not in the initiation of cardiomegaly. This finding implied that preventing PAOD development in dialysis or even in CKD patients would halt further cardiac remodeling. However, this idea requires further study to validate the effect.
CVD is a common co-morbidity that contributes significantly to the death rate in patients with ESRD26,27. In clinical practice, CTR is an easy and quick method of assessing a patient’s heart condition. Various previous studies have demonstrated that the CTR had a considerable impact on predicting mortality in patients on HD, and it was suggested to be a first-line approach to evaluate the presence of cardiac disease in a patient with CHD5,24,28. In this study, the factors that determined CTR >0.6 in CHD patients were identified after adjusting for multiple variables, including old age, previous CVD history, non-smoking status and lower transferrin saturation. Increased vascular stiffness at older ages is independently associated with severe cardiomegaly29. Smoking is negatively associated with severe cardiomegaly, potentially due to the enlarged chest cavity of smokers. Therefore, we did not consider smoking to be a protective factor against cardiomegaly development. In this study, Hb level was not associated with severe cardiomegaly, but transferrin saturation, which reflects iron storage, was an exacerbating factor in cardiomegaly progression. Interestingly, the association between PAOD and severe cardiomegaly was significant, especially in patients without anemia or CVD history. A reasonable explanation would be that anemia and CVD might be strong risks for cardiomegaly development. Therefore, the association of PAOD and severe cardiomegaly was attenuated in such groups. Regarding the gender difference, we hypothesized that estrogen or androgen might play an important role, but this idea requires further study for confirmation.
Our study had certain limitations. First, the study utilized a cross-sectional design. Therefore, causation between PAOD and cardiomegaly cannot be inferred. Based on pathophysiology, we hypothesize that vascular atherosclerosis developed initially and then led to further cardiomegaly. However, further cohort studies are needed to verify this causation. Second, we did not assess intra- or interobserver variability for the measurement of ABI and CTR. Nevertheless, ABI and CTR measurements are commonly and easily performed and are reliable. Finally, this work was a single-center study, which might not be applicable to all CHD populations. Nevertheless, the results of data analysis on the association between risk factors and CTR were compatible with the findings of previous studies6,18.
Conclusion
PAOD was strongly related to severe cardiomegaly in CHD patients. This finding suggested that treating PAOD beyond the traditional approach might be beneficial in delaying the progression of cardiomegaly.
Additional Information
How to cite this article: Liou, K.-Y. et al. Association between peripheral arterial occlusive disease and cardiothoracic ratio in patients on chronic hemodialysis. Sci. Rep. 6, 38458; doi: 10.1038/srep38458 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hammermeister, K. E., Chikos, P. M., Fisher, L. & Dodge, H. T. Relationship of cardiothoracic ratio and plain film heart volume to late survival. Circulation 59, 89–95 (1979).
Philbin, E. F. et al. The relationship between cardiothoracic ratio and left ventricular ejection fraction in congestive heart failure. Digitalis Investigation Group. Arch Intern Med 158, 501–506 (1998).
Frishman, W. H. et al. Cardiomegaly on chest x-ray: prognostic implications from a ten-year cohort study of elderly subjects: a report from the Bronx Longitudinal Aging Study. Am Heart J 124, 1026–1030 (1992).
Spinar, J. et al. Non-invasive prognostic factors in chronic heart failure. One-year survival of 300 patients with a diagnosis of chronic heart failure due to ischemic heart disease or dilated cardiomyopathy. Int J Cardiol 56, 283–288 (1996).
Chen, K. H. et al. Cardiothoracic ratio, malnutrition, inflammation, and two-year mortality in non-diabetic patients on maintenance hemodialysis. Kidney Blood Press Res 31, 143–151 (2008).
Chen, K. H. et al. Cardiothoracic ratio association with mortality in patients on maintenance peritoneal dialysis. Ther Apher Dial 15, 81–88 (2011).
Wada, Y. et al. Clinical and Pathological Impact of Tissue Fibrosis on Lethal Arrhythmic Events in Hypertrophic Cardiomyopathy Patients With Impaired Systolic Function. Circ J 79, 1733–1741 (2015).
Ko, S. H. & Bandyk, D. F. Interpretation and significance of ankle-brachial systolic pressure index. Semin Vasc Surg 26, 86–94 (2013).
de Vinuesa, S. G. et al. Subclinical peripheral arterial disease in patients with chronic kidney disease: prevalence and related risk factors. Kidney international. Supplement, S44–47 (2005).
Leskinen, Y., Salenius, J. P., Lehtimaki, T., Huhtala, H. & Saha, H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. American journal of kidney diseases: the official journal of the National Kidney Foundation 40, 472–479 (2002).
Cheung, A. K. et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney international 58, 353–362 (2000).
Newman, A. B. et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arteriosclerosis, thrombosis, and vascular biology 19, 538–545 (1999).
Ono, K. et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. Journal of the American Society of Nephrology: JASN 14, 1591–1598 (2003).
Chen, S. C. et al. Ankle brachial index as a predictor for mortality in patients with chronic kidney disease and undergoing haemodialysis. Nephrology 15, 294–299 (2010).
Tsai, M. H., Liou, H. H., Leu, J. G., Yen, M. F. & Chen, H. H. Sites of peripheral artery occlusive disease as a predictor for all-cause and cardiovascular mortality in chronic hemodialysis. PLoS One 10, e0128968 (2015).
Lin, C. Y., Leu, J. G., Fang, Y. W. & Tsai, M. H. Association of interleg difference of ankle brachial index with overall and cardiovascular mortality in chronic hemodialysis patients. Ren Fail 37, 88–95 (2015).
Chen, J. et al. Ankle Brachial Index and Subsequent Cardiovascular Disease Risk in Patients With Chronic Kidney Disease. J Am Heart Assoc 5 (2016).
Asakawa, T. et al. Association between the Hemoglobin Level and Cardiothoracic Ratio in Patients on Incident Dialysis. Cardiorenal Med 4, 189–200 (2014).
Shinzato, T. et al. Determination of Kt/V and protein catabolic rate using pre- and postdialysis blood urea nitrogen concentrations. Nephron 67, 280–290 (1994).
Foley, R. N. et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int 58, 1325–1335 (2000).
Lai, S. et al. Cardiac, Inflammatory and Metabolic Parameters: Hemodialysis versus Peritoneal Dialysis. Cardiorenal Med 5, 20–30 (2015).
Paoletti, E., Bellino, D., Cassottana, P., Rolla, D. & Cannella, G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46, 320–327 (2005).
Hayashi, C. et al. Ankle brachial pressure index and carotid intima-media thickness as atherosclerosis markers in Japanese diabetics. Diabetes Res Clin Pract 66, 269–275 (2004).
Sodhi, H. S., Shrestha, S. K., Rauniyar, R. & Rawat, B. Prevalence of peripheral arterial disease by ankle-brachial index and its correlation with carotid intimal thickness and coronary risk factors in Nepalese population over the age of forty years. Kathmandu Univ Med J (KUMJ) 5, 12–15 (2007).
Brevetti, G., Giugliano, G., Brevetti, L. & Hiatt, W. R. Inflammation in peripheral artery disease. Circulation 122, 1862–1875 (2010).
Ortiz, A. et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383, 1831–1843 (2014).
Shinzato, T. et al. Report of the annual statistical survey of the Japanese Society for Dialysis Therapy in 1996. Kidney Int 55, 700–712 (1999).
Yen, T. H., Lin, J. L., Lin-Tan, D. T. & Hsu, K. H. Cardiothoracic ratio, inflammation, malnutrition, and mortality in diabetes patients on maintenance hemodialysis. The American journal of the medical sciences 337, 421–428 (2009).
El-Abbadi, M. & Giachelli, C. M. Arteriosclerosis, calcium phosphate deposition and cardiovascular disease in uremia: current concepts at the bench. Curr Opin Nephrol Hypertens 14, 519–524 (2005).
Acknowledgements
The authors report no conflicts of interest. All authors are responsible for the content and writing of this paper. We thank our patients for contributing to this study.
Author information
Authors and Affiliations
Contributions
All authors discussed the results and commented on the manuscript. M.T. and H.L. designed the study. K.L. and Y.F. collected the data. J.L. supervised the collection of the data. H.X. and T.Y. performed the structural characterizations. M.T. and H.L. performed data analysis. K.L., Y.F. and M.T. co-wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liou, KY., Liou, HH., Fang, YW. et al. Association between peripheral arterial occlusive disease and cardiothoracic ratio in patients on chronic hemodialysis. Sci Rep 6, 38458 (2016). https://doi.org/10.1038/srep38458
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38458
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.