Abstract
Animals move their heads and eyes to compensate for movements of the body and background, search, fixate, and track objects visually. Avian saccadic head/eye movements have been shown to vary considerably between species. We tested the hypothesis that the configuration of the retina (i.e., changes in retinal ganglion cell density from the retinal periphery to the center of acute vision-fovea) would account for the inter-specific variation in avian head/eye movement behavior. We characterized retinal configuration, head movement rate, and degree of eye movement of 29 bird species with a single fovea, controlling for the effects of phylogenetic relatedness. First, we found the avian fovea is off the retinal center towards the dorso-temporal region of the retina. Second, species with a more pronounced rate of change in ganglion cell density across the retina generally showed a higher degree of eye movement and higher head movement rate likely because a smaller retinal area with relatively high visual acuity leads to greater need to move the head/eye to align this area that contains the fovea with objects of interest. Our findings have implications for anti-predator behavior, as many predator-prey interaction models assume that the sensory system of prey (and hence their behavior) varies little between species.
Similar content being viewed by others
Introduction
Animals move their eyes and heads for multiple reasons: to compensate for body movements and changes in the visual background, search for objects of interest in the environment, fixate and track moving objects, etc1. More specifically, in birds head and eye movements have a saccadic nature2,3,4. Head movements are more common than eye movements during gaze shifts in birds5. However, eye movements contribute more to gaze shifting during head turns of smaller compared to those of larger amplitude6.
The accepted view has been that eye movements are negligible or very limited in birds due to their relatively large eye size and consequently reduced mobility within the orbits7. However, recent studies have shown that many species of birds, including those belonging to the most diverse avian Order (Passeriformes), have quite large degrees of eye movement8,9. Additionally, the rate at which birds move their heads also varies substantially between bird species10. However, there has been little empirical testing at the comparative level to explain the diversity in eye and head movement behavior controlling for the effects of shared evolutionary history. Addressing this gap can help us understand the evolution of visual strategies to search for food and avoid predators, key-components of predator-prey interactions11.
The way the retina spatially samples the visual environment has been suggested to have a role in head and eye movements1. Retinal ganglion cells transfer information from the retina to the visual centers in the brain, with regions of the retina with higher ganglion cell density (i.e., centers of acute vision, such as foveae) providing more detailed spatial information about portions of the visual space than regions of the retina with lower cell density12. The incidence and patterns of eye and head movements vary in species with and without centers of acute vision (respectively, heterogeneous and homogenous cell density across the retina)1,13. Because the center of acute vision provides higher quality spatial information but occupies a reduced region of the retina, some motion is expected in the form of eye or head movements to align the center of acute vision to objects of interest14,15. Therefore, head and eye movements are necessary, among other tasks, to track objects with more accuracy and obtain more detailed spatial information, ultimately leading to enhanced behavioral responses (i.e., increase chances of capturing mobile prey, finding a safe spot while escaping a predator, etc.).
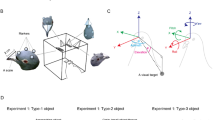
The avian retina is characterized by an increase in cell density from the retinal periphery to the center of acute vision16 (Fig. 1a–d). Interestingly, the rate of change in ganglion cell density varies between species17 (Fig. 1b,e). It has been hypothesized that species in which the change in cell density is more pronounced across the retina (Fig. 1a,b) would rely more on the center of acute vision to gather high quality information than species with a more gradual change in cell density (Fig. 1d,e)17,18. This hypothesis assumes that relatively high visual acuity occurs above a certain ganglion cell density threshold, which is lower than the peak cell density found in the center of acute vision (Fig. 1b,e). In species with a more pronounced rate of change in cell density, the relative size of the retinal area with high visual acuity would be smaller (Fig. 1b,c), compared to species with a more gradual change in cell density (Fig. 1e,f), leading to a greater need to move the center of acute vision through eye and/or head movements. We consequently predicted that the degree of eye movement and the head movement rate would increase in species with a more pronounced change in ganglion cell density across the retina (Fig. 1b,c) than in species with a less pronounced change (Fig. 1e,f).
RG cell density increases from the retinal periphery to the fovea (i.e. center of acute vision); in this graph shown as changes in cell density from the nasal to the temporal part of the retina (a,d) through a sampling transect (red-dotted line). Assuming that above a threshold RG cell density animals will perceive objects with relatively high resolution, two extreme patterns of RG cell density changes can be identified: a, d (shown here as schematic representations of topographic maps with isodensity lines delimiting areas with different cell density). First, a steep change in RG cell density (b) will lead to a relatively smaller area of high visual resolution (c). Second, a smooth change in RG cell density (e) will lead to a relatively larger area with high visual resolution (f). Species with a smaller retinal area with high visual resolution (c) are expected to be more dependent on head/eye movements to get snapshots of a visual scene with high acuity than species with a larger retinal area with high visual resolution (f). Shown are the relative position of the center of acute vision (i.e., fovea, invagination of the retinal tissue) and schematic representations of a potential avian predator perceived by the species with the different types of retinal configuration. Drawing by Gabriela Sincich.
The position of the center of acute vision also varies between bird species12,16, thus leading to our second prediction. If the center of acute vision is not at the center of the retina, but localized in one specific acentric region, we would expect a stronger association between head/eye movement behavior and retinal configuration in the retinal region containing the center of acute vision because the changes in ganglion cell density (and hence visual resolution) would be more prominent there than in other regions of the retina.
In this study, we tested for the first time the relationship between retinal configuration and head/eye movement behavior in 29 bird species known to have a single fovea, controlling for the effects of evolutionary history. This single-foveate retinal configuration is representative of most of the bird species studied so far11. Additionally, we established the average position of the center of acute vision in these single-foveate species.
Results
The averaged Cartesian coordinates of the fovea in the 29 bird species studied were: x, −0.145 ± 0.013 (in the temporal region); and y, 0.057 ± 0.017 (in the dorsal region; Fig. 2a). The 95% confidence intervals of both coordinates did not overlap with zero: x, −0.172, −0.119; and y, 0.023, 0.092 (Fig. 2a), indicating the avian fovea is on average not at the center of the retina but it is located dorso-temporally in species with a single fovea (Fig. 2a).
The slope in cell density change differed significantly among the different retinal regions: dorsal, 3.63 ± 0.28; temporal, 3.48 ± 0.22; ventral, 3.01 ± 0.20; nasal, 2.48 ± 0.17 (F3,84 = 32.50, P < 0.001). The dorsal and temporal slopes did not differ significantly (P = 0.631), but they were significantly higher than the ventral and nasal slopes (P < 0.01). Consequently, the dorsal and the temporal areas of the avian retina have a more pronounced change in cell density from the periphery to the fovea. Additionally, we found that species with higher head movement rates also showed higher degree of eye movement (F2,27 = 3.99, P = 0.031, R2 = 0.13, χ = 0).
Head movement rate was positively and significantly associated with the temporal slope in cell density change (F2,27 = 3.39, P = 0.049, R2 = 0.11, χ = 0; Fig. 3a). However, head movement rate was not significantly related to the overall (F2,27 = 2.27, P = 0.123, R2 = 0.08, χ = 0), nasal (F2,27 = 1.34, P = 0.279, R2 = 0.08, χ = 0), dorsal (F2,27 = 1.25, P = 0.303, R2 = 0.04, χ = 0), and ventral (F2,27 = 2.77, P = 0.080, R2 = 0.09, χ = 0) slopes in cell density change.
We found a positive and significant association between the degree of eye movement and the overall slope in cell density across all retinal regions (F2,27 = 13.00, P < 0.001, R2 = 0.33, χ = 0). Similar significant associations were found considering each retinal region separately: nasal (F2,27 = 16.66, P < 0.001, R2 = 0.38, χ = 0; Fig. 3c), temporal (F2,27 = 5.43, P = 0.010, R2 = 0.17, χ = 0; Fig. 3c), dorsal (F2,27 = 12.10, P < 0.001, R2 = 0.31, χ = 0; Fig. 3d), and ventral (F2,27 = 15.46, P < 0.001, R2 = 0.36, χ = 0; Fig. 3e).
Discussion
Previous studies found that the incidence of eye movements and patterns of head and eye movements are different in species with a center of acute vision present than those without one (reviewed in ref. 1). Ours is the first comparative study showing that the way the retina is configured in bird species with a single fovea (i.e., the type of center of acute vision with the highest spatial resolving power14) can influence head and eye movement behaviors. However, the relationships we found varied in different regions of the retina.
The avian fovea on average is placed off the retinal center. More specifically, the fovea is commonly found in the dorso-temporal region of the retina, and thus projects into the fronto-ventral region of the head (Fig. 2b,c). This suggests that the spot that provides the highest spatial visual resolution is pointing slightly below and towards the edges of the beak but without necessarily projecting into the binocular field due to the lateral placement of the orbits19. The fovea placement may facilitate the visual exploration of the substrate when the head is down looking for food by integrating the retinal inputs of the right and left foveae with the frontal binocular input through small amplitude lateral movements of the head9.
Our proxy of retinal configuration provided an estimate of the rate of change in spatial resolving power from the retinal periphery to the center of acute vision. In general, we found that species with a more pronounced change in spatial resolving power from the retinal periphery to the fovea tended to have higher amplitude of eye movement and higher head movement rates (but see below). Our results are consistent with the hypothesis that if the change in cell density from the periphery to the fovea is more pronounced, the retinal region above the threshold of relatively high visual acuity would be smaller and the need to move the head/eye to align this region with objects of interest would be higher compared to a more gradual change in cell density that would lead to a relatively larger retinal region above the threshold of relatively high visual acuity (Fig. 1b,e,18). As a result, species with higher rate of change in cell density across the retina may rely more on the center of acute vision to track objects, compensate for body and background movements, etc. This dependence on high visual acuity can translate into two mechanisms to move the center of acute vision: greater amplitude of eye movements and higher rates to move the head around, as both traits were positively associated in our studied species.
However, spatial resolving power may not be the only factor accounting for the patterns observed, as the densities of photoreceptors involved in both chromatic and achromatic vision in birds also increase from the retinal periphery to the fovea20. Therefore, it is also possible that chromatic and achromatic visual contrast and contrast sensitivity are also associated with the changes in spatial resolving power across different parts of the retina. This reinforces the idea that the avian fovea is more than a center of high chromatic resolution, but one also of achromatic and motion vision20. Overall, our findings open up the need for testing alternative retinal mechanisms driving changes in head and eye movement behavior.
Despite being significant in all retinal regions, the association between degree of eye movement and change in cell density was the weakest in the temporal region (17% compared to >30% in the other three regions). On the other hand, the association between head movement rate and change in cell density was only significant in the temporal region of the retina. We speculate that the region of the retina with the fovea may drive head movement behavior, which is more predominant in birds5, while the non-foveate regions of the retina may drive the different types of eye movements that to a large degree compensate for head movements1. This suggests that different retinal regions may play different roles in triggering eye and/or head movements, possibly influencing their orientations. Given the comparative nature of our data, measuring the orientation of head and eye movements would have been logistically challenging with the almost 30 species studied. A recent study using an avian eye tracker found that European starlings are capable of quickly moving their eyes in multiple directions (ventral, dorsal, nasal, temporal) to track a stimulus4. However, in the absence of a stimulus, starlings scan their visual space making spontaneous eye movements along an axis tilted downwards anteriorly and upwards posteriorly, which appears related to the visual exploration of their beaks while using their probing foraging technique4. Other bird species move their eyes along a horizontal axis (chicken Gallus gallus21; zebra finch Taeniopygia guttata3) or a combination of a tilted and a horizontal axes (pigeon Columba livia22). This degree of variation in the orientation of spontaneous eye movements could be related to the type of center of acute vision (fovea, area, visual streak, or combination of them) as well as the ecology of the species. The availability of new eye tracking technology6,23 could allow us to test the potential interactions between retinal morphology, head/eye movement behavior, and habitat visual complexity.
Our findings suggest that some components of anti-predator behavior (e.g., scanning) are influenced by how the avian visual system is configured. This is relevant because the predictions of many models on anti-predator behavior implicitly assume little variation in the sensory system of prey, which contradicts recent empirical evidence9,11. The implication is that some species may be at higher predation risk than others in certain habitats because of the association between retinal configuration and behavior. For instance, species with more pronounced changes in cell density across the retina may need to increase their head movement rates to a much greater extent than species with less pronounced cell density changes in visually complex habitats (e.g., wooded area) after detecting some cues that may be related to the presence of a predator (e.g., sounds of rustling leaves). Under these conditions, it is likely that the rate at which high visual resolution portions of the visual space are captured is lower in species with more pronounced changes in cell density due to their smaller retinal area with high visual acuity (Fig. 1c), potentially leading to a higher perceived risk of predation, longer flight initiation distances, and longer times at the refuge. Overall, incorporating some of the inter-specific variability in the prey visual system may help develop more realistic predictions about the interspecific variation in anti-predator behavior.
Methods
We used 29 species that were determined to have a single fovea by cross sections of the retina (i.e., tissue invagination) or by visual inspection of wholemounted retinas (i.e., torus shape on the surface of the retina characteristic of a fovea) (Appendix 1). The species included in the study inhabit different habitats (11 in open woodlands, 6 in forests, 5 in scrubs, 3 in urban areas, 2 in grasslands, 2 in marshes), and have different diets (12 insectivores, 12 granivores, 5 omnivores) (Appendix 1). Some of the raw data on some of the species used in this study were published previously (Appendix 1), and unpublished data were obtained on species in Indiana, USA. All procedures were performed in accordance with the relevant guidelines and regulations and were approved by Purdue Animal Care and Use Committee (protocol# 09-018).
Previous publications explained in detail the methods used to collect the data for each species on degree of eye movement24, head movement rate19, and change in cell density from the retinal periphery to the fovea16. Here, we briefly describe these methods.
We estimated the mean degree of eye movement (°) for each species by measuring between 2 and 13 individuals per species, depending on the availability of individuals. We used a visual field apparatus with the head of the animal at the center24. We examined the retinal field with a Keeler Professional ophthalmoscope every 10° from below (120°) to above (60°) the bill (i.e., coordinate system where 0° was directly above the head and 90° was in front of the head). We measured the degree (amplitude) of eye movement at each elevation by encouraging the animal to converge and diverge maximally its eyes using sounds. The difference between the converged and diverged eye positions was considered the degree of eye movement.
We estimated head movement rates (number of changes in any kind of head position while head-up per min) from videos by measuring between 9 and 12 individuals per species (for a similar approach see refs 10, 19 and 25). We obtained videos from two sources: the Macaulay Library (http://macaulaylibrary.org) and our own recordings. We recorded individuals in rural and suburban areas in Tippecanoe County (IN, USA) using a JVC Everio camcorder between 0700 and 1700 hrs. We minimized the chances of re-sampling the same individual by following the individual that had been recorded for a few minutes and moving in the opposite direction at least 50 m before finding a new individual. We recorded individuals showing scanning and foraging behavior, but avoided individuals that were singing, flying, or interacting with conspecifics. We measured the number of changes in head position (lateral, up, down, etc.) while the animal was in a head-up body posture per min (head-movement rate). We did not discriminate between different types of head movements or their amplitude because videos were recorded from different angles.
We extracted, wholemounted, and stained retinas for retinal ganglion cells following the procedures presented in ref. 26. After euthanasia, the eyes were removed and axial length was measured with calipers (0.01 mm accuracy). We then hemisected each eye at the ora serrata, removed vitreous humor using forceps and spring scissors, and placed the eyecup in 4% paraformaldehyde. After fixation, we processed the retina following the procedures in refs 10 and 26. Ganglion cells were stained with cresyl violet. We used an Olympus BX51 microscope at 1000x and an Olympus S97809 camera to capture images at different sites across each retina using Stereo Investigator (MBF Bioscience, Williston, Vermont). Ganglion cells were then counted to estimate density (cells/sq.mm) in 50 mm × 50 mm counting frames in each of the sites using ImageJ (http://imagej.nih.gov/ij/). Other cell types within the ganglion cell layer (e.g. amacrine, glial cells, etc.) were selectively excluded from our counts by morphological identification27,28,29. We built topographic maps showing the variation in ganglion cell distribution across the retina following ref. 26.
Using the topographic maps, we measured the position of the fovea with a Cartesian coordinate system (x, y) relative to the center of the retina (method detailed in ref. 16). Positive x-values indicate temporal and negative x-values indicate nasal, and positive y-values indicate dorsal and negative y-values indicate ventral positions of the fovea. We measured gradients in ganglion cell density on the topographic maps by establishing transects across the nasal, temporal, dorsal, and ventral retinal axes, centered on the fovea16. Sampling points were laid out and the averaged cell density recorded. We plotted these cell density values in each axis and then fitted a trend line. The slope of this line was used as the proxy of the change in cell density from the retinal periphery to the fovea (details in ref. 16), with higher and lower slope values indicating a more and less pronounced cell density change, respectively. We estimated the averaged nasal, temporal, ventral, dorsal, and overall (all four axes) slopes based on 2 to 5 topographic maps per species. This proxy was independent of the size of the retina (which is positively associated with body size30), as the relationship between (log) eye axial length and the averaged slope across all axes was not significant (F2,27 = 0.43, P = 0.658, R2 = −0.02, χ = 1), controlling for phylogenetic relatedness (see below).
Statistical analysis
Head movement rate was log transformed to meet model assumptions and we present means ± standard errors throughout. We first established the differences in nasal, temporal, ventral, and dorsal slopes using a general linear model and conducted pairwise comparisons with Tukey-tests. To assess the relationship between retinal configuration and eye and head movement behaviors, we conducted phylogenetic generalized least squares models (PGLS31) to account for the effects of shared evolutionary history of these species using a purpose-built phylogeny based on a maximum likelihood analysis of DNA sequence data (see below). We ran the PGLS analyses using the Caper package32 in R33. Each of the 29 species was represented by a single averaged data point in our dataset. We corroborated that our results met the model assumptions by visually inspecting the distribution of residuals and the fitted vs. the residual values. We also checked for outliers (samples with values >3 or <−3) but did not to detect any.
To control for potential similarities arising from phylogenetic relatedness (and not selection pressure), we constructed an evolutionary tree for our focal species using DNA sequence data from GenBank. A multigene data set (seven mtDNA genes plus 1 nDNA gene; see Appendix 2) was purpose-built for the analysis by downloading all sequence data for the focal species (from GenBank release number 182) and mining homologous genes using the Perl Script GenBankStrip.pl v2.1. Thereafter, for each gene, the individual sequences were aligned to one another using MUSCLE v3.8.3134 for non-coding genes and MUSCLE as driven by the Perl script transAlign v1.335 for protein-coding genes (all Perl scripts can be downloaded from http://www.uni-oldenburg.de/ibu/systematik-evolutionsbiologie/programme/). All automated alignments were then corrected afterwards by eye as needed and pruned down to the set of focal taxa using the Perl script seqCleaner.pl v1.2. The Mississippi Alligator, Alligator mississippiensis, was also added as the outgroup species to define the root of the phylogenetic tree. Phylogenetic analysis of the combined data set (6661 bp for eight genes; accession numbers provided in Appendix 2) was performed in a maximum likelihood (ML) framework using the program RAxML v7.2.836. A combined rapid bootstrap algorithm (with 1000 replicates) and ML search37 was used to derive the final tree, with the optimal model of evolution being fitted to each individual gene partition.
Additional Information
How to cite this article: Moore, B. A. et al. Does retinal configuration make the head and eyes of foveate birds move?. Sci. Rep. 7, 38406; doi: 10.1038/srep38406 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Land, M. F. Eye movements of vertebrates and their relation to eye form and function. J Comp Phys A 201, 195–214 (2015).
Dawkins, M. S. What are birds looking at? Head movements and eye use in chickens. Anim Behav 63, 991–998 (2002).
Voss, J. & Bischof, H.-J. Eye movements of laterally eyed birds are not independent. J Exp Biol 212, 1568–1575 (2009).
Tyrrell, L. P., Butler, S. R. & Fernández-Juricic, E. Oculomotor strategy of an avian ground forager: tilted and weakly yoked eye saccades. J Exp Biol 218, 2651–2657 (2015).
Gioanni, H. Stabilizing gaze reflexes in the pigeon (Columba livia): 1. Horizontal and vertical optokinetic eye (OKN) and head (OCR) reflexes. Exp Brain Res 69, 567–582 (1988).
Yorzinski, J. L., Patricelli, G. L., Babcock, J. S., Pearson, J. M. & Platt, M. L. Through their eyes: selective attention in peahens during courtship. J Exp Biol 216, 3035–3046 (2013).
Land, M. F. Motion and vision: why animals move their eyes. J Comp Physiol A – Sens Neural Behav Physiol 185, 341–352 (1999).
Troscianko, J., von Bayern, A. M. P., Chappell, J., Rutz, C. & Martin, G. R. Extreme binocular vision and a straight bill facilitate tool use in New Caledonian crows. Nat Commun 3, 1110, doi: 10.1038/ncomms2111 (2012).
Moore, B. A., Pita, D., Tyrrell, L. P. & Fernández-Juricic, E. Vision in avian emberizid foragers: maximizing both binocular vision and fronto-lateral visual acuity. J Exp Biol 218, 1347–1358 (2015).
Moore, B. A., Doppler, M., Young, J. E. & Fernández-Juricic, E. Interspecific differences in the visual system and scanning behavior of three forest passerines that form heterospecific flocks. J Comp Phys A 199, 263–277 (2013).
Fernández-Juricic, E. Sensory basis of vigilance behavior in birds: synthesis and future prospects. Behav Processes 89, 143–152 (2012).
Collin, S. P. Behavioural ecology and retinal cell topography. In: Archer, S., Djamgoz, M. B., Loew, E. R., Partridge, J. C., Vallerga, S. (Eds.), Adaptive Mechanisms in the Ecology of Vision. Kluwer Academic Publishers, Dordrecht, pp. 509–535 (1999).
Martinez-Conde, S. & Macknik, S. L. Fixational eye movements across vertebrates: comparative dynamics, physiology, and perception. J Vis 28, 1–16 (2008).
Walls, G. L. The evolutionary history of eye movements. Vision Res 2, 69–80 (1962).
Land, M. F. & Tatler, B. W. Looking and acting: vision and eye movements in natural behaviour. Oxford University Press, Oxford (2009).
Moore, B. A. et al. A novel method to measure retinal specialization traits from topographic maps for comparative analysis. J Vis 12, 1–24 (2012).
Fernández-Juricic, E. et al. Visual systems and vigilance behaviour of two ground-foraging avian prey species: white-crowned sparrows and California towhees. Anim Behav 81, 705–713 (2011).
Dolan, T. & Fernández-Juricic, E. Retinal ganglion cell topography of five species of ground foraging birds. Behav Brain Res 75, 111–121 (2010).
Tyrrell, L. P., Moore, B. A., Loftis, C. & Fernández-Juricic, E. Looking above the prairie: localized and upward acute vision in a native grassland bird. Sci Rep 3, Article number: 3231 (2013).
Baumhardt, P. E., Moore, B. A., Doppler, M. & Fernández-Juricic, E. Do American goldfinches see their world like passive prey foragers? A study on visual fields, retinal topography, and sensitivity of photoreceptors. Brain Behav Evol 83, 181–198 (2014).
Schwarz, J. S., Sridharan, D. & Knudsen, E. I. Magnetic tracking of eye position in freely behaving chickens. Front Syst Neurosci 7, 91 (2013).
Bloch, S., Rivaud, S. & Martinoya, C. Comparing frontal and lateral viewing in the pigeon. III. Different patterns of eye movements for binocular and monocular fixation. Behav Brain Res 13, 173–182 (1984).
Tyrrell, L. P., Butler, S. R., Yorzinski, J. L. & Fernández-Juricic, E. A novel system for bi-ocular eye-tracking in vertebrates with laterally placed eyes. Methods Ecol Evol 5, 1070–1077 (2014).
Fernández-Juricic, E., Gall, M. D., Dolan, T., Tisdale, V. & Martin, G. R. The visual fields of two ground foraging birds, House Finches and House Sparrows, allow for simultaneous foraging and anti-predator vigilance. Ibis 150, 779–787 (2008).
Fernández-Juricic, E. et al. Testing the terrain hypothesis: Canada geese see their world laterally and obliquely. Brain Behav Evol 77, 147–158 (2011).
Ullmann, J. F. P., Moore, B. A., Temple, S. E., Fernández-Juricic, E. & Collin, S. P. The retinal wholemount technique: a window to understanding the brain and behaviour. Brain Behav Evol 9, 26–44 (2012).
Freeman, B. & Tancred, E. The number and distribution of ganglion cells in the retina of the brush-tailed possum, Trichosurus vulpecula. J Comp Neurol 177, 557–567 (1978).
Ehrlich, D. Regional specialization of the chick retina as revealed by the size and density of neurons in the ganglion cell layer. J Comp Neurol 195, 643–657 (1981).
Stone, J. The wholemount handbook. A guide to the preparation and analysis of retinal wholemounts. Maitland Publishing, Sydney (1981).
Kiltie, R. A. Scaling of visual acuity with body size in mammals and birds. Funct Ecol 14, 226–234 (2000).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999).
Orme, D. et al. Caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5. Available at: http://cran.r-project.org/web/packages/caper/(2013).
R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing (2011). Available online at http://www.R-project.org/
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004).
Bininda-Emonds, O. R. P. transAlign: using amino acids to facilitate the multiple alignment of protein-coding DNA sequences. BMC Bioinformatics 6, 156 (22Jun05) (2005).
Stamatakis, A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006).
Stamatakis, A. & Hoover, P. & Rougemont, J., A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 75, 758–771 (2008).
Acknowledgements
This work was supported by the National Science Foundation [IOS 1146986].
Author information
Authors and Affiliations
Contributions
B.A.M. and E.F.J. conceived the study. L.P.T., O.R.P.B.E. and D.P. provided intellectual input. B.A.M., L.P.T. and D.P. captured the subjects. B.A.M., L.P.T. and D.P. performed the experiments. E.F.J., B.A.M. and O.R.P.B.E. ran the statistical analyses. B.A.M. and E.F.J. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Moore, B., Tyrrell, L., Pita, D. et al. Does retinal configuration make the head and eyes of foveate birds move?. Sci Rep 7, 38406 (2017). https://doi.org/10.1038/srep38406
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38406
This article is cited by
-
The visual fields of the Harpy Eagle (Harpia harpyja)
Journal of Ornithology (2023)
-
The relative sizes of nuclei in the oculomotor complex vary by order and behaviour in birds
Journal of Comparative Physiology A (2023)
-
Foveal shape, ultrastructure and photoreceptor composition in yellow-legged gull, Larus michahellis (Naumann, 1840)
Zoomorphology (2021)
-
The potential role of Arhgef33 RhoGEF in foveal development in the zebra finch retina
Scientific Reports (2020)
-
Blind free-living kiwi offer a unique window into the ecology and evolution of vertebrate vision
BMC Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.