Abstract
Short day length-induced alteration of potassium (K) localization in perennial trees is believed to be a mechanism for surviving and adapting to severe winters. To investigate the relationship between cesium (Cs) and K localizations, a model tree poplar, hybrid aspen T89, was employed. Under short day length conditions, the amount of 137Cs absorbed through the root and translocated to the root was drastically reduced, but 42K was not. Potassium uptake from the rhizosphere is mediated mainly by KUP/HAK/KT and CNGC transporters. In poplar, however, these genes were constantly expressed under short-day conditions except for a slight increase in the expression a KUP/HAK/KT gene six weeks after the onset of the short-day treatment. These results indicated that the suppression of 137Cs uptake was triggered by short day length but not regulated by competitive Cs+ and K+ transport. We hypothesize that there are separately regulated Cs+ and K+ transport systems in poplar.
Similar content being viewed by others
Introduction
In 2011, the Fukushima Daiichi Nuclear Power Plant accident released a large amount of radionuclides into the environment. Due to its long half-life, radioactive cesium (137Cs) was considered the main contaminant. To estimate the transfer process of 137Cs in the terrestrial environment, we focused on its behavior in woody plants because the transfer process within the forest ecosystem is much slower than it is in other areas1. After the forest contamination by 137Cs, depositions to tree canopies, leaf- and/or bark uptake, acropetal branch translocation, etc. were energetically investigated2,3,4. Little is known about the physiology of Cs transfer and distribution within trees. Cesium chemically resembles potassium (K) but it is not an essential nutrient for plant growth. Avery reported that Cs+ inhibits the inward-K+ channels in the plasma membrane and is therefore considered toxic to plants5. In general, rhizosphere Cs+ consists mostly of stable 133Cs and its concentration is <200 μM, which is not toxic to plants6. Cesium uptake and translocation within the plant body are thought to be mediated by K+ transport systems6. Arabidopsis HAK5 (AtHAK5) is a K+ uptake transporter in roots and is up-regulated under K+ deficiency7,8. The AtHAK5 T-DNA insertion mutant, athak5, showed a significantly decreased K content and a tolerance to 300 μM Cs+ treatment under low K conditions9,10. AtCNGC2 demonstrated cation transport activity using transgenic transfected human embryonic kidney cells. AtCNGC1 is the candidate gene for Cs+ uptake and was identified by quantitative trait locus analysis in Arabidopsis11,12.
In this study, we investigated 137Cs and 42K localizations using a model tree, poplar, under both long- and short photoperiods. To estimate the 137Cs retention time within the forest ecosystem, the Cs content of each tree organ must be determined under seasonal conditions since Cs is circulated via root uptake, translocation, and leaf abscission. Poplar is a perennial deciduous tree with a characteristic seasonal cycle of growth and dormancy. The phase shift from growth to dormancy is a winter adaptation13. The transition of meristems into dormant buds is crucial to protect them against hazardous frosts. Woody plants shift their growth stage when they perceive changes in photoperiod and temperature14. The initiation of cold acclimation under short day length increases endogenous abscisic acid levels and reduces endogenous gibberellic acid levels15,16,17. In beech tree (Fagus sylvatica L.) leaf senescence, leaf K content decreases before shedding and the recovered K is deposited in the stem cortex and pith18. Japanese native poplar (Populus maximowiczii) also showed a decrease in leaf K concentration following dormant bud formation19. An increase in K+ concentration in xylem sap was observed during the winter season in field-grown Populus nigra20. These behaviors imply the existence of re-translocation mechanisms for K, and it is assumed that the potassium is transported to the organs that require it once it is resorbed. Potassium is transported through various systems within the plant body21. Epstein et al. showed that K+ absorption in barley roots is mediated by both a high-affinity- and a low-affinity biphasic transport process22. The high-affinity transport system (HATS) is up-regulated by a decrease in external K+ concentration. The low-affinity transport system (LATS), however, operates even when there is sufficient external K+ 23. Potassium ion uptake by the root symplast via HATS is mediated by the KUP/HAK/KT transporter family. There are thirteen such transporters in Arabidopsis24,25 and twenty-seven in rice26. Non-selective cation transport mechanisms such as voltage-independent cation channels (VICC) are categorized as LATS. In Arabidopsis, AtCNGC encodes VICC type channels. Twenty types of AtCNGC are present in the Arabidopsis genome.
Based on the above, it is assumed that the expression and function of K+ permeable transporters also regulate Cs+ translocation in various plant species and situations. Therefore, we investigated the relationship between the change in K localization induced by short day length and the behavior of Cs absorbed from the rhizosphere. To this end, 137Cs and 42K accumulations and gene expression patterns of major K+ transporters were analyzed using a model tree poplar, hybrid aspen T89.
Results
Amount of 137Cs in shoots was down-regulated under short-day conditions
Under a controlled growth cycle system in Populus alba L., the shift from long-day (LD) to short-day (SD) conditions decreased phosphate in the lower leaves27. This change suggests the existence of mechanisms for the re-translocation of phosphate from older- to younger (upper) leaves in response to with seasonal changes. Furukawa et al. indicated Ca2+ transport from root to shoot in Populus maximowiczii is also regulated by the shift from LD to SD19. Based on these facts, the uptake of Cs+ within the root and its behavior within the plant body in LD and SD conditions were compared. To measure seasonal variations in Cs+ uptake, a 137Cs+ solution was added to the growth media under LD3, LD9, and SD6 conditions (see Methods).
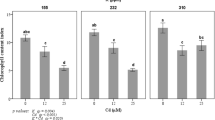
Figure 1A shows the localization of 137Cs by root absorption under LD3, LD9, and SD6 conditions. In the LD3 plants, 137Cs was localized entirely and the radiation intensity around the apex was highest there. The LD9 plants were the same age as the SD6 plants and showed the same 137Cs behavior as the LD3 plants. In the SD6 plants, 137Cs was localized mainly in the stem and root and the total 137Cs was lower than that for the other plants. In LD3, LD9, and especially SD6 plants, all the nodes showed high amounts of 137Cs. The quantity of 137Cs in the shoots of SD6 plants was about 36.3% and 23.6% lower than that in the LD3 and LD9 shoots, respectively (Fig. 1B). On the other hand, the amount of 137Cs in the roots was similar for all three conditions. Cesium-137 accumulated mainly (48.8%) in the leaves under LD3 conditions (Fig. 1C). In LD9 conditions, 137Cs also accumulated to a large extent in the leaves (42.5%). Nevertheless, under SD6 conditions, the leaf 137Cs content was 32.1%, and organs containing the most 137Cs were the stems (39.7%). For the shoot apices, 137Cs levels were lower in SD6 plants than they were in LD3 and LD9, but the difference was not significant. The concentrations of 137Cs were highest in the apices of the LD3 and LD9 plants (Fig. 1D). Nevertheless, the decreases in the 137Cs concentrations in the apices and the leaves under SD6 conditions were significant, and the transition to SD suppressed Cs transport into the apices and the leaves.
Effect of short-day transition on 137Cs uptake activity in poplar.
(A) Localization of root-applied 137Cs under LD3, LD9, and SD6 conditions. The upper images are photos and the lower images are autoradiographs. Poplars were treated with 137Cs for 48 h. In the autoradiographs, color change from blue to red indicates 137Cs accumulation. Bar indicates 1 cm. (B) Alteration of the total amounts of 137Cs in poplar under transition to SD conditions. Poplars in each photoperiod were treated with 137Cs for 48 h. (C) Cesium-137 accumulations in each organ after 48 h treatment under LD3, LD9, and SD6 conditions. (D) Cesium-137 concentrations in each organ after 48 h treatment under LD3, LD9, and SD6 conditions. Three plants were tested for each photoperiod. Error bars indicate standard deviation.
Potassium-42 uptake was constant under LD and SD conditions
Based on the 137Cs uptake activity assays, it was expected that the amount of 42K absorbed through the root would also be down-regulated by the transition to SD. Poplar roots were treated with exogenous 42K and the amounts of 42K in the shoots and roots under LD3, SD2, SD4, and SD6 conditions were measured after 24 h incubation (Fig. 2). No difference was found in the amount of 42K in the roots among four conditions. The amount of 42K in the shoots at the early stage of SD was almost equivalent to that in the shoots at LD3. In contrast, the amount of 42K in the SD6 plant was slightly higher than it was in the other conditions, but the difference was not significant. These data suggest that the demand for K in the rhizosphere neither increased nor decreased by the transition to SD in the poplar for up to six weeks.
A comparison of Figs 1B and 2 indicates that 137Cs accumulation significantly decreased under SD6 condition, but 42K accumulation remained almost constant through the SD transition. This fact indicates that the decrease in Cs levels in the shoot is not explained by a simple reduction in transpiration rate under SD conditions and that important K+ uptake systems in poplar might be independently regulating Cs accumulations in it. It is also implied that the transporter responsible for Cs+ uptake in poplar might have only limited involvement in K+ uptake since no decrease in K accumulation was observed when Cs accumulation was low.
PttHAK-like1 was up-regulated by transition to short-day
We investigated under SD conditions the expression patterns of some candidate genes related to K+ transport. We focused on the KUP/HAK/KT family K+ transporters and a cyclic-nucleotide-gated channel (CNGC) type K+ channels. As for KUP/HAK/KT family genes, we concentrated on three genes in poplar. One of these was Populus tremula K+ uptake transporter 1 or PtKUP1 (Accession number, AJ299422; POPTR_0003s13370) which was identified in hybrid aspen28. PtKUP1 was used in a complementation test with a K+ -uptake-deficient E. coli mutant. The addition of Cs+ to the culture media strongly inhibited the growth of E. coli expressing PtKUP128. We also looked at two KUP/HAK/KT family transporters resembling AtHAK5. Populus trichocarpa, whose genome was elucidated in 200629, has nine AtHAK5 homolog genes in its genome, including PtKUP1. To evaluate the similarity between these putative K+ transporters, we constructed a phylogenetic tree of these genes and AtHAK5 homologs reported to be involved in K+ and Cs+ transport. We used their amino acid sequences (Fig. S1A). An AtHAK5 homolog in barley, HvHAK1, also up-regulated its expression under K deficiency, and transgenic yeast expressing the HvHAK1 gene showed an enhanced growth rate30. Like HvHAK1, rice OsHAK5 also exhibits a high homology to AtHAK531. The poplar gene POPTR_0010s10450 had the highest amino acid sequence homology with AtHAK5. POPTR_0001s00580 had the second highest. We identified POPTR_0010s10450 and POPTR_0001s00580 orthologues in the hybrid aspen T89 and named them PttHAK-like1 and PttHAK-like2, respectively.
CNGC (cyclic-nucleotide-gated channel) may be a non-selective K+ channel which mediates K+ uptake by the root symplast21. In Arabidopsis, the CNGC channel AtCNGC2 shows K+ permeability32. A quantitative trait locus analysis indicated that AtCNGC1 is associated with shoot K and Cs concentrations in Arabidopsis12,33. In P. trichocarpa, nine genes were selected as AtCNGC1 homologs based on their amino acid sequences (see Supplementary Fig. S1B). Proteome BLAST analysis showed that POPTR_0012s01690 and POPTR_0015s02090 scored significantly higher than did others and the orthologues in hybrid aspen T89 were named as PttCNGC1-like1 and PttCNGC1-like2, respectively.
To determine which K+ uptake related gene is the most abundantly expressed among these five transporters, we evaluated the expression level of each gene under LD3 conditions. In poplar roots, no obvious differences were found in the expression levels of the genes selected (Fig. 3A). There may be redundancy in the expression of these K+ influx transporters under LD3 conditions. During the transition to the SD conditions, the expression of PtKUP1 did not significantly change (Fig. 3B). PttHAK-like1 showed steady expression until the transition to SD4 conditions and was up-regulated by about 1.5-fold under SD6 conditions (Fig. 3C). PttHAK-like2 expression tended to decrease in SD2 and SD4 plants but maintained statistically steady-state transcription levels through SD transition (Fig. 3D). The expressions of PttCNGC1-like1, PttCNGC1-like2 were also relatively constant under SD conditions (Fig. 3E,F).
Effect of transition to SD on the transcriptional expression of the KUP/HAK/KT and CNGC1 homolog genes in poplar root.
Total RNA was isolated from the root and gene transcript levels were analyzed using qRT-PCR. UBIQUTIN was used as a reference gene. (A) Comparison of poplar K+ transport related genes under normal growth conditions (LD3). Three plants were tested for this analysis. Error bars indicate standard deviation. (B–F) Change in the expression levels of K+ transport related genes under transition to SD. (B) PtKUP1, (C) PttHAK-like1, (D) PttHAK-like2, (E) PttCNGC1-like1, and (F) PttCNGC1-like2. All gene expression levels were normalized by that for LD3. Error bars indicate standard deviation. * Indicates significant difference from the LD3 expression level (**<0.01). All primers used are listed in Table 1.
There was a small but statistically significant increase in the expression level of PttHAK-like1 under SD6 conditions but the amount of K absorbed through the root did not change with the transition to SD (Figs 2 and 3C). This inconsistency may be accounted for by the low elevation level of PttHAK-like1 expression and the significant differences in the amino acid sequence of the poplar genes. To confirm, we sequenced the entire PttHAK-like1 gene from the hybrid aspen T89. AtHAK5 and POPTR_0010s10450 in P. trichocarpa and PttHAK-like1 in hybrid aspen T89 and their alignment are shown in Supplementary Fig. S2. The AtHAK5 amino acid sequence showed 44.5% homology to POPTR_0010s10450 and 44.2% to PttHAK-like1. For the poplar, PttHAK-like1 and POPTR_0010s10450 shared 97.8% homology. The GEGGTFALY domain (AtHAK5-type transporters) is important for K+ and Cs+ selectivity34. Of these three genes, the GEGGTFALY domain was completely conserved and, consequently, there is no obvious explanation for the functional divergence.
Despite the steady 42K uptake manner through seasonal transitions, Cs accumulation activity was down-regulated under SD6 conditions. Therefore, the Cs+ and K+ transport systems are probably separately regulated in poplar.
Discussion
Potassium is one of the most abundant essential plant nutrients. It is required for metabolism, photosynthesis, the tricarboxylic acid (TCA) cycle, glycolysis, and amino acid biosynthesis35. Maintaining enough K within the plant body is therefore quite important. For example, in K-deficient sunflowers, the carbon flux into the TCA cycle decreased due to changes in carbon distribution36. Potassium deficiency also inhibited sugar translocation in several plants37. Thus, the amount of K is closely tied to processes that maintain homeostasis in plants such as charge balance, pH regulation, and osmotic potential35. Potassium is the dominant solute in the xylem- and phloem saps of Ricinus communis and the circulation of K is required for plant growth and development38.
In this study, the relative amounts of 42K accumulated were compared over seasonal transitions. It was found that 42K accumulation remained constant until SD6. Dormant buds formed up to four weeks after the onset of the short-day treatment (data not shown); therefore, K re-translocation should have already started at SD6. Nevertheless, the results showed that 42K accumulation from root uptake and the expression of genes related to the root K+ uptake were almost constant (Figs 2 and 3B–F). It has been reported that the induction of AtHAK5 was enhanced by K+ deficiency7,8 or by Cs+ applications when there was sufficient K+ 39. Therefore, the slight increase in PttHAK-like1 expression under SD6 might be a response to K+ starvation during the long growth period.
Despite the constant K accumulation pattern under SD conditions, Cs accumulation drastically decreased in SD6 plants (Fig. 1A,B). Cesium ion uptake and translocation are considered to be regulated by the plant K+ transport system but no down-regulation in the genes related to K+ uptake was identified during SD transition (Fig. 3B–F). It is known that plant mineral uptake mechanisms are regulated by protein activity level as well as gene expression6. Further nutrient transport activity analysis is necessary but these results suggest the possible existence of a novel uptake transporter which carries Cs+ much more efficiently than it does K+.
Since a decrease in Cs was observed only in the shoot (Fig. 1B), attention should be given to the transporters involved in Cs+ transfer between the root cells adjacent to the xylem and the xylem vessels themselves. Potassium-42 translocation from the root to the shoot was not affected by the transition to SD (Fig. 2). Therefore, K+ xylem loading might not be down-regulated, and there could be a Cs+ re-uptake pathway from the xylem sap to the root cells. This type of regulation was hypothesized for the Zn2+ transport system adjacent to root xylem vessels, and it may serve to keep shoot Zn2+ concentrations below toxic levels40. This mechanism would only be plausible if Cs+ specific transporters exist near the root xylem vessels—and these have not yet been found.
We did not find the K+ uptake transporter which was obviously up- or down regulated in the transition to SD. We used Arabidopsis eFP Browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), which provides detailed information about the Arabidopsis gene expression site and various gene induction factors such as organic- and inorganic stressors. Expressions of the homologous Arabidopsis genes AtKUP1, AtHAK5, and AtCNGC1 are not changed by short-day treatment or by exogenous abscisic acid. These expression patterns are consistent with our results and imply that short day length-induced regulation of K+ uptake is also unnecessary in poplar.
Unlike K accumulation, Cs accumulation did not remain constant, but drastically changed by day length transition. Therefore, the Cs+ uptake and translocation mechanisms differ from those for K+ in poplar. It was not determined why Cs accumulation was down-regulated but K accumulation was constant under the transition to SD conditions. It is clear, however, that Cs accumulation was affected by photoperiod.
Methods
Plant material and growth conditions
Hybrid aspen T89 (Populus tremula x tremuloides) (kindly provided by Prof, B. Sundberg, Swedish University of Agricultural Sciences, Sweden) were cultured in sterile pots in half-strength Murashige & Skoog (MS) medium under light- and temperature-controlled conditions (light 16 h, darkness 8 h, 23 °C; light intensity 37.5 μmol m−1 s−1). Each month, all plants were cut about five centimeters below the shoot apex and replanted in fresh MS medium.
Measurement of 137Cs and 42K distributions in poplar
Poplars were grown under long-day (LD) conditions for three- and nine weeks in light- and temperature-controlled conditions (LD3 and LD9). Long-day conditions were as follows: light-period 16 h (light intensity 37.5 μmol m−1 s−1), dark-period 8 h, temperature 23 °C. To investigate the effects of seasonal transitions, the culture conditions were shifted to short-day (SD) for an additional two, four, and six weeks (SD2, SD4, and SD6) after the end of LD3 cultivation. Short-day conditions were as follows: light-period 8 h (light intensity 37.5 μmol m−1 s−1), dark-period 16 h, temperature 23 °C. 137CsCl (25 kBq, with 0.1 μM 133CsCl) or 42K (8 kBq, with 0.1 μM 39KCl) solutions were then added to the growth media to trace root absorption. The 42KCl solution was prepared using an 42Ar+-42K+ generator41,42. The purity of the 42K+ was verified from the gamma-ray spectra emitted by the test solutions using a germanium detector (GEM-type, ORTEC, USA). The decay of the 42K+ spectral peak (1525 keV) was monitored for 7 d as described in Kobayashi et al.43. The half-lives of the test solutions were measured with a liquid scintillation counter (LSC-6100, Hitachi Aloka Medical, Japan) and were theoretically identical to the actual half-life of 42K. Plants grown under LD and SD conditions were incubated with radioisotopes under the same photoperiods. Incubation times were 48 h and 24 h for the 137Cs and 42K experiments, respectively. Shoots and roots were separated and dried for 3 d at 50 °C. In the 137Cs assay using SD6 plants, plants were cut into four parts: apex (shoot apex and top three leaves), leaf (remaining leaves and petioles), stem, and root. To measure 137Cs and 42K radioactivity, the gamma counters AccuFLEX γ7001 (Hitachi Aloka Medical, Japan) and ARC-300 (Hitachi Aloka Medical, Japan) were used, respectively. The details of handling and measuring 137Cs and 42K were described in Kobayashi et al.43. Cesium-137 distribution was also investigated autoradiographically with a laser imaging scanner (FLA-9500, GE Healthcare, UK) in LD3, LD9, and SD6 plants. Significant differences between 42K and 137Cs quantities for each organ type and under each photoperiod were evaluated using one-way ANOVA.
Acquisition of K influx transporter homologous gene nucleotide sequences
Full-length AtHAK5 (At4G13420) and AtCNGC1 (At5G53130) coding sequences were obtained from the Arabidopsis sequence database (TAIR; https://www.arabidopsis.org/). Eight homologous HAK5 genes and nine homologous CNGC1 genes were identified in poplar from the plant genomic resource (Phytozome; https://phytozom.jgi.doe.gov/pz/portal.html). OsHAK1 (Os04g0401700) and OsHAK5 (Os01g0930400) coding sequences were identified from RAP-DB, (http://rapdb.dna.affrc.go.jp/). The HvHAK1 (Accession number: AF025292) coding sequence was searched using nucleotide BLAST in NCBI (http://www.ncbi.nlm.nih.gov/).
Constructing the phylogenetic tree
The full-length coding sequences for the Populus HAK5 and CNGC1 homologs were converted to amino acid sequences, and then phylogenetic trees were created using the Maximum Likelihood method in MEGA application (Molecular Evolutionary Genetics Analysis, ver. 5.05).
Gene expression analysis
Plant roots were flash-frozen in liquid nitrogen then pulverized using a mixer mill (QIAGEN, Germany). Total RNA was extracted using RNeasy Plant Mini Kit (QIAGEN). Quantitative real-time reverse-transcription PCR (qRT-PCR) was performed using One Step SYBR PrimeScript RT-PCR Kit ІІ (Takara, Japan) and 7300 Real Time PCR System (Applied Biosystems, USA). Three biological replicates were run for each photoperiod. An ubiquitin gene (Accession number: AF240445) was used as a reference gene in hybrid aspen T89. The Ubiqutin primers for qRT-PCR were the following: UBIQUTIN-F (5′-TGAACCAAATGATACCATTGATAG-3′) and UBIQUTIN-R (5′-GTAGTCGCGAGCTGTCTTG-3′). The gene expression analysis primers for PtKUP1, PttHAK-like1, PttHAK-like2, PttCNGC1-like1, and PttCNGC1-like2 are listed in Table 1. Significant differences between each gene expression level were confirmed by one-way ANOVA.
Cloning of the PttHAK-like1 coding sequence
The full-length coding sequence of PttHAK-like1 was cloned. The whole root of Hybrid aspen T89 grown until SD6 was harvested and stored at −80 °C. Total RNA was extracted using RNeasy Plant Mini Kit, and cDNA was synthesized from the extracted RNA with a ReverTra Ace (TOYOBO, Japan). The primers used for cloning PttHAK-like1 were the following: PttHAK-like1: (5′-ATGGAAGGAGATGATGATCG-3′) and (5′-TTAGACCATGTATGTCATCCC-3′). The full-length coding sequence was amplified by PrimeSTAR GXL DNA Polymerase (Takara, Japan) and purified by Wizard SV Gel and PCR Clean-Up System (Promega, USA). PttHAK-like1 was inserted into a pGEM T-Easy cloning vector by TA cloning (Promega, USA). The nucleotide sequences of PttHAK-like1 were determined using a DNA sequencer (3130 Genetic Analyzer; Applied Biosystems, USA) and a Big-Dye terminator v3.1 sequencing standard Kit (Applied Biosystems, USA). The sequence was analyzed with Finch TV and BioEdit.
Additional Information
How to cite this article: Noda, Y. et al. Short day length-induced decrease of cesium uptake without altering potassium uptake manner in poplar. Sci. Rep. 6, 38360; doi: 10.1038/srep38360 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Shaw, G. Radionuclides in forest ecosystems. Radioactivity in the Environment. 10, 127–155 (2007).
Kato, H., Onda, Y. & Gomi, T. Interception of the Fukushima reactor accident-derived 137Cs, 134Cs, and 131I by coniferous forest canopies. Geophys. Res. Lett. 39, doi: 10.1029/2012GL052928 (2012).
Takata, D. Distribution of radiocesium from the radioactive fallout in fruit trees. In: Nakanishi, T. M., Tanoi, K. (ed) Agricultural implications of the Fukushima nuclear accident (Springer, New York 143–162 2013).
Kanasashi, T. et al. Radiocesium distribution in sugi (Cryptomeria japonica) in eastern Japan: translocation from needles to pollen. J. Environ. Radioact. 139, 398–406 (2015).
Avery, S. V. Cesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J. Ind. Microbiol. 14, 76–84 (1995).
White, P. J. & Broadley, M. R. Mechanisms of cesium uptake by plants. New Phytol. 147, 241–256 (2000).
Ahn, S. J., Shin, R. & Schachtman, D. P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 134, 1135–1145 (2004).
Jung, J. Y., Shin, R. & Schachtman, D. P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 21, 607–621 (2009).
Gierth, M., Mäser, P. & Schroeder, J. I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 137, 1105–14 (2005).
Qi, Z. et al. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a cesium uptake pathway in Arabidopsis. J. Exp. Bot. 59, 595–607 (2008).
Leng, Q., Mercier, R. W., Yao, W. & Berkowitz, G. A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 121, 753–761 (1999).
Kanter, U. et al. Cesium and strontium accumulation in shoots of Arabidopsis thaliana: Genetic and physiological aspects. J. Exp. Bot. 61, 3995–4009 (2010).
Jansson, S., Bhalerao, R. P. & Groover, A. T. Genetics and genomics of populus. (Springer, New York 2010).
Welling, A., Moritz, T., Palva, E. T. & Junttila, O. Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol. 129, 1633–1641 (2002).
Olsen, J. E. et al. Ectopic expression of oat phytochrome A in hybrid aspen changes critical day length for growth and prevents cold acclimatization. Plant J. 12, 1339–1350 (1997).
Welling, A., Kaikuranta, P. & Rinne, P. Photoperiodic induction of dormancy and freezing tolerance in Betula pubescens. Involvement of ABA and dehydrins. Physiol. Plant. 100, 119–125 (1997).
Mølmann, J. A. et al. Low night temperature and inhibition of gibberellin biosynthesis override phytochrome action and induce bud set and cold acclimation, but not dormancy, in PHYA overexpressors and wild-type of hybrid aspen. Plant Cell Environ. 28, 1579–1588 (2005).
Eschrich, W., Fromm, J. & Essiamah, S. Mineral partitioning in the phloem during autumn senescence of beech leaves. Trees 2, 73–83 (1988).
Furukawa, J., Kanazawa, M. & Satoh, S. Dormancy-induced temporal up-regulation of root activity in calcium translocation to shoot in Populus maximowiczii. Plant Root 6, 10–18 (2012).
Furukawa, J. et al. Seasonal fluctuation of organic and inorganic components in xylem sap of Populus nigra. Plant Root 5, 56–62 (2011).
Ahmad, I. & Maathuis, F. J. M. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 171, 708–714 (2014).
Epstein, E., Rains, D. W. & Elzam, O. E. Resolution of dual mechanisms of potassium absorption by barley roots. Proc. N. A. S. 49, 684–692 (1963).
Maathuis, F. J. M. & Sanders, D. Regulation of K+ absorption in plant root cells by external K+: interplay of different plasma membrane K+ transporters. J. Exp. Bot. 48, 451–458 (1997).
Rubio, F., Santa-Maria, G. E. & Rodriguez-Navarro, A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 109, 34–43 (2000).
Mäser, P. et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667 (2001).
Gupta, M. et al. KT/HAK/KUP potassium transporters gene family and their whole life cycle expression profile in rice (Oryza sativa). Mol. Genet. Genomics 280, 437–452 (2008).
Kurita, Y. et al. Establishment of a shortened annual cycle system; a tool for the analysis of annual re-translocation of phosphorus in the deciduous woody plant (Populus alba L.). J. Plant Res. 127, 545–551 (2014).
Langer, K. et al. Poplar potassium transporters capable of controlling K+ homeostasis and K+ -dependent xylogenesis. Plant J. 32, 997–1009 (2002).
Tuskan, G. A. et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604 (2006).
Santa-María, G. E., Rubio, F., Dubcovsky, J. & Rodríguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9, 2281–2289 (1997).
Yang, T. et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 166, 945–959 (2014).
Hua, B. G., Mercier, R. W., Leng, Q. & Berkowitz, G. A. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiol. 132, 1353–1361 (2003).
Harada, H. & Leigh, R. A. Genetic mapping of natural variation in potassium concentrations in shoots of Arabidopsis thaliana. J. Exp. Bot. 57, 953–960 (2006).
Alemán, F. et al. The F130S point mutation in the Arabidopsis high-affinity K+ transporter AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front. Plant Sci. 5, 430, doi: 10.3389/fpls.2014.00430.eCollection (2014).
Amtmann, A. & Rubio, F. Potassium in plants. eLS, doi: 10.1002/9780470015902.a0023737 (2012).
Yamada, S. et al. Effect of potassium nutrition on current photosynthesized carbon distribution to carbon and nitrogen compounds among rice, soybean and sunflower. J. Plant Nutr. 25, 1957–1973 (2002).
Amtmann, A., Troufflard, S. & Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133, 682–691 (2008).
Marschner, H., Kirkby, E. A. & Engels, C. Importance of cycling and recycling of mineral nutrients within plants for growth and development. Botanica. Acta. 110, 265–273 (1997).
Adams, E., Abdollahi, P. & Shin, R. Cesium inhibits plant growth through jasmonate signaling in Arabidopsis thaliana. Int. J. Mol. Sci. 14, 4545–4559 (2013).
Burleigh, S. H., Kristensen, B. K. & Bechmann, I. E. A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 52, 1077–1088 (2003).
Homareda, H. & Matsui H. Biochemical utilization of 42Ar-42K Generator. Radioisotopes 35, 543–546 (1986).
Aramaki, T. et al. Application of 42K to Arabidopsis Tissues Using Real-Time Radioisotope Imaging System (RRIS). Radioisotopes 64, 169–176 (2015).
Kobayashi, N. I. et al. Tracer experiment using 42K+ and 137Cs+ revealed the different transport rates of potassium and caesium within rice roots. Functional Plant Biology 43, 151–160 (2015).
Acknowledgements
This work was financially supported in part by KAKENHI Grant Number 24110007 awarded to J.F. We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
Y.N. and J.F. contributed equally to this work. Y.N. and J.F. proposed and organized the project. Y.N., J.F., T.A., N.N., K.T., and S.S. discussed and designed the experiment. Y.N., J.F., T.A., N.N., A.H., and K.T. carried out the experiments. Y.N., J.F., T.A., N.N., A.H., K.T. T.M.N., and S.S. analyzed and interpreted the data. Y.N. and J.F. wrote the main manuscript text. All the authors revised the manuscript and participated in discussions of the research.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Noda, Y., Furukawa, J., Aohara, T. et al. Short day length-induced decrease of cesium uptake without altering potassium uptake manner in poplar. Sci Rep 6, 38360 (2016). https://doi.org/10.1038/srep38360
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38360
This article is cited by
-
A Seasonal Change of Active Ingredients and Mineral Elements in Root of Astragalus membranaceus in the Qinghai-Tibet Plateau
Biological Trace Element Research (2021)
-
Effects of environmental conditions, low-level potassium, ethylenediaminetetraacetic acid, or combination treatment on radiocesium-137 decontamination in Napier grass
Environmental Science and Pollution Research (2021)
-
Wood structure of Populus alba formed in a shortened annual cycle system
Journal of Wood Science (2018)
-
Photoperiod and Soil Munition Constituent Effects on Phytoaccumulation and Rhizosphere Interactions in Boreal Vegetation
Water, Air, & Soil Pollution (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.