Abstract
The associations of the metabolic syndrome (MetS) with intraocular pressure and primary open angle glaucoma (OAG) have been reported. This study aimed to determine whether a difference in association exists between OAG and metabolic risk factors according to obesity status among Korean adults. A total of 8,816 participants (≥40 years) in the Korea National Health and Nutrition Examination Survey were classified into obese, body mass index (BMI) ≥ 25 kg/m2 and non-obese, BMI < 25 kg/m2. The prevalence of MetS was 40.1% in non-obese OAG and 66.0% in obese OAG. The prevalence of OAG increased with increasing number components for MetS in total population and in non-obese subjects (P < 0.001, respectively), while the prevalence of OAG was not associated with number of components for MetS in obese subjects (P = 0.14). In non-obese individuals, subjects with high triglycerides, high blood pressure (BP), and MetS were more likely to have OAG compared with those without high triglycerides, high BP, and MetS after adjusting for potential confounders. However, MetS or its components exhibited no significant association with glaucoma status in obese individuals. Our study provides understanding on the differences in association of OAG with MetS and its components according to obesity status.
Similar content being viewed by others
Introduction
Glaucoma is the second leading cause of global blindness following cataract and accounts for approximately 8% of blindness cases1. Among subtypes of glaucoma, open angle glaucoma (OAG) is reported to be the most common2,3,4; more specifically, normal tension glaucoma (NTG) predominates primary open angle glaucoma (POAG) in Asians. NTG was reported to comprise about 77% of OAG in Asians and is characterized by normal baseline intraocular pressure (IOP) and slow progression5, is asymptomatic until late stages, and is therefore difficult to detect if screening examinations are not performed.
Of the many risk factors of OAG, IOP is considered as a predominant risk factor worldwide. Recently, associations between IOP and components of metabolic syndrome (MetS) have been reported in both genders in diverse ethnic groups6,7,8. Moreover, associations between OAG and components of MetS have also been reported9. Components such as blood pressure (BP) showed differential association with OAG according to the level of BP, with low diastolic and high systolic BP being associated with OAG10,11. Increasing evidence suggests strong association between OAG and MetS12,13. Like many other metabolic diseases, the relationship with obesity has been previously evaluated, and numerous studies reported that lower body mass index (BMI) is associated with increased risk of OAG14,15.
Obesity, a growing public health problem especially in Asian countries16,17, is thought to cause metabolic abnormalities, such as insulin resistance, dyslipidemia, hypertension, which are risk factors for cardiovascular disease and type II diabetes18. However, there has been recent interest in the heterogeneity of cardiovascular disease risk within the traditional body mass index (BMI) categories: A substantial proportion of overweight or obese adults are metabolically healthy, whereas a considerable proportion of normal-weight adults exhibit a clustering of metabolic abnormalities19,20. This subgroup of metabolically abnormal but non-obese individuals are reported to be associated with high risk of cardiovascular disease19,21,22. It has been commonly reported that despite lower BMI, Asians tend to have higher percent body fat and are more insulin resistant than Caucasians23,24, with relatively high prevalence of metabolically obese but normal weight population16,25.
Considering that OAG is affected by hemodynamic risk factors9,10,11, the disease may also show differential association with obesity and MetS. Despite the possible clinical significance, the associations between OAG and metabolic risk factors according to obesity status have not been addressed in large population-based studies. Therefore, the present study was conducted to investigate the associations between metabolic risk factors and OAG in Korean adults aged 40 years or older and stratified by obesity status, focusing on possible differences in these associations.
Results
The prevalence of OAG was 3.90% overall, 4.22% among non-obese participants, and 3.25% among obese participants. The study population included 210 underweight (BMI < 18.5 kg/m2), 3,203 normal weight (18.5 ≤ BMI < 23 kg/m2), 2,324 overweight (23 ≤ BMI < 25 kg/m2), and 3,079 obese (BMI ≥ 25 kg/m2) participants, who were grouped according to the recommendations for Asians by the WHO Pacific Regional Office26,27. The general and clinical characteristics of the subjects are shown according to presence of glaucoma and obesity in Table 1. In both non-obese and obese groups, age, IOP, household income, education level, diagnosis of hypertension and systolic BP were significantly different between non-glaucoma and glaucoma subjects. Spherical equivalent, TGs, HDL-cholesterol, and diagnosis of MetS in the non-obese group were also significantly different between non-glaucoma and glaucoma subjects.
In total population, the diagnosis of hypertension, the prevalence of high FPG, the prevalence of high TGs, and the presence of MetS were significantly higher in glaucoma subjects compared with non-glaucoma subjects (P < 0.001, 0.043, 0.002, and <0.001, respectively, Table 2). However, contrasting results on diagnosis of MetS and its components were found in non-glaucoma and glaucoma subjects according to obesity status.
In the non-obese group, the diagnosis of hypertension, prevalence of high TGs, prevalence of low HDL-cholesterol, and presence of MetS were significantly higher in glaucoma subjects compared with non-glaucoma subjects (P < 0.001, <0.001, 0.009, and <0.001, respectively) However, among obese individuals, only the diagnosis of hypertension was significantly higher in glaucoma subjects than non-glaucoma subjects (P = 0.017).
Table 3 shows that, in total population, subjects with hypertension, high TGs, and MetS were significantly more likely to have glaucoma after adjusting for surveyed year, age, and sex (Model 1, odds ratio (OR), 1.58; 95% confidence interval (CI), 1.13–2.21; OR, 1.43; 95% CI, 1.08–1.90; OR, 1.37; 95% CI, 1.03–1.83, respectively), which still remained significant after additional adjustment for IOP, household income, exercise, education level, smoking status, alcohol consumption, BMI, and dyslipidemia medication use (Model 2). Similarly, in non-obese individuals, subjects with hypertension, high TGs, and MetS were also significantly more likely to have glaucoma (Model 2, OR, 1.88; 95% CI, 1.29–2.74; OR, 1.65, 95% CI, 1.12–2.43; OR, 2.00, 95% CI, 1.34–3.00, respectively). Unlike non-obese individuals, MetS components in obese individuals exhibited no statistically significant association with glaucoma.
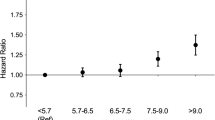
The prevalence of glaucoma according to the number of MetS components in non-obese and obese individuals is presented in Fig. 1. The prevalence of glaucoma significantly increased with increasing number of components for MetS among total population and non-obese subjects (p-value for trend <0.001 and <0.001), while the prevalence of glaucoma was not significantly associated with the number of components for MetS among obese subjects (p-value for trend = 0.145).
Prevalence of glaucoma according to the number of MetS components in total study participant, non-obese and obese participants.
Among non-obese participants, the prevalence of glaucoma increased with increasing number of MetS components (p for trend <0.001), while the prevalence of glaucoma was not associated with the number of MetS components among obese individuals (p for trend = 0.145).
Discussion
To the best of our knowledge, this is the first large, population-based study to examine the associations between metabolic risk factors and OAG according to obesity status among an Asian adult population. In this cross-sectional study comprising Korean adults aged 40 years or older who participated in KNHANES during the years 2010–2012, we found a positive association between elevated blood pressure, high TGs, and MetS and OAG among the non-obese population after adjusting for potential confounders (Table 3). Moreover, the prevalence of OAG was positively associated with the number of MetS components in the non-obese-population, whereas no significant association was observed in the obese population (Fig. 1).
The obese population in our study not only showed no association between OAG and MetS, but also had a lower prevalence of OAG itself than the non-obese population. This is in agreement with previous studies which revealed that higher BMI is associated with lower risk of OAG among Asians15 as well as Caucasians14. In our study, the prevalence of OAG was 4.22% among non-obese subjects, 3.25% among obese subjects, and 3.90% in the total study population. This is similar to the prevalence published in other epidemiologic studies in Korea (Namil study, 3.5%)3 and Japan (Tajimi study, 3.9%)4.
Although the exact mechanism underlying this phenomenon is not known, a few factors may have played a role in the protective effect against OAG among the obese population, effective enough to overcome the adverse influence of MetS and it’s components on the development of OAG. First, leptin is primarily synthesized in adipose tissue, and in addition to its well-known action on the hypothalamus for appetite and metabolism control, neuroprotective activity in the brain28 has also been reported. The degeneration and progressive loss of retinal ganglion cells are pathophysiological features of glaucoma29. Leptin receptors were found on axons of such retinal ganglion cells30, with leptin acting as a neuroprotective agent in glaucoma31. Plasma leptin level is known to increase proportionally with an increase in adipose tissue32. Second, estrogen, which is increased in both men and women in the obese population33, has a neuroprotective effect through estrogen receptors in retinal ganglion cells34 as well as increasing ocular blood flow35. Prior studies using animal models revealed retinal protective effect of 17-beta-estradiol on retinal ganglion cells34, and decreased retinal nerve fiber thickness was found in postmenopausal women not on hormone replacement therapy compared with those who did36. In addition, increased neuropeptide Y release which is known to be associated with obesity37 has been reported to inhibit the decrease in the number of ganglion cells38. Animal models showed neuroprotective role of neuropeptide Y against necrotic and apoptotic retinal ganglion cell death induced by glutamate38, which is the main excitatory neurotransmitter in the retina39.
In our study, individuals with high TGs were significantly more likely to have OAG in the non-obese and total population (Table 3). This result is consistent with a previous nationwide cohort study carried out in Asia which reported higher prevalence of high TGs among OAG patients40. Since dyslipidemia is a well-established risk factor for atherosclerosis41, high TGs may contribute to OAG development through decreased retinal and choroidal perfusion and ischemic damage of the retinal ganglion cells. In the same context, the use of statins is reported to have a neuroprotective effect against OAG through improved perfusion, as demonstrated in an ischemia-reperfusion animal model42. Although the definition of high TGs included individuals on treatment for high TGs in the present study, high TGs in the non-obese and total population were still strongly associated with OAG even after additional adjustment for dyslipidemia medication use in Model 2 of Table 3. In contrast, high TGs in the obese population were not associated with increased prevalence of OAG.
Low HDL cholesterol was not associated with increased prevalence of OAG in all three groups after adjustment for confounding variables including medication use for dyslipidemia (Table 3). Similarly, in other studies conducted in Asia, which did not divide the study groups according to obesity, no association was found between low HDL cholesterol and OAG43,44. Among the young Korean population aged between 10 and 39 years with normal baseline IOP, low HDL cholesterol has been identified as a significant variable increasing the risk of OAG, suggesting a possible difference in the association in younger age groups6.
In the present analysis, non-obese individuals with hypertension were more likely to be associated with OAG than were those without hypertension after adjustment for confounding variables such as IOP and BMI, while no such association was observed among obese individuals. The association between high systolic BP and increased prevalence of OAG has been verified in many previous studies10,11, as high systolic BP can result in low perfusion pressure due to reduced vessel diameter in the short-term and arteriosclerosis in the long-term45. Systemic use of beta-blocker and nitrate to lower blood pressure has been previously addressed to be associated with lower IOP27, possibly providing a protective effect against glaucoma. In this study, we further found that individuals with high BP or those on antihypertensive medications were associated with higher prevalence of OAG, independent of IOP.
High prevalence of myopia is one of the well-known ocular characteristics of East Asians, with a prevalence of 35.7% among Korean adults over 40 years of age, which is higher than Western Europe and United States, and similar to China and Japan46. Despite the well-established association between myopia and OAG47, when divided into groups according to obesity status, OAG subjects were significantly more myopic than non-OAG subjects only in the non-obese group, and not in the obese group. Moreover, although smoking is one of the recognized risk factors of glaucoma48, there was no difference in smoking status between non-OAG and OAG subjects when stratified by obesity status.
OAG in the present study included both POAG with high IOP and NTG, as IOP was not taken into account in the diagnosis. In addition to the well-known relationship between IOP and glaucoma, the association between IOP and MetS has also been previously established in both men6 and women7 of various ethnic groups8. However, our study is interesting in that the relationship between OAG and MetS was evaluated according to obesity, independent of IOP.
Our study should be interpreted with consideration of the following limitations. First, causality cannot be determined because this was a cross-sectional analysis. Second, instead of separately studying the effect of statin use on OAG, subjects with statin use were defined as having dyslipidemia in the diagnosis of MetS components. However, despite the reported protective effect of statin use in glaucoma49, subjects with high TGs were more likely to be associated with OAG in the non-obese group and all study participants even after further adjusting for dyslipidemia medication use, implying that the association is powerful enough to overcome the protective effect of statins. Third, MetS components were defined using single lipid and glucose measurement, which may have biased the results. Fourth, the relationship between OAG and metabolic risk factors were not separately evaluated for male and female, despite the fact that sex hormones may play a role in the pathogenesis of OAG. Further investigations on the association between OAG and metabolic syndrome components in the non-obese population according to gender may yield additional interesting results. Fifth, we included both POAG and NTG in the analysis. Although adjustment for IOP was carried out to minimize the effect of confounding, since NTG is much more prevalent among East Asians3,4, a separate analysis may also provide interesting findings. Finally, OAG was diagnosed based on FDT results instead of using the standard automated perimetry (SAP). This may be responsible for slightly higher prevalence of OAG in this study (3.9%) compared with other epidemiologic study in Korea using SAP (3.5%)3, as FDT has a higher false positive rate than SAP17. Despite these limitations, the present study was based on a nationally representative sample of the Korean adult population, which is a major strength. Furthermore, it is the first large population-based study to examine the associations between OAG and components of MetS according to obesity status.
In conclusion, high TGs, high BP, and MetS were independently associated with increased prevalence of OAG in non-obese individuals, while such associations were not found in obese individuals. Moreover, the prevalence of OAG increased with increasing number of MetS components among the non-obese population, whereas such trend was not observed among the obese population. This indicates a difference in association of components of MetS and glaucoma according to obesity status, and highlights a possible need for special attention to metabolically obese but normal weight adults, especially those with high BP and high TGs, on screening and detecting glaucoma.
Patients and Methods
Study Population
This study was conducted using data from the Korea National Health and Nutrition Examination Survey V (KNHANES) 2010–2012. KNHANES is a cross-sectional, nationally representative sample of the non-institutionalized Korean civilian population aged one year or older and employs a rolling sampling design that implements a complex, stratified, multistage probability sample design. KNHANES is organized regularly by the Korean Ministry of Health and Welfare to monitor the health and nutritional status of people in South Korea50,51. It is comprised of a health interview survey, health examination survey, and nutrition survey. The ophthalmologic survey was first introduced in KNHANES in 2008 and involves ophthalmologic interviews and examinations for participants aged 3 years or older. In this study, a total of 12,881 individuals aged 40 years or older who completed the ophthalmologic survey were selected. Of these 12,881 subjects, we excluded individuals who had missing data in the health interview survey or health examination survey. After exclusion, a total of 8,816 subjects were selected as the study population. The KNHANES V was conducted according to the Declaration of Helsinki and was carried out by specially trained interviewers or examiners who were not provided with any prior information about the participants, and all participants signed an informed consent form. This study was approved by the Institutional Review Board of the St. Vincent’s hospital, the Catholic University of Korea.
Measurements
In the health interview survey, trained interviewers recorded information about demographic and health-related characteristics including age, education, residence, and household income. Information about lifestyle characteristics, including smoking, drinking alcohol, and exercise, were recorded by self-reported questionnaires. Residence was categorized as urban (8 major cities in South Korea: Seoul, Gyeonggi, Busan, Daegu, Incheon, Gwangju, Daejeoun, Ulsan) or rural (8 other provinces in South Korea). Household income was divided into quartiles after being adjusted for the number of family members. Participants were grouped in the low income group if their household income was categorized into the lowest quartile. Education level was categorized into four groups by highest education achieved: elementary school, middle school, high school, and university. Subjects were categorized in the high education group if their highest education was high school or university. Smoking status was categorized into three groups: non-smokers, current smokers, and ex-smokers. Alcohol consumption status was also classified into three groups: non-drinker, mild-to-moderate drinker (<30.0 g alcohol/day), and heavy drinker (≥30.0 g alcohol/day). Regular exercise was defined as moderate-intensity physical activity (e.g., carrying a light load, cycling at a regular pace, playing tennis) for at least 20 minutes at a time and at least three times a week.
Height was measured to the nearest 0.1 cm using a portable stadiometer (SECA 225, SECA Deutschland, Hamburg, Germany) while the participants were standing barefoot. Weight was measured to the nearest 0.1 kg using an electronic scale (GL-6000-20, CAS KOREA, Seoul, Korea) while the participants wore a lightweight gown. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference (WC) was measured after normal expiration to the nearest 0.1 cm using a measuring tape (SECA 200, SECA Deutschland) at the mid-level between the lower margin of the ribcage and the iliac crest at the mid-axillary line. BP was measured from the right arm using a standard mercury sphygmomanometer (Baumanometer, WA Baum Co., New York, NY, USA) after 5 minutes of rest in the sitting position. Systolic and diastolic BPs were measured three times at 30 second intervals, and the average of the final two values was used for analysis. Hypertension was defined as systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or use of antihypertensive medications.
Venous blood samples were collected from participants who had been fasting for at least 8 hours. Fasting plasma glucose (FPG), high-density lipoprotein (HDL) cholesterol, total cholesterol, and triglycerides (TGs) were measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. Diabetes mellitus was defined as FPG ≥ 126 mg/dL or use of insulin or anti-hyperglycemic medications.
All ophthalmologic examinations were performed using a slit-lamp (Haag-Streit model BQ-900; Haag-Streit AG, Koeniz, Switzerland) by ophthalmologists. The participants underwent ophthalmologic interviews, visual acuity measurements, slit-lamp examinations of the anterior segment, and IOP measurements. IOP was measured once per eye with a Goldmann applanation tonometer during slit-lamp examination. A digital non-mydriatic retinal camera and a Nikon D-80 digital camera (Nikon Inc., Tokyo, Japan) were used to obtain fundus images from all participants, without pupil dilation. Visual field testing by frequency-doubling technology (FDT) (Humphrey Matrix; Carl Zeiss Meditec, Inc., Dublin, CA) using the N-30-1 screening test was performed if the participants were suspected of having glaucoma and met any of the following criteria: (1) IOP ≥ 22 mmHg or glaucomatous optic disc; (2) vertical cup-to-disc ratio (VCDR) or horizontal cup-to-disc ratio (HCDR) ≥ 0.5; (3) optic disc hemorrhage; (4) retinal nerve fiber layer defect; or (5) non-adherence to the ISNT rule (i.e., normally, the neuroretinal rim thicknesses are in the following order: inferior > superior > nasal > temporal). FDT was repeated if the rate of fixation errors was >0.33 or if the false-positive rate was >0.33. Only one eye from each subject was used for statistical analysis. The data from the eye diagnosed with OAG were used for analysis, and the worse eye was chosen for analysis in bilateral cases. For control subjects, the right eye was chosen for analysis because the IOPs in the right and left eyes were highly correlated (Pearson’s correlation = 0.79, P < 0.001).
Definition of glaucoma
We defined OAG as “glaucoma” in this study. OAG was diagnosed based on the International Society of Geographical and Epidemiological Ophthalmology Criteria. OAG was defined as the presence of an open angle (peripheral anterior chamber depth >1/4 corneal thickness) and satisfaction of any one of the following category 1 or 2 criteria: Category 1 required both a visual field defect consistent with glaucoma and either a VCDR ≥ 0.7 (97.5th percentile) or a VCDR difference ≥0.2 between eyes (97.5th percentile) [if FDT results are present and have fixation error and false-positive error ≤1]; Category 2 required a VCDR ≥ 0.9 (99.5th percentile) or a VCDR difference ≥0.3 between eyes (99.5th percentile) [if FDT results are absent or if FDT results have fixation error and false-positive error ≥2]. IOP was not taken into consideration when establishing the diagnosis of OAG.
Definitions of obesity and MetS
In this study, obesity was defined as a BMI ≥ 25 kg/m2, as recommended for Asians by the WHO Western Pacific Regional Office26,27. MetS was diagnosed according to the National Cholesterol Education Program Adult Treatment Panel III’s modified definition of MetS52. The WC criteria were modified to be suitable for the Asian population53. MetS was diagnosed when more than three of the following risk factors were present: (1) WC ≥ 90 cm for men or WC ≥80 cm for women; (2) serum TGs ≥ 150 mg/dL or treatment for high TGs; (3) HDL cholesterol <40 mg/dL for men, HDL cholesterol <50 mg/dL for women, or undergoing treatment for HDL cholesterol abnormalities; (4) systolic BP ≥ 130 mmHg, diastolic BP ≥ 85 mmHg, or use of antihypertensive medication; (5) FPG ≥ 100 mg/dL or use of antihyperglycemic medication.
Statistical analysis
Statistical analyses were conducted using the SAS (version 9.3; SAS Institute, Inc., Cary, NC, USA) survey procedure and the sampling weights of KNHANES to acquire nationally representative prevalence estimates. All analyses performed in this study were adjusted for survey year to minimize the variations with survey year. The data in this study are presented as mean ± SE for continuous variables or proportion (SE) for categorical variables. General and clinical characteristics of subjects were compared according to the presence of glaucoma and obesity. Variables with skewed distributions were analyzed after logarithmic transformations. We performed a priori subgroup analysis by obesity status, which was verified by interaction between MetS and obesity (P for interaction = 0.134). Diagnoses of MetS and its components were also compared according to the presence of glaucoma and obesity status. Multiple logistic regression analysis was used to estimate the magnitude of the associations of MetS and its components with glaucoma according to obesity status after adjusting for surveyed year, age, and sex in Model 1. Model 2 additionally adjusted for IOP, household income, exercise, education level, smoking status, alcohol consumption, BMI, and dyslipidemia medication use. Odds ratios (ORs) for glaucoma according to the presence of MetS and its components were calculated separately in the non-obese and obese groups, as well as two groups together in total. Differences in prevalence of glaucoma according to number of MetS components were compared in non-obese and obese subjects. A p-value <0.05 was considered statistically significant.
Additional Information
How to cite this article: Kim, H.-A. et al. Differential Association of Metabolic Risk Factors with Open Angle Glaucoma according to Obesity in a Korean Population. Sci. Rep. 6, 38283; doi: 10.1038/srep38283 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Pascolini, D. & Mariotti, S. P. Global estimates of visual impairment: 2010. The British journal of ophthalmology 96, 614–618, doi: 10.1136/bjophthalmol-2011-300539 (2012).
Cho, H. K. & Kee, C. Population-based glaucoma prevalence studies in Asians. Survey of ophthalmology 59, 434–447, doi: 10.1016/j.survophthal.2013.09.003 (2014).
Kim, C. S., Seong, G. J., Lee, N. H. & Song, K. C. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 118, 1024–1030, doi: 10.1016/j.ophtha.2010.10.016 (2011).
Iwase, A. et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 111, 1641–1648, doi: 10.1016/j.ophtha.2004.03.029 (2004).
Anderson, D. R., Drance, S. M. & Schulzer, M. Natural history of normal-tension glaucoma. Ophthalmology 108, 247–253 (2001).
Kim, Y. H. et al. High intraocular pressure is associated with cardiometabolic risk factors in South Korean men: Korean National Health and Nutrition Examination Survey, 2008-2010. Eye (London, England) 28, 672–679, doi: 10.1038/eye.2014.43 (2014).
Park, B. J., Park, J. O., Kang, H. T. & Lee, Y. J. Elevated intraocular pressure is associated with metabolic syndrome in postmenopausal women: the Korean National Health and Nutrition Examination Survey. Menopause (New York, N.Y.) 20, 742–746, doi: 10.1097/GME.0b013e31827ce3c6 (2013).
Wygnanski-Jaffe, T. et al. Metabolic syndrome: a risk factor for high intraocular pressure in the Israeli population. International journal of ophthalmology 8, 403–406, doi: 10.3980/j.issn.2222-3959.2015.02.34 (2015).
Newman-Casey, P. A., Talwar, N., Nan, B., Musch, D. C. & Stein, J. D. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology 118, 1318–1326, doi: 10.1016/j.ophtha.2010.11.022 (2011).
Memarzadeh, F., Ying-Lai, M., Chung, J., Azen, S. P. & Varma, R. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Investigative ophthalmology & visual science 51, 2872–2877, doi: 10.1167/iovs.08-2956 (2010).
Zheng, Y. et al. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the singapore malay eye study. Investigative ophthalmology & visual science 51, 3399–3404, doi: 10.1167/iovs.09-4867 (2010).
Poh, S., Mohamed Abdul, R. B., Lamoureux, E. L., Wong, T. Y. & Sabanayagam, C. Metabolic syndrome and eye diseases. Diabetes research and clinical practice 113, 86–100, doi: 10.1016/j.diabres.2016.01.016 (2016).
Tham, Y. C. & Cheng, C. Y. Associations between Chronic Systemic Diseases and Primary Open Angle Glaucoma: An Epidemiological Perspective. Clinical & experimental ophthalmology, doi: 10.1111/ceo.12763 (2016).
Ramdas, W. D. et al. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Archives of ophthalmology (Chicago, Ill.: 1960) 129, 767–772, doi: 10.1001/archophthalmol.2010.373 (2011).
Nangia, V. et al. Prevalence and associated factors of glaucoma in rural central India. The Central India Eye and Medical Study. PloS one 8, e76434, doi: 10.1371/journal.pone.0076434 (2013).
Park, Y.-M. M. & Liu, J. In Metabolic Syndrome: A Comprehensive Textbook (ed Rexford, S. Ahima ) 87–100 (Springer International Publishing, 2016).
Kim, C. S. et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea national health and nutrition examination survey (1998-2011). Diabetes & metabolism journal 38, 35–43, doi: 10.4093/dmj.2014.38.1.35 (2014).
Mokdad, A. H. et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama 289, 76–79 (2003).
Wildman, R. et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Archives of internal medicine 168, 1617–1624 (2008).
Lee, S. H. et al. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008–2010. Clinical endocrinology 82, 213–220, doi: 10.1111/cen.12502 (2015).
Kim, M. K. et al. Normal weight obesity in Korean adults. Clinical endocrinology 80, 214–220, doi: 10.1111/cen.12162 (2014).
Mark Park, Y. M. et al. Diet Quality and Mortality Risk in Metabolically Obese Normal-Weight Adults. Mayo Clinic proceedings 91, 1372–1383, doi: 10.1016/j.mayocp.2016.06.022 (2016).
Wang, J. et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. The American journal of clinical nutrition 60, 23–28 (1994).
Lear, S. A., Kohli, S., Bondy, G. P., Tchernof, A. & Sniderman, A. D. Ethnic variation in fat and lean body mass and the association with insulin resistance. The Journal of clinical endocrinology and metabolism 94, 4696–4702, doi: 10.1210/jc.2009-1030 (2009).
Lee, S. H. et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: the Chungju Metabolic disease Cohort (CMC) study. Clinical endocrinology 75, 475–481, doi: 10.1111/j.1365-2265.2011.04085.x (2011).
Organization, W. H. The Asia-Pacific perspective: redefining obesity and its treatment (Sydney: Health Communications, Australia, 2000).
Wen, C. P. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public health nutrition 12, 497–506, doi: 10.1017/s1368980008002802 (2009).
Zhang, F., Wang, S., Signore, A. P. & Chen, J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke; a journal of cerebral circulation 38, 2329–2336, doi: 10.1161/strokeaha.107.482786 (2007).
Qu, J., Wang, D. & Grosskreutz, C. L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Experimental eye research 91, 48–53, doi: 10.1016/j.exer.2010.04.002 (2010).
Gariano, R. F. & Gardner, T. W. Retinal angiogenesis in development and disease. Nature 438, 960–966, doi: 10.1038/nature04482 (2005).
Gupta, A. Leptin as a neuroprotective agent in glaucoma. Medical hypotheses 81, 797–802, doi: 10.1016/j.mehy.2013.08.023 (2013).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England journal of medicine 334, 292–295, doi: 10.1056/nejm199602013340503 (1996).
Zumoff, B. Relationship of obesity to blood estrogens. Cancer research 42, 3289s–3294s (1982).
Zhou, X. et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Developmental neurobiology 67, 603–616, doi: 10.1002/dneu.20373 (2007).
Toker, E., Yenice, O., Akpinar, I., Aribal, E. & Kazokoglu, H. The influence of sex hormones on ocular blood flow in women. Acta ophthalmologica Scandinavica 81, 617–624 (2003).
Deschenes, M. C. et al. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Investigative ophthalmology & visual science 51, 2587–2600, doi: 10.1167/iovs.09-3710 (2010).
Kim, H. J., Min, K. B. & Min, J. Y. Neuropeptide Y gene-by-psychosocial stress interaction effect is associated with obesity in a Korean population. Psychoneuroendocrinology 69, 10–15, doi: 10.1016/j.psyneuen.2016.03.003 (2016).
Santos-Carvalho, A., Elvas, F., Alvaro, A. R., Ambrosio, A. F. & Cavadas, C. Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell death & disease 4, e636, doi: 10.1038/cddis.2013.160 (2013).
Kishida, K. & Naka, K. I. Amino acids and the spikes from the retinal ganglion cells. Science (New York, N.Y.) 156, 648–650 (1967).
Lin, H. C., Chien, C. W., Hu, C. C. & Ho, J. D. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: a case-control study. Ophthalmology 117, 2088–2095, doi: 10.1016/j.ophtha.2010.03.003 (2010).
Bansal, D., Nayakallu, R. S., Gudala, K., Vyamasuni, R. & Bhansali, A. Agreement between Framingham Risk Score and United Kingdom Prospective Diabetes Study Risk Engine in Identifying High Coronary Heart Disease Risk in North Indian Population. Diabetes & metabolism journal 39, 321–327, doi: 10.4093/dmj.2015.39.4.321 (2015).
Honjo, M. et al. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Archives of ophthalmology (Chicago, Ill.: 1960) 120, 1707–1713 (2002).
Tan, G. S., Wong, T. Y., Fong, C. W. & Aung, T. Diabetes, metabolic abnormalities, and glaucoma. Archives of ophthalmology (Chicago, Ill.: 1960) 127, 1354–1361, doi: 10.1001/archophthalmol.2009.268 (2009).
Wang, S. et al. Dyslipidemia and eye diseases in the adult Chinese population: the Beijing eye study. PloS one 7, e26871, doi: 10.1371/journal.pone.0026871 (2012).
Costa, V. P. et al. Ocular perfusion pressure in glaucoma. Acta ophthalmologica 92, e252–266, doi: 10.1111/aos.12298 (2014).
Yoon, K. C. et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean journal of ophthalmology: KJO 25, 421–433, doi: 10.3341/kjo.2011.25.6.421 (2011).
Shen, L. et al. The Association of Refractive Error with Glaucoma in a Multiethnic Population. Ophthalmology, doi: 10.1016/j.ophtha.2015.07.002 (2015).
Chiotoroiu, S. M., Pop de Popa, D., Stefaniu, G. I., Secureanu, F. A. & Purcarea, V. L. The importance of alcohol abuse and smoking in the evolution of glaucoma disease. Journal of medicine and life 6, 226–229 (2013).
Stein, J. D. et al. The relationship between statin use and open-angle glaucoma. Ophthalmology 119, 2074–2081, doi: 10.1016/j.ophtha.2012.04.029 (2012).
Ko, S. H. et al. Higher prevalence and awareness, but lower control rate of hypertension in patients with diabetes than general population: the fifth korean national health and nutrition examination survey in 2011. Diabetes & metabolism journal 38, 51–57, doi: 10.4093/dmj.2014.38.1.51 (2014).
Park, Y. M. et al. Glycaemic and haemoglobin A1c thresholds for detecting diabetic retinopathy: the fifth Korea National Health and Nutrition Examination Survey (2011). Diabetes research and clinical practice 104, 435–442, doi: 10.1016/j.diabres.2014.04.003 (2014).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645, doi: 10.1161/circulationaha.109.192644 (2009).
Alberti, K. G., Zimmet, P. & Shaw, J. The metabolic syndrome–a new worldwide definition. Lancet 366, 1059–1062, doi: 10.1016/s0140-6736(05)67402-8 (2005).
Acknowledgements
The authors thank Professor Ki Ho Park, Department of Ophthalmology, Seoul National University College of Medicine and Professor Chan Yun Kim, Department of Ophthalmology, Institute of Vision Research, Yonsei University College of Medicine for reviewing this article. We report this result on behalf of the Epidemiologic Survey Committee of the Korean Ophthalmological Society. Also, we are grateful to Dr. Srishti Shrestha for critical review of this manuscript.
Author information
Authors and Affiliations
Contributions
Hyun-Ah Kim wrote the main manuscript text. Kyungdo-Han performed the statistical analysis. The critical revision of the manuscript was done by Yun-Ah Lee, Jin A Choi, Yong-Moon Park. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kim, HA., Han, K., Lee, YA. et al. Differential Association of Metabolic Risk Factors with Open Angle Glaucoma according to Obesity in a Korean Population. Sci Rep 6, 38283 (2016). https://doi.org/10.1038/srep38283
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38283
This article is cited by
-
Metabolic syndrome as an independent risk factor for glaucoma: a nationally representative study
Diabetology & Metabolic Syndrome (2023)
-
Girl Power in Glaucoma: The Role of Estrogen in Primary Open Angle Glaucoma
Cellular and Molecular Neurobiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.