Abstract
We conducted a meta-analysis of analytic and observational studies to evaluate the association between smoking and epiretinal membrane (ERM). The pertinent studies were identified via a literature search using three databases (MEDLINE, Cochrane Library, Embase) and the reference lists of retrieved studies. Cohort, case-control and cross-sectional studies meeting the predefined criteria were included. We extracted the odds ratios (OR) and 95% confidence intervals (CI) from each study. Overall risk estimates were pooled using random-effects models. Subgroup analyses based on several stratified factors were also performed. Two cohort studies and six cross-sectional studies involving 46,837 subjects were included. The pooled effect of all eight studies showed an unexpected significant decreased association between smoking and the occurrence of ERM (OR, 0.72; 95% CI 0.61–0.84; p = 0.29, I2 = 17.9%). Subgroup analyses supported this finding, except for the age-unadjusted group (OR, 0.87; 95% CI 0.63–1.22), the ERM classification group (cellophane macular reflex (CMR) OR, 0.93; 95% CI 0.68–1.28; preretinal macular fibrosis (PMF) OR, 0.74; 95% CI 0.41–1.32), the Asian group (OR, 0.75; 95% CI 0.52–1.09) and the past smoker group (OR, 1.02; 95% CI 0.85–1.22). The pooled effects from the current literature suggested a declining association between smoking and ERM, which requires further studies to confirm.
Similar content being viewed by others
Introduction
Epiretinal membrane (ERM) is a common retinal condition that typically occurs in people aged 50 years and older and is characterized by the proliferation of abnormal tissues on the inner retinal surface1,2, which results in mild to moderate visual impairment with an impact on quality of life3. Electron microscopy has shown glial cells, retinal pigment epithelium (RPE) cells, fibrocytes, myofibroblasts, and fibrous astrocytes to be involved4. The early form of ERM, in which there is a patch or patches of irregular increased reflection from the inner retinal surface is sometimes called cellophane macular reflex (CMR) and is usually asymptomatic. Meanwhile, the more severe form, which includes surface wrinkling retinopathy and is termed preretinal macular fibrosis (PMF)5,6, can cause significant loss of visual acuity and visual symptoms via tangential traction in the macula area7.
In recent years, studies among different ethnic groups have provided population-based prevalence data on ERM, ranging from 1.0% to 28.9%5,8,9,10,11,12,13,14,15,16,17,18. Despite much effort in epidemiologic, clinical and laboratory research, the pathogenesis and risk factors for ERM remain incompletely understood. There is still a great discrepancy in existing studies regarding possible risk factors, other than age, for primary ERM5,11,17.
It is know that ERM formation is a fibrotic process that occurs on the surface of the retina1, with most ERMs containing fibroblasts and myofibroblasts4,19. Evidence has shown that retinal Müller cells, hyalocytes, and RPE cells have the ability to differentiate into a myofibroblastlike phenotype that is responsible for excessive collagen production and deposition, as well as the contractile activity of ERMs20,21,22. The retinal (outer) side of the ERM usually consists of an extracellular matrix (ECM) layer containing bundles of extracellular fibrils, which are usually randomly oriented. The exact origin of the collagenous components in the ECM of ERM remains unclear1. Meanwhile, smoking is a well-known risk factor for a wide range of diseases, including vascular disease, lung cancer, and chronic obstructive pulmonary disease23,24,25. Additionally, smoking-associated fibrosis has recently been described by several researchers26,27. Checa et al.28 demonstrated that cigarette smoke extract induced the expression of a variety of profibrotic genes, and the activation of TGF-β1, resulting in the excessive accumulation of ECM and the activation of myofibroblasts, which also plays a crucial role in the pathogenesis of ERM1. Thus, it is of great interest to examine whether smoking behavior is related to ERM.
Methods
Search Strategy
We followed the MOOSE checklist to conduct this meta-analysis. (see Supplementary Material). We conducted a systematic search through three databases, including MEDLINE (1950 to October 2015), the Cochrane Library (up to October 2015), and Embase (1980 to October 2015), using the following MeSH terms and keywords: smoking OR tobacco OR cigarette OR lifestyle OR risk factor OR epidemiology, combined with epiretinal membrane OR cellophane macular reflex OR preretinal macular fibrosis. References in the retrieved publications were reviewed to identify other pertinent studies. No language restrictions were imposed.
Study Selection
Studies were considered eligible if they met the following criteria: (1) they were cohort or case–control or cross-sectional studies published as original articles; (2) they estimated the relationship between smoking and the risk of ERM with the odds ratio (OR) and 95% confidence interval (CI) or provided any other calculable information; and (3) they defined OR of smoking as current smoker VS none/never smoker or current smoker VS none/never or past/former/ever smoker. When articles with overlapping participants were available, we selected the most recent study. We contacted the authors of related articles for necessary data such as ORs of association between smoking and ERM and ORs concerning smoking status and other stratified factors to determine whether the article met our inclusion criteria. We excluded the literature sources such as conference abstracts, as they lacked adequate information for evaluation of their validity and reliability.
Two of the authors (Q-HT and S-ZW) reviewed the titles and abstracts independently to exclude any obviously irrelevant studies. If uncertainty regarding suitability remained, full manuscripts were subsequently obtained. Any disagreement was resolved by discussion.
Data Extraction and Study Quality Evaluation
For each study, the following characteristics were extracted: (1) last name of first author, (2) publication year, (3) country in which the study was conducted, (4) study design, (5) study period, (6) population type and sample size, (7) mean age or age range of study subjects, (8) smoking status, (9) ERM classification, (10) ERM definition and grating, (11) ERM prevalence and (12) adjusted variables. Methodological quality was assessed using the validated Newcastle–Ottawa quality assessment scale (for cohort studies)29 and the Cross-Sectional Study Quality scale (for cross-sectional studies)30.
Data Synthesis
The association between smoking and ERM was evaluated by calculating pooled ORs and 95% CIs. We used age-adjusted or gender-adjusted ORs whenever possible. However, several articles did not provide adjusted ORs (shown in Table 1), in which case we used the unadjusted ORs. Smoking was defined as current smoker VS none/never smoker or current smoker VS none/never or past/ever smoker, except for the subgroup analysis of smoking status where smoking is defined as current smoker VS past smoker. The significance of the pooled OR was determined by Z test (P < 0.05 was considered statistically significant, unless otherwise specified). ERMs were classified as either early or late stage, commonly corresponding to CMR or PMF, respectively. Subgroup analysis was performed based on study design, subject race, smoking status, ERM classification and age adjustment. In addition, sensitivity analysis was also performed.
Heterogeneity among the included studies was tested the Cochran Q and I2 statistics31. Significant heterogeneity was detected if the P value was <0.1 or I2 was >50%32. According to the Cochrane Reviewers’ Handbook Version 4.2.2, results from random-effects model are more conservative and they approximate results more precisely from the fixed-effects model when no significant heterogeneity is detected, so we used a random-effects model throughout all analyses. Potential publication bias was assessed by Egger’s test33 and Begg’s test34. A sensitivity analysis, which removed each study one at a time, was conducted to confirm the stability of the findings. All statistical analyses were performed using commercially available software (STATA 12.0; StataCorp LP). The level of significance was set to P < 0.05, unless otherwise specified.
Results
Identification and Selection of Studies
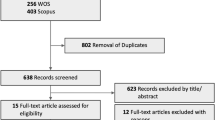
Our search of three databases identified 329 articles. After screening of the titles and abstracts, 311 unrelated articles were excluded as irrelevant. In further evaluation of the remaining 18 articles, 10 were excluded for the following reasons: 613,14,35,36,37,38 did not report the risk factor of smoking; 35,11,39 could not provide essential data after contacting the corresponding authors and 115 did not meet the definition of smoking. In the end, two cohort studies10,18 and six cross-sectional studies9,12,16,17,40,41 met our criteria (Fig. 1).
Study Characteristics and Study Quality Evaluation
The detailed characteristics of the eight selected studies are summarized in Table 1. The eight studies involved 46,837 participants from 1992 to 2013. In terms of the participants’ ethnicities, four studies were conducted on Asian populations, three were conducted among Caucasians and one was a multi-ethnic study. All the studies were volunteers-based or population-based. Participants in all studies were 40 years of age or older. Smoking status was mainly divided into “never/none”, “past/former/ever” and “current”. ERM classification mostly involved “any ERM”, “CMR” and “PMF”. Various diagnostic methods (e.g., fundus photography, optical coherence tomography (OCT) or indirect ophthalmoscopy) were used. And the prevalence of any ERM in different studies ranged from 3.4% to 28.9%. Several studies provided age, gender or other variable-adjusted ORs, while others did not. Study quality evaluation can be seen in the supplemental materials. (Table S1 for cross-sectional studies, Table S2 for cohort studies).
Association Between Smoking and ERM
In the overall analysis, we detected that smoking was associated with a significantly decreased risk of ERM (OR, 0.72; 95% CI 0.61–0.84) without significant heterogeneity (P = 0.293, I2 = 17.9%) (Fig. 2). We then conducted subgroup analyses based on the following stratified factors:
-
1
Study design: In the study-specific analysis, significant association was also identified in the cohort group (OR, 0.78; 95% CI 0.65–0.94; P = 1.00, I2 = 0.0%) and cross-sectional group (OR, 0.69; 95% CI 0.54–0.88; P = 0.206, I2 = 32.4%) (Figure S1).
-
2
Population ethnicity: In consideration of difference in hereditary susceptibility or environmental factors, we pooled estimates based on ethnicity. Although smoking was not associated with ERM in Asians (OR, 0.75; 95% CI 0.52–1.09; P = 0.163, I2 = 41.4%), a significantly decreased risk between smoking and ERM was found in Caucasians (OR, 0.72; 95% CI 0.61–0.85; P = 0.336, I2 = 8.2%) (Figure S2).
-
3
Smoking status: We also sought to investigate whether smoking has a lasting impact on ERM pathology. To this end, comparisons between past smokers or current smokers and those who had never smoked were undertaken. Current smokers displayed a significantly decreased risk of ERM (OR, 0.73; 95% CI 0.62–0.86; P = 0.416, I2 = 0.0%), while a nonsignificant relationship was found in past smokers (OR, 1.02; 95% CI 0.85–1.22; P = 0.285, I2 = 20.4%) (Figure S3).
-
4
ERM classification: We pooled estimates in terms of different stages of ERM, although no significant correlation with smoking was revealed in the early-stage group (CMR) (OR, 0.93; 95% CI 0.68–1.28; P = 0.041, I2 = 68.7%) or in late-stage group (PMF) (OR, 0.74; 95% CI 0.41–1.32; P = 0.007, I2 = 68.9%) (Figure S4).
-
5
Adjustment for age: Increasing age has been consistently demonstrated to be a risk factor for ERM, we assessed the outcomes in both the age-adjusted group (OR, 0.62; 95% CI 0.51–0.76; P = 0.899, I2 = 0.0%) and the group not adjusted for age (OR, 0.87; 95% CI 0.63–1.22; P = 0.231, I2 = 31.8%) (Figure S5).
Publication Bias and Sensitivity Analysis Study
Begg’s rank correlation test and Egger’s linear regression test indicated no evidence of significant publication bias among the included studies (Begg, P = 0.23; Egger, P = 0.815), and funnel plots showed a symmetric distribution (Fig. 3A). Sensitivity analysis showed no significant changes after removing one study at a time, indicating the stability of these findings (Fig. 3B).
Discussion
Our meta-analysis showed that there was a significant decreasing association between smoking behavior and risk of ERM in cohort and cross-sectional studies. Similar significant relationships were also found in the stratified analyses except for ERM classification, Asian ethnicity, past smoker status and age-unadjusted subgroups.
ERM consists of a sheet of fibrotic tissue that varies in thickness from a single layer of collagen with interspersed cells to a thicker, multilayered fibrocellular proliferation42,43. Although the pathogenesis of ERM has not yet been fully elucidated, the formation and progression of ERM can be regarded as a fibrotic process because the pathologic findings include increased ECM protein deposition and membrane contraction in which myofibroblasts play a crucial role1. Several studies have demonstrated that exposure to cigarette smoke extracts can provoke activation of the TGF β pathway and the epithelial-mesenchymal transition (EMT)44,45, and thereby up-regulate numerous genes involved in fibrosis and the accumulate of ECM28 and these processes are also known to plays a crucial roles in the pathogenesis of ERM. However, the results of epidemiologic studies of the relationship between smoking and risk of ERM have remained inconclusive.
The unexpected protective association between smoking and ERM was also observed in other studies5,15,16,40. Although no adequate explanation has been provided, some researchers have suggested several possible reasons for this finding. Kawasaki et al.16 attributed this unexpected protective association to the substantial difference in the proportion of male and female current smokers in their study participants. As a result, 92.7% of current smokers were men. In addition, current smokers were significantly younger than non-smokers or past smokers. After adjusting for age and gender, the inverse association between current smoking and ERM was attenuated (unadjusted OR 0.39 (CI 0.26 to 0.59); age–gender-adjusted OR 0.60 (CI 0.38 to 0.94)). However, residual influence from the substantial gender difference in the proportion of current smokers cannot be excluded. Another explanation proposed by McCarty et al.40 was a survival effect caused by higher mortality among male current smokers. And in addition, Duan et al.9 have demonstrated that neither the number of cigarettes smoked per day nor smoking years was significantly associated with ERMs, suggesting that this negative association with smoking could have been a spurious finding or could have been due to decreased survival among smokers. Thus, no consensus exists, and further evidence is needed.
Studies have reported significant discrepancy in the prevalence of ERM among different ethnic groups. Some studies have indicated that the prevalence of ERM in the Caucasian population is much higher than in the Asian population9,13,14. In our meta-analysis, we also discovered different associations among ethnic groups with smoking behavior. The underlying explanation for these racial/ethnic variations is unclear. One reason for the discrepancies between studies may be ethnic differences in the background pigmentation of the fundus, leading to differences in the ophthalmoscopic visibility of the ERM13. Meanwhile, this discrepancy could also be attributed to systematic differences in the definition and detection methods of CMR and PMF as well as differences in the grading of these retinal conditions according to Kawasaki et al. Another intriguing possibility is the higher rates of smoking among Asians. Indeed, it has been reported that smoking rates are higher in China and Japan than in Australia and the United States46,47,48,49. Owing to the apparent inverse association between smoking and ERMs, the higher prevalence of cigarette smoking may explain the lower rates of ERMs in our study9. However, such a hypothesis remains to be verified.
We also detected a bit smaller ORs of age-adjusted studies than age-unadjusted studies. Several studies have observed the reverse associations between current smoking and ERM, which they tended to explain by age constitution and survival effect. And the reverse association was attenuated after adjusting age in the research of Kawasaki et al.16, which is different from our subgroup analysis. If the hypothesis was real, it’s reasonable to assume the adjusted ORs would decrease, compared with unadjusted ORs within same study. However, among different studies and combined effects, it’s still so complicated to reach such a conclusion that adjusted one would be attenuated. One possible explanation is the heterogeneities of methodologies, subject ethnicities and statistical analyses between different studies. Although, P values and I2 didn’t show statistical significance in most situations, they did exist and could affect the results in some degree. Considering few studies provided both age-adjusted and age-unadjusted ORs, we could neither test the hypothesis nor simply compare the absolute values of ORs among studies and combined effects.
Several limitations in our work cannot be ignored. First, there were only eight studies with two different study designs included in our meta-analysis. However, the heterogeneity of total pooled estimates was not significant, which indicated good homogeneity among the included studies. Moreover, sensitivity analysis showed no significant changes after removing one study at a time, indicating the stability of these findings. Still, we should be cautious about generalizing our conclusions to other conditions. Secondly, we defined ORs of smoking as current smoker VS none/never smoker or current smoker VS none/never or past/former/ever smoker, which arbitrarily combined the effects of none smoker and past smoker. Coincidentally, subgroup analysis based on smoking status supported our assumption that differences between the two groups were not significant. Self-reported questionnaires and medical records were widely adopted in these including articles, so unclearly definition of smoking status and recall bias could affect the association. While, obscure smoking status definition of McCarty et al. may also attenuate the difference between none smoker and past smoker. Still, we can not get to a conclusion whether it is a fact or statistical spuriousness. Third, the participants were population-based or volunteer-based. It is a common view that volunteers might be more conscientious about maintaining a healthy lifestyle, which could dilute the observed association. Fourth, we failed to evaluate the dosage– response association owing to insufficient data on smoking consumption and different durations. Furthermore, the study of McCarty et al.40 did not clearly define smoking status, we contacted Dr. McCarty to confirm this definition, but received no reply. To take full use of the existing literature, we still included this study in our analysis. Despite the ambiguous definition, the sensitivity analysis showed an unchanged result, which reduced the risk.
In conclusion, this meta-analysis of analytic and observational studies revealed an unexpected beneficial effect of smoking related to ERM. We provide additional evidence on the association between smoking and ERM and also discuss the reason for this unusual association. Owing to the limitations of our work, we should be conservative in interpreting the pooled estimates. Further high-quality research that specifically adresses daily cigarette consumption and smoking duration is warranted to confirm our findings and provide a possible explanation for this association.
Additional Information
How to cite this article: Wang, S.-Z. et al. The Association Between Smoking and Epiretinal Membrane. Sci. Rep. 6, 38038; doi: 10.1038/srep38038 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bu, S. C., Kuijer, R., Li, X. R., Hooymans, J. M. & Los, L. I. Idiopathic epiretinal membrane. Retina 34, 2317–35 (2014).
Sidd, R. J., Fine, S. L., Owens, S. L. & Patz, A. Idiopathic preretinal gliosis. Am J Ophthalmol 94, 44–8 (1982).
Okamoto, F., Okamoto, Y., Fukuda, S., Hiraoka, T. & Oshika, T. Vision-related quality of life and visual function after vitrectomy for various vitreoretinal disorders. Invest Ophthalmol Vis Sci 51, 744–51 (2010).
Smiddy, W. E. et al. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology 96, 811–20, discussion 821 (1989).
Fraser-Bell, S., Ying-Lai, M., Klein, R., Varma, R. & Los Angeles Latino Eye, S. Prevalence and associations of epiretinal membranes in latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci 45, 1732–6 (2004).
Klein, R., Klein, B. E., Wang, Q. & Moss, S. E. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc 92, 403–25, discussion 425–30 (1994).
Bouwens, M. D. & Van Meurs, J. C. Sine Amsler Charts: a new method for the follow-up of metamorphopsia in patients undergoing macular pucker surgery. Graefes Arch Clin Exp Ophthalmol 241, 89–93 (2003).
Cheung, N., Wang, J. J. & Wong, T. Y. Variations in prevalence estimates of epiretinal membranes. Eye (Lond) 21, 1131, author reply 1131–2 (2007).
Duan, X. R. et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci 50, 2018–23 (2009).
Kawasaki, R. et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye (Lond) 23, 1045–51 (2009).
Koh, V. et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci 53, 1018–22 (2012).
Miyazaki, M. et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama study. Graefes Arch Clin Exp Ophthalmol 241, 642–6 (2003).
You, Q., Xu, L. & Jonas, J. B. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye (Lond) 22, 874–9 (2008).
Zhu, X. F. et al. Prevalence and risk factors of idiopathic epiretinal membranes in Beixinjing blocks, Shanghai, China. PLoS One 7, e51445 (2012).
Mitchell, P., Smith, W., Chey, T., Wang, J. J. & Chang, A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology 104, 1033–40 (1997).
Kawasaki, R. et al. Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol 92, 1320–4 (2008).
Ng, C. H. et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology 118, 694–699 (2011).
Aung, K. Z. et al. The prevalence and risk factors of epiretinal membranes: The Melbourne collaborative cohort study. Retina 33, 1026–1034 (2013).
Zhao, F. et al. Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome: analysis of flat-mounted internal limiting membrane specimens. Retina 33, 77–88 (2013).
Guidry, C., Bradley, K. M. & King, J. L. Tractional force generation by human muller cells: growth factor responsiveness and integrin receptor involvement. Invest Ophthalmol Vis Sci 44, 1355–63 (2003).
Kohno, R. I. et al. Possible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contraction. Br J Ophthalmol 93, 1020–6 (2009).
Parapuram, S. K. et al. Differential effects of TGFbeta and vitreous on the transformation of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 50, 5965–74 (2009).
Hecht, S. S. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol 3, 461–9 (2002).
Shah, R. S. & Cole, J. W. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther 8, 917–32 (2010).
Taylor, J. D. COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther 23, 376–83 (2010).
Katzenstein, A. L., Mukhopadhyay, S., Zanardi, C. & Dexter, E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol 41, 316–25 (2010).
Yousem, S. A. Respiratory bronchiolitis-associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol 19, 1474–9 (2006).
Checa, M. et al. Cigarette Smoke Enhances the Expression of Profibrotic Molecules in Alveolar Epithelial Cells. PLoS One 11, e0150383 (2016).
Downs, S. H. & Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52, 377–84 (1998).
Whiting, P., Rutjes, A. W., Reitsma, J. B., Bossuyt, P. M. & Kleijnen, J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3, 25 (2003).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003).
Alderson, P. G. S. & Higgins, J. P. T. eds Cochrane reviewer’s handbook4.2.2 [updated MSTVH2004]. In: Cochrane Library, Issue1. Chichester: wiley, 102–153 (2004).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994).
Fraser-Bell, S., Guzowski, M., Rochtchina, E., Wang, J. J. & Mitchell, P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology 110, 34–40 (2003).
Meuer, S. M. et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology 122, 787–95 (2015).
Horiguchi, M. [The Epidemiology and Surgical Indication of Epiretinal Membrane]. Nihon Ganka Gakkai Zasshi 119, 441–4 (2015).
Noda, Y. et al. [Prevalence of Epiretinal Membrane Using Optical Coherence Tomography]. Nihon Ganka Gakkai Zasshi 119, 445–50 (2015).
Wang, S. B. et al. Prevalence and risk factors of epiretinal membrane in a cohort with cardiovascular disease risk, compared with the Blue Mountains Eye Study. Br J Ophthalmol (2015).
McCarty, D. J. et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol 140, 288–94 (2005).
Ye, H. et al. Prevalence and associations of epiretinal membrane in an elderly urban Chinese population in China: the Jiangning Eye Study. Br J Ophthalmol (2015).
Kritzenberger, M. et al. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 58, 953–65 (2011).
Snead, D. R. et al. Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch Clin Exp Ophthalmol 242, 853–62 (2004).
Eurlings, I. M. et al. Cigarette smoke extract induces a phenotypic shift in epithelial cells; involvement of HIF1alpha in mesenchymal transition. PLoS One 9, e107757 (2014).
Liu, Y., Gao, W. & Zhang, D. Effects of cigarette smoke extract on A549 cells and human lung fibroblasts treated with transforming growth factor-beta1 in a coculture system. Clin Exp Med 10, 159–67 (2010).
Giovino, G. A. et al. Surveillance for selected tobacco-use behaviors-United States, 1900–1994. MMWR CDC Surveill Summ 43, 1–43 (1994).
Gu, D. et al. Cigarette smoking and exposure to environmental tobacco smoke in China: the international collaborative study of cardiovascular disease in Asia. Am J Public Health 94, 1972–6 (2004).
Hill, D. J. & White, V. M. Australian adult smoking prevalence in 1992. Aust J Public Health 19, 305–8 (1995).
Yang, G. et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA 282, 1247–53 (1999).
Acknowledgements
This study was supported by grant 201101C8000001 from the Foundation for Science and Technology Innovation in rural Ningbo.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y.X. and S.W. Performed the experiments: S.W., Q.T. and H.W. Analyzed the data: H.W. and Q.L. Contributed reagents/materials/analysis tools: Y.X. and Q.L. Wrote the paper: S.W., Q.T. and H.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, SZ., Tong, QH., Wang, HY. et al. The Association between Smoking and Epiretinal Membrane. Sci Rep 6, 38038 (2016). https://doi.org/10.1038/srep38038
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38038
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.