Abstract
Sphingans, a group of structurally closely related bacterial exopolysaccharides produced by members of the genus Sphingomonas, can be applied in a variety of industries such as food, cement, and personal care applications due to their high viscosity. A high sphingan-producing-bacterium, Sphingomonas sp. WG can secret large quantity of sphingan designated as WL. To enhance the production of WL, a three-stage control strategy was applied and the highest WL production can reach 33.3 g/L. The rheological analysis showed that the aqueous solution of WL had high viscosity, typical shearing-thinning behavior and great stability to high temperature, a wide range of pH (1 to 14), and high salinity. WL was composed principally of carbohydrate with 6.52% O-acyl groups. The carbohydrate portion of WL contained about 13% glucuronic acid and some neutral sugars including mannose, glucose and rhamnose in the molar ratio of 1:2.28:2.12. Partial acid hydrolysis of WL produced a new oligosaccharide WL-1. Structural resolution revealed that WL-1 consisted of α-L-Rha-(1→4)-β-L-Rha-(1→4)-β-D-Glc-(1→3)-α-D-Glc with β-D-Man substituent at the third glucose residue and carboxyl and O-acyl groups. These findings will broaden the applications of this novel sphingan in food, ink, oil and other industries.
Similar content being viewed by others
Introduction
Microbial exopolysaccharides (EPS) are ubiquitous and important biopolymers with a wide range of commercial applications in various industries such as food, pharmaceutical, and cosmetic industries. One of the best known microbial EPS is xanthan gum produced by Xanthomonas campestris. It has been widely used in food, oil, agriculture and pharmaceutical industries as a thickener, viscosifier, stabilizer and emulsifier1. Due to their diversity and novelty of both structure and function, EPS has attracted much attention. Within the microbial polysaccharides, sphingans are structurally closely related bacterial EPS produced by members of the genus Sphingomonas. The known sphingans including gellan (S-60), welan (S-130), rhamsan (S-194) and diutan (S-657) present excellent rheological properties. They have great potential for many industrial applications, including food, ink, enhanced oil recovery, cement and personal care application. For example, compared with the commonly used water-soluble polymer xanthan gum, welan gum showed higher oil displacement efficiency and viscoelasticity at the same condition though its molecular weight (MW) is 6.6 × 105 Da while the MW of xanthan gum is 2.0 × 106 Da2.
Most sphingans have the conserved linear repeating tetrasaccharide backbone structure (glucose-glucuronic acid-glucose-rhamnose/mannose) with glucosyl, rhamnosyl, mannosyl or acetyl group as different side-chain substituents. For example, gellan gum has glyceryl and acetyl substituents without sugar side chain while welan gum carries acetyl and an L-rhamnosyl or L-mannosyl as side group3. Recent studies have found that the backbone structures of sphingans also exhibit the complexity and some novel sphingans have been identified. Different from the normal sphingans exclusively containing glucuronic acid (such as gellan gum, welan gum) or deoxyglucuronic acid (like sphingan I-886), the sphingan PS-EDIV produced by Sphingomonas pituitosa DSM 13101 contained both glucuronic acid and deoxyglucuronic acid, a statistical replacement of every 10th-20th deoxyglucuronic by glucuronic acid4. Another interesting example is the sphingan secreted by Sphingomonas sp. CS101 that does not contain glucuronic acid and consists of glucose, mannose, fucose, and rhamnose in a molar ratio of 2.1:1.1:1.0:0.15. The EPS from Sphingomonas paucimobilis P4 has a trisacchrides structure (D-glucose-D-glucose-L-rhamnose) in which glucuronic acid is also absent6. The structural variations have obvious influence on the physiochemical characteristics and also develop a broad range of commercial applications of different sphingans. Novel sphingans with excellent rheological properties such as high viscosity and good thermal stability will also amplify the application of this group of EPS.
However, currently the yields of sphingans are still low, which greatly inhibits their applications. The production of gellan gum was below 10 g/L produced by three different strains S. paucimobilis E2 (DSM 6314), NK 2000 and GS1 and the highest production reported was about 35.7 g/L by the strain S. paucimobilis ATCC 314617. The production of welan gum is usually 20 g/L8 and can reach 26 g/L9,10 by the mutation breeding of producing strain or the optimization of fermentation process, however, its yield is much lower than that of xanthan gum (about 35–45 g/L)1. Thus, high production of sphingans will be of great importance for their applications.
Sphingomonas sp. WG, screened from sea mud samples of the Jiaozhou Bay, exhibited high EPS production capacity. This strain is in the cluster of sphingan-secreting strains and closest to the welan gum producing strain Sphingomonas sp. ATCC 31555 according to the phylogenetic analysis based on the similarity of 16 S rRNA sequences (GenBank accession No. KM590521) (Fig. 1). In the present study, in order to obtain a high yield of sphingan WL produced by Sphingomonas sp. WG, a three-stage control strategy was applied in the fermentation process. Considering that the behavior of EPS in aqueous solution is important to increase its applications in various industrial processes, the rheological properties of WL aqueous solution as well as the effects of temperature, pH and inorganic salts were also studied. Moreover, the structural data of WL were presented at great length because the structural properties of sphingans have a great influence on their physiochemical characteristics and industrial applications.
Phylogenetic relationship between strain Sphingomonas.
WG and Sphingomonas species based on 16 S rRNA gene sequences. The tree was constructed with the MEGA6 program using the neighbour-joining method. The numbers at the branches were bootstrap values (confidence limits). Erythrobacter longus was selected as an outgroup to root the tree. Scale bar, 0.01 substitutions/site.
Results and Discussion
Three-stage control fermentation and purification of the sphingan WL
In order to achieve high sphingan yields, a three-stage control strategy was applied in the fermentation process of sphingan WL by Sphingomonas sp. WG. The main controlled parameter was dissolved oxygen (DO) concentration considering that the oxygen transfer might be limited by the high viscosity of the broth in the polysaccharide production. Different opinions existed regarding the effects of DO concentration on the synthesis of gellan gum. Banik and Santhiagu observed that higher DO tension resulted in higher yield and MW of gellan gum11. However, Giavasis et al. found that high agitation did not enhance the gellan gum production while moderate agitation yielded the highest MW and concentration of gellan gum12. The effect of oxygen supply was also investigated in welan gum fermentation and a moderate DO concentration (about 20%) was suitable for both higher welan gum synthesis and its MW. The enhanced metabolic flux of G-1-P to welan gum at 20% DO concentration might result in the increased MW and welan gum concentration13. Therefore, the DO concentration was controlled during the three stages of WL fermentation process. Another important factor in this strategy was the addition of the precursors. In the process of sphingan fermentation, addition of precursors like UDP-glucose, UDP-glucuronicacid, dTDP-rhamnose and other substances might enhance its yield. For example, with supplementation of 200 μM glucose-6-phosphate and fructose-6-phosphate, welan gum production was enhanced by 18%14. So, two precursors, rhamnose and mannose (18 g, respectively) were added in the WL synthesis stage (the second stage). The last factor considered was the utilization of main nutrients, carbon sources and nitrogen sources, therefore, glucose and yeast extract were added at the late stage of the fermentation process. All taken into account, the strategy was applied and the results were shown in Fig. 2. During the first stage (0–24 h), the DCW increased in an exponential way after a very short lag time and reached 6.56 g/L at 24 h. However, the DO level reduced sharply due to the rapid growth of cell and then was maintained above 20% by adjusting the aeration rate and agitation speed. In this period, the yield of WL increased at a slow rate and reached about 5.00 g/L at 24 h. In the second stage (24–48 h), the DCW increased slowly and retained at about 8.2 g/L. The DO concentration fluctuated but was maintained above 30%. The yield of WL increased rapidly and reached 25.2 g/L at 48 h. In the third stage (78–90 h), the feeding of glucose and yeast extract aroused a sustainable increase of WL production and the highest yield can reach 33.3 g/L at the end of fermentation with a high WL productivity up to 0.37 g/L/h. The viscosity of fermentation broth was over 70,000 mPa·s and the liquid state became a jelly-like state after standing for several minutes. The high WL production capacity of Sphingomonas sp. WG will be of great importance for the application of this sphingan.
Subsequently, WL was extracted and purified from fermentation broth. Precipitation of the polysaccharide with alcohol such as methanol, ethanol and isopropanol is the most commonly-used method for recovery of polysaccharide in industry. Thus, ethanol was chosen in our work to extract the sphingan. Furthermore, in order to enhance the recovery of WL, isothiazolinone at a final concentration of 0.2 g/L was added to the broth to kill the bacterial cells and inhibit enzymes from degrading WL before physical extraction with ethanol. The efficiency of total recovery reached 95% after precipitation, filtration and lyophilization. The purified WL will be used in subsequent experiments to elucidate its chemical and rheological properties which have great influence on its application potential.
Rheological property analysis of WL solution
Rheological properties of WL solution were measured. As shown in Fig. 3a, an enhancement of viscosity with WL concentration at the same shear rate was observed. As welan gum and other sphingans2, all WL solutions showed a typical non-Newtonian pseudoplastic behavior or a strong shearing-thinning behavior. The viscosity of all solutions decreased when the shear rate increased and the degree of shear thinning was markedly increased as concentration of WL solution increased. This behavior may be due to the orientation or deformation of macromolecular network in the direction of flow caused by the shearing of EPS solution15. Furthermore, WL solutions exhibited good stability under diverse conditions including high temperature, wide range of pH and high concentration of salts (Fig. 3b–d). The viscosity of WL solutions at different concentrations (0.2%, 0.6% and 1.0%) showed little changes when the temperature increased from 30 °C to 100 °C, indicating that WL had good thermal stability. The viscosity of WL solution maintained at a high level in the range of pH 1–14. Compared with the highest viscosity, the lowest viscosity of WL solution decreased about 13% at pH 1. Generally, the repulsive interactions of anionic groups along the backbone of EPS chain lead to the high viscosity of EPS. However, when salts were added, cations with the opposite charge shielded the charge-charge repulsions, which resulted in a decrease in viscosity and even phase separation15,16. Interestingly, our results showed that the salts had minor effect on its viscosity. The viscosity of the WL solution exhibited a little increase when NaCl (2 to 4%) or CaCl2 (2 to 8%) was added to the broth. When supplemented with 2% (w/v) CaCl2, the apparent viscosity of WL solution was increased by 9%. When the concentration of salts further increased, the viscosity decreased slightly and remained 90 and 86% when 10% (w/v) NaCl and CaCl2 was added, respectively. These results showed that the WL was of good salt resistance and this might be caused by the good hydrophilicity of main chain structure of EPS that made the space structure stable even when the charge of the EPS was shielded16.
Rheological property analysis of crude WL solution.
(a) The viscosity of different concentrations of WL solution under different shear rates. (b) The effect of temperature on the viscosity of WL solution of different concentrations (0.2%, 0.6%, and 1.0% (w/v)). (c) The effect of pH (1–14) on the viscosity of 0.8% (w/v) WL solution (25 °C). (d) The effect of different concentrations of NaCl and CaCl2 (0-10%) on the viscosity of 0.8% (w/v) WL solution (25 °C).
The excellent rheological properties, good thermal and pH stability and salt resistance of WL suggest that this sphingan will have good potential for biotechnological applications. Its typical shear-thinning behavior has several potential advantages in food, ink and other applications. For example, the pseudoplasticity of EPS will enhance food sensory qualities such as mouth feel, flavor release in final products and favors industrial operations like mixing and pumping17. Similar to welan gum18, the addition of WL might also provide shear-thinning flow characteristics to the ink, that is, the ink supplemented with WL is a viscous liquid and it will switch to thin, readily flowable liquid at shear rates generated in writing process. Furthermore, the good resistance to salts will be beneficial for its use in oil industry. For example, WL can be used in enhanced oil recovery process as polymer flooding or in deep wells to control the viscosity of aqueous oil drilling muds and fluids in oil field with high salinity.
Chemical properties of WL
Normally, sphingans have conserved linear repeating tetrasaccharide backbone structure and different side chains. Structural variations of side chains have obvious effects on the physiochemical characteristics and also lead to different commercial applications of different sphingans. For example, the acyl group residue of gellan gum can affect the gelling process and the hardness of the gel. Gellan gum without acyl residue (Gelrite®) is always used as the substitute of agar to solidify nutrient media in thermopiles cultivation19 while gellan gum with low acyl content (Kelcogel®F) can be used as a gelling agent in food and personal care applications20. Welan gum, with both acyl group and L-rhamnosyl or L-mannosyl as side chains, does not gel but it shows excellent rheological properties and good stability over a broad range of pH (2–12) and temperature (up to 150 °C) and can be applied in petroleum, concrete, food and many other industries21. Therefore, the chemical properties of WL were further analyzed in detail.
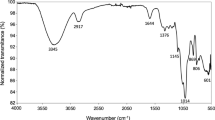
At first, WL was scanned between 4000 and 400 cm−1 and the FT-IR spectrum was found to be well resolved, which indicated that the WL had typical polysaccharide features (Fig. 4a). The spectra revealed an O-H absorption peak at 3416 cm−1, which was mainly caused by the stretching vibration of O-H existed in the hydrogen bond of the polysaccharide. The bands at 2974 cm−1 and 2932 cm−1 were attributable to CH2 asymmetric stretching. The signal at 1726 cm−1 showed that WL contained O-acetyl groups. The absorption peaks at 873 and 810 suggested that WL contained mannose22,23.
The compositional analysis of WL was also carried out. According to the ultraviolet-visible absorbance (UV-Vis) spectrum of the sample, there were no absorption peaks at 260 nm and 280 nm, indicating that WL did not contain nucleic acid or protein (Fig. 4b). The analysis of phenolsulfuric acid and sulfuric acid carbazole methods showed that the total sugar and glucuronic acid content of WL were about 70 and 13%, respectively. The WL also contained acetyl groups with content of 6.52%. The monosaccharide composition of WL was determined by GC-MS after WL was totally hydrolyzed by acid and was transformed into acetyl derivatives (Supplementary Fig. S1). WL was found to be mainly composed of D-mannose, D-glucose and L-rhamnose in a molar ratio of 1:2.28:2.12. The composition of WL was consistent with the composition of welan gum reported by other scientists24.
WL has a high molecular mass over 106 Da and exhibits a high viscosity at low concentrations, therefore, the commonly-used analytical techniques such as NMR and mass spectrometry might not be effective enough in their precise structure elucidation23. Thus, WL was first partially hydrolyzed by 0.2 mol/L trifluoroacetic acid (TFA) and obtained one single symmetrical peak WL-1 using a Sephacryl S-100 High Resolution gel column, which indicated that it was a homogeneous oligosaccharide (Fig. 5a). The structure of this oligosaccharide was investigated in next experiment to provide overall structure information on the sphingan WL.
Structural characterization of WL-1.
(a) Sephacryl High Resolution (S-100) chromatogram of WL-1 fraction from partial acid hydrolysis of WL. (b)1H NMR spectrum (400 MHz) of WL-1 in D2O. The anomeric protons were labeled as A-E. (c) 13C NMR and DEPT spectra of WL-1 in D2O. Acetone-d6 was used as internal standard. (d) Proposed structure of the oligosaccharide WL-1. It should be pointed out that WL-1 also contained carboxyl groups and O-acyl groups.
The positions of glycosidic linkages in WL-1 were analyzed by Smith degradation and GC-MS methods. The periodate oxidation analysis revealed that 0.29 mol of NaIO4 was consumed, and nearly no formic acid (only 0.04 mol) was formed per molar of sugar residues, confirming no existence of (1→)- or (1→6)-linked glycosidic bonds. Then the oxidized sample was treated by Smith degradation and converted into alditol acetate derivatives for GC-MS analysis. The results confirmed the presence of erythritol and glucose which indicated the existence of (1→ 4)-linked and (1→3)-linked glycosidic bonds, respectively25.
Finally, the structure of WL-1 dissolved in D2O was analyzed according to the 1D and 2D NMR spectra obtained on Bruker Ascend 400 MHz NMR spectrometer. In the anomeric region of 1H-NMR spectrum (Fig. 5b), five major proton signals occurred at δ 5.06 (s), 4.83 (s), 4.72 (d, JH-1,H-2 = 7.96 Hz), 4.51 (d, JH-1,H-2 = 8.08 Hz), and 4.48 (d, JH-1,H-2 = 7.92 Hz) ppm indicating a regular oligosaccharide unit and were attributed to α, α, β, β, β-configuration respectively. The two upfield doublets at δ 1.29 and 1.31 ppm were assigned to methyl protons, which indicated H-6 of two rhamnose residues. The chemical shift at δ 2.10 ppm suggested that one monosaccharide unit was acetylated. Other sugar protons were observed in the δ 3.2 to 4.0 ppm region. The 13C-NMR spectrum of WL-1 showed five anomeric carbon signals at δ 104.6, 104.0, 103.7, 95.3, and 95.0 ppm (Fig. 5c). As shown in the DEPT spectrum, three CH2OH groups had the carbon signals at δ 61.5, 62.1, 62.5 ppm, which were assigned to the C-6 non-substituted glucose or mannose residues26. The two CH3 groups (C-6 of Rha) had the carbon signals at δ 18.6, 22.3 ppm. Other sugar ring carbons linked to oxygen were observed in the δ 68.4 to 82.8 ppm region.
The 1H-1H COSY spectrum (Supplementary Fig. S2) gave various proton correlations of sugar residues. The direct C-H couplings of polysaccharide were assigned by the 1H-13C HSQC spectrum (Supplementary Fig. S3). In the 1H-13C HSQC spectrum, the anomeric proton signals at δ 5.06, 4.83, 4.72, 4.51, and 4.48 were clearly attributed to the anomeric carbon signals at δ 95.3, 95.0, 104.6, 103.7 and 104.0 ppm, respectively. Combining with the analysis of the 1H-1H COSY, 1H-13C HSQC spectra and the comparison with the chemical shift data of similarly substituted sugar residues, the assignment of main signals of five sugar residues could be completed23,27.
The 1H-13C HMBC (Supplementary Fig. S4) spectrum confirmed the repeating units of monosaccharide sequences in the oligosaccharide chain. The key cross signal of the H-1 (residue E) at δ 4.48 ppm and the C-3 (residue A) at δ 81.9 ppm indicated the linkage →3,4)-β-D-Glc-(1→3)-α-D-Glc. The H-1 (residue D) signal at δ 4.51 ppm of (1→)-linked β-D-Man was correlated to the C-3 (residue E) signal at δ 80.2 ppm of →3,4)-β-D-Glc-(1→residue, and the H-1 (residue C) signal at δ 4.51 ppm of the →4)-β-D-Rha-(1→was related to the C-4 (residue E) signal at δ 82.8 ppm of the →3,4)-β-D-Glc-(1→. These data indicated that the residue E was linked with residues A, D, and C by a 1,3-O glycosidic bond, 1,3-O glycosidic bond, and 1,4-O glycosidic bond respectively. Besides, the cross peaks of H-1/C-5 and H-1/C-3 were observed in the residue A. Although the carbonyl signals were not observed in the 13C NMR spectrum, some H-C correlations were clearly shown in the 1H-13C HMBC spectrum. Combining with the uronic acid content and acetyl content assays, these data indicated that the documented oligosaccharide also contained carboxyl groups and O-acyl groups. The complete proton and carbon chemical shifts were presented in Table 1 and the proposed structure of WL-1 was shown in Fig. 5d. The WL-1 perhaps consisted of α-L-Rha-(1→4)-β-L-Rha-(1→4)-β-D-Glc-(1→3)-α-D-Glc with β-D-Man substituent at the third glucose residue, carboxyl groups and O-acyl groups. This structure was different from the conserved structure of sphingans, indicating WL should be a novel sphingan with unique properties.
In conclusion, a high sphingan production up to 33.3 g/L was obtained in fermentation of Sphingomonas sp. WG by a three-stage control strategy. The sphingan WL it secreted displayed excellent rheological properties and good thermal and pH stability and salt resistance. Although the composition of WL was very similar to other sphingans, WL had a novel structure according to the structure of oligosaccharide WL-1 deriving from partial acid hydrolysis of WL, which may endow the EPS with unique properties. Sphingomonas sp. WG has great potential for industrial production and will broaden the application of sphingans in food, ink, oil and other industries.
Methods
Microorganism and culture media
Sphingomonas sp. WG was screened from sea mud samples of the Jiaozhou Bay and has been deposited in China Center for Type Culture Collection (CCTCC) under the number M2013161. YEPD medium contained (g/L): glucose 20, peptone 20, yeast extract 10. Twenty grams of agar were added to allow the solidification of culture media when necessary. The composition of fermentation medium was as follows (g/L): maltose 50, yeast extract 4.0, corn steep liquor 2.0, bionitrogen 15, MgSO4 8, K2HPO4 2 and Na2HPO4 2.5.
Fermentation of WL
Sphingomonas sp. WG was activated in YEPD medium and then was inoculated in a Bioengineering NLF-22 Bioreactor containing 15 L fermentation medium. The initial culture conditions were described as follows: temperature 30 °C, pH 7.5, impellor speed 300 rpm, and the aeration rate 0.48 vvm. A three-stage strategy was performed to achieve the high yield of EPS. During the first stage of the fermentation process (0–24 h), the DO concentration was maintained above 20% by adjusting the aeration rate and agitation speed. In the second stage (24–48 h), the DO level was above 30% and 18 g rhamnose and 18 g mannose were added to the fermentation broth. In the third stage (48–90 h), the DO level was constantly maintained above 30%. Glucose and yeast extract were fed to the fermentation broth at a ratio of 10:1 and the total amount of feeding glucose and yeast extract was 40 g/L and 4 g/L, respectively.
In the fermentation process, DCW, the production of WL and DO concentration were detected at certain intervals. To determine the DCW, a certain amount of distilled water was added to the broth to reduce the viscosity at first. The cell mass was separated by the centrifugation of diluted broth at 10,000 × g for 30 min and the pellet was washed twice with distilled water and dried in a hot-air oven at 80 °C until a constant weight. The DCW was determined gravimetrically and expressed in g/L. To determine the yield of WL, the broth was immersed in boiling water bath for 15 min and centrifuged at 10,000× g for 30 min at 4 °C to completely remove the biomass. Then the WL was precipitated by adding two volumes of ice-cold alcohol to the cell-free supernatant overnight at 4 °C. The precipitate formed was recovered by centrifugation at 10,000× g for 30 min, dried in a hot-air oven at 80 °C for 6 h, and then weighted28. The DO concentration was measured using the oxygen electrode on the bioreactor. The viscosity of fermentation broth was performed using a Brookfield Viscometer DV-III with an ultra-low adaptor (Brookfield Engineering Laboratories) equipped with a programmable rheometer and circulating baths with a programmable controller (TC-502P, Brookfield Engineering Laboratories). The LV3 spindle was set to rotate at 0.5 rpm and 25 °C.
Extraction and purification of WL
To kill the bacterial cells and inhibit enzymes from degrading EPS, the fermentation broth was pretreated with isothiazolinone at a final concentration of 0.2 g/L. Crude WL was then precipitated by mixing the fermentation broth with acidic ethanol whose pH was adjusted by oxalic acid at a ratio of 1:1 for about 20 min. It was then filtered by the plate and frame filter presses. The crude WL was successively washed three times with equal volume of acetone and water, respectively. In order to remove the proteins, cell debris and salt, the plate and frame filter presses were applied again and WL powder was obtained by lyophilization.
Rheological properties analysis of WL solution
WL solutions at different concentrations (0.2%, 0.4%, 0.6%, 0.8% and 1.0%) were prepared to investigate the rheological properties. At first, the viscosity of WL solutions under different shear rates ranging from 0.5 to 100 rpm was measured using a Brookfield viscometer DV-III with an ultra-low adapter and the spindle LV3 at 25 °C. To investigate the thermal stability of WL, WL solutions (0.2%, 0.6% and 1.0%) were incubated for 20 min at 20, 30, 40, 50, 60, 70, 80, 90 and 100 °C, respectively. The pH of 0.8% WL solutions was gradually adjusted to 2 to 14 by 10 mol/L HCl and NaOH with gentle homogenization for 1 h and then incubated overnight at 25 °C. The influence of salts was also studied by the addition of 200 g/L NaCl and 200 g/L CaCl229. The viscosity of all above solutions was measured using a Brookfield viscometer DV-III with the spindle LV3 at a shear rate of 20 rpm.
Analysis of FT-IR and UV-Vis spectra of WL
One milligram of WL was converted into KBr discs and analyzed with a Nicolet 6700 FT-IR Spectrometer (Thermo Scientific). UV-Vis spectrum of WL solution at proper concentration was recorded on a UV-visible spectrophotometer (UV-2450) in the range of 190–800 nm.
Compositional analysis of WL
The total sugar content was determined by the phenolsulfuric acid method using a glucose solution as the standard30. The content of glucuronic acid was measured by the sulfuric acid carbazole method31 using glucuronic acid as the standard. The acetyl content was determined as follows: one milliliter WL aqueous solution at a concentration of 1 mg/mL was sequentially treated with 2 mL of alkaline hydroxylamine reagent, 1 mL of 4 mol/L hydrochloric acid to change the pH to 1.2 ± 0.2, 1 mL of acidic ferric chloride reagent and the OD at 540 nm was detected. In the control group, the addition sequence of hydrochloric acid and acidic ferric chloride was reversed32. Acetylcholine chlorid was chosen as the standard. All the measurements were repeated three times.
Monosaccharide composition of WL was analyzed by GC method. Fifty milligram of WL was hydrolyzed with 15 mL of 2 mol/L TFA at 110 °C for 4 h. To convert the obtained monosaccharides into their acetylated aldononitriles, 0.5 mL pyridine and 10 mg hydroxylamine hydrochloride were added to the hydrolyzed products and incubated at 90 °C for 30 min in a preheated water bath shaker, 0.5 mL of acetic anhydride was subsequently added and incubated at 90 °C for another 30 min33. The derivatives were detected using an Agilent Technologies 7890 A gas chromatograph equipped with a FID detector and a HP-5 capillary column (30 m by 0.2 mm). The parameters were: nitrogen as carrier gas; injection volume of 1 μL; 10:1 (v/v) split; injector at 280 °C; the oven temperature programming from 150 °C to 280 °C at 10 °C per min and then at 280 °C for 20 min. Monosaccharide components and composition percentage were determined using glucose, fructose, mannose, galactose and rhamnose as the standards. Inositol was chosen as the internal reference.
Partial acid hydrolysis of WL and purification of oligosaccharide WL-1
Fifty milligram of WL was hydrolyzed with 15 mL of 0.1 mol/L TFA for 3 h at 100 °C and the hydrolysate was evaporated at 40 °C to dryness. The residual TFA was removed by the vacuum evaporation with methanol three times. To obtain hydrolyzed WL products, 10 mL distilled water was added and the dissolved compounds were recovered by centrifugation. The recovered compounds were fractionated with a Sephacryl S-100 High Resolution gel column (16 mm × 100 cm) (GE Healthcare) eluted with water at a rate of 2 mL/min. Only one peak was resolved, collected and then concentrated at 50 °C under vacuum evaporation and freeze-dried to obtain pure WL-1.
Smith degradation
Smith degradation was applied to selectively degrade the polysaccharide to deduce the structure of glycosidic linkages25. Twenty five milligram of WL was oxidized with 25 mL of 15 mmol/L NaIO4 at 4 °C. When the optical density at 223 nm became stable, ethylene glycol was added to the mixture to stop the reaction. The formation of formic acid was then determined by the sodium hydroxide titration. The remaining reaction product was dialyzed against distilled water for 72 h, concentrated by the vacuum evaporation method at 50 °C and reduced by 70 mg NaHB4 for 24 h. The pH was adjusted to 6–7 with 0.1 mol/L of acetic acid and the mixture was dialyzed against distilled water for 72 h and freeze-dried. The obtained product was hydrolyzed with 2 mol/L TFA at 110 °C for 4 h and converted into acetylated aldononitriles as above described33. Finally, its composition was analyzed by GC-MS on Agilent Technologies 7890 A gas chromatograph equipped with a 5975 C MSD detector and a HP-5 capillary column (30 m by 0.2 mm). The parameters were set as follows: nitrogen as carrier gas; injection volume of 1 μL; 10:1 (v/v) split; injector at 280 °C; the oven temperature programming from 150 °C to 280 °C at 10 °C per min and then at 280 °C for 20 min; ion source at 250 °C; MSD detector in the EI mode and scan range from 45 to 550 amu.
NMR spectroscopy analysis
The pure WL-1 (20 mg/mL) was dissolved in D2O in a 5 mm NMR tube. 1H NMR and 13C NMR were carried out on Bruker Ascend 400 MHz NMR spectrometer system at 25 °C. The deuterated acetone was chosen as the internal reference. Distortionless enhancement by DEPT, COSY, HSQC and HMBC were also performed in order.
Additional Information
How to cite this article: Li, H. et al. The preparation and characterization of a novel sphingan WL from marine Sphingomonas sp. WG. Sci. Rep. 6, 37899; doi: 10.1038/srep37899 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hsu, C. H. & Lo, Y. M. Characterization of xanthan gum biosynthesis in a centrifugal, packed-bed reactor using metabolic flux analysis. Process Biochem. 38, 1617–1625 (2003).
Xu, L., Xu, G., Liu, T., Chen, Y. & Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 92, 516–522 (2013).
Fialho, A. M. et al. Occurrence, production, and applications of gellan: current state and perspectives. Appl. Microbiol. Biotechnol. 79, 889–900 (2008).
Schultheis, E. et al. Structural characterization of the exopolysaccharide PS-EDIV from Sphingomonas pituitosa strain DSM 13101. Appl. Microbiol. Biotechnol. 78, 1017–1024 (2008).
Seo, E. J. et al. Isolation of an exopolysaccharide-producing bacterium, Sphingomonas sp. CS101, which forms an unusual type of sphingan. Biosci. Biotechnol. Biochem. 68, 1146–1148 (2004).
Lobas, D. et al. Structure and physical properties of the extracellular polysaccharide PS-P4 produced by Sphingomonas paucimobilis P4 (DSM 6418). Carbohydr. Res. 251, 303–313 (1994).
Prajapati, V. D., Jani, G. K., Zala, B. S. & Khutliwala, T. A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr. Polym. 93, 670–678 (2013).
Kaur, V., Bera, M. B., Panesar, P. S., Kumar, H. & Kennedy, J. F. Welan gum: microbial production, characterization, and applications. Int. J. Biol. Macromol. 65, 454–461 (2014).
Zhu, P. et al. Screening and characterization of Sphingomonas sp. mutant for welan gum biosynthesis at an elevated temperature. Bioprocess Biosyst. Eng. 37, 1849–1858 (2014).
Li, H. et al. Enhanced welan gum production using a two-stage agitation speed control strategy in Alcaligenes sp. CGMCC2428. Bioprocess Biosyst. Eng. 34, 95–102 (2011).
Banik, R. M. & Santhiagu, A. Improvement in production and quality of gellan gum by Sphingomonas paucimobilis under high dissolved oxygen tension levels. Biotechnol. Lett. 28, 1347–1350 (2006).
Giavasis, I., Harvey, L. M. & Mcneil, B. The effect of agitation and aeration on the synthesis and molecular weight of gellan in batch cultures of Sphingomonas paucimobilis. Enzyme Microb. Technol. 38, 101–108 (2006).
Li, H. et al. Effects of dissolved oxygen and shear stress on the synthesis and molecular weight of welan gum produced from Alcaligenes sp. CGMCC2428. Process Biochem. 46, 1172–1178 (2011).
Li, H., Xu, H., Xu, H., Li, S. & Ouyang, P. K. Biosynthetic pathway of sugar nucleotides essential for welan gum production in Alcaligenes sp. CGMCC2428. Appl. Microbiol. Biotechnol. 86, 295–303 (2010).
Zhang, L. M., Zhou, J. F. & Hui, P. S. Thickening, shear thinning and thixotropic behavior of a new polysaccharide-based polyampholyte in aqueous solutions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 259, 189–195 (2005).
Han, M., Du, C., Xu, Z. Y., Qian, H. & Zhang, W. G. Rheological properties of phosphorylated exopolysaccharide produced by Sporidiobolus pararoseus JD-2. Int. J. Biol. Macromol. 88, 603–613 (2016).
Velasco, S. E. et al. Chemical and rheological properties of the beta-glucan produced by Pediococcus parvulus 2.6. J. Agric. Food Chem. 57, 1827–1834 (2009).
Wang, A. & Chadwick, B. W. Ink composition. United States patent 5769931 (1998).
Lin, C. C. & Casida, L. E. GELRITE as a gelling agent in media for the growth of thermophilic microorganisms. Appl. Environ. Microbiol. 47, 427–429 (1984).
Sutherland, I. W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 11, 663–674 (2001).
Li, H., Xu, H., Li, S., Feng, X. & Ouyang, P. Optimization of exopolysaccharide welan gum production by Alcaligenes sp. CGMCC2428 with Tween-40 using response surface methodology. Carbohydr. Polym. 87, 1363–1368 (2012).
Sun, H. H. et al. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 78, 117–124 (2009).
Jia, W. et al. Structure of oligosaccharide F21 derived from exopolysaccharide WL-26 produced by Sphingomonas sp. ATCC 31555. Carbohydr. Polym. 90, 60–66 (2012).
Kang, K. S. & Veeder, G. T. Heteropolysaccharide S-130. United States patent 4342866 (1982).
Chen, C., Zhang, B., Fu, X. & Liu, R. H. A novel polysaccharide isolated from mulberry fruits (Murus alba L.) and its selenide derivative: structural characterization and biological activities. Food Funct. 7, 2886–2897 (2016).
Chen, Y. et al. Structure and antioxidant activity of an extracellular polysaccharide from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Carbohydr. Polym. 87, 218–226 (2012).
Duus, J., Gotfredsen, C. H. & Bock, K. Carbohydrate structural determination by NMR spectroscopy: modern methods and limitations. Chem. Rev. 100, 4589–4614 (2000).
Li, S., Xu, H., Li, H. & Guo, C. Optimizing the production of welan gum by Alcaligenes facalis NX-3 using statistical experiment design. Afr. J. Biotechno. 9, 1024–1030 (2010).
Liu, S. B. et al. Optimization of fermentation conditions and rheological properties of exopolysaccharide produced by deep-sea bacterium Zunongwangia profunda SM-A87. PLoS One 6, e26825 (2011).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Bitter, T. & Muir, H. M. A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334 (1962).
Hestrin, S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 180, 249–261 (1949).
Chen, Y., Xie, M. Y., Wang, Y. X., Nie, S. P. & Li, C. Analysis of the monosaccharide composition of purified polysaccharides in Ganoderma atrum by capillary gas chromatography. Phytochem. Anal. 20, 503–510 (2009).
Acknowledgements
This work was financially supported by the 863 Program (2015AA020925), Natural Science Foundation of China (41276135 and 31172010), Program for New Century Excellent Talents in University (NCET-13–1031), Chinese Postdoc Fund (2014M551981), Shandong Provincial Natural Science Foundation (ZR2015CL003), and Fundamental Research Funds for the Central Universities (15CX05015 A). Special thanks to Robert K. Thomas FRS from University of Oxford for his contribution in improving the manuscript.
Author information
Authors and Affiliations
Contributions
H.Z. conceived the idea, directed the work and drafting the manuscript. H.L. designed and performed most of experiments, guided data analysis and interpretation and wrote a draft of the manuscript. X.J. and W.L.Z. performed the rheological property analysis. Y.J.S. and Z.M.F. performed the chemical properties analysis. S.W.S. participated in the structural elucidation. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, H., Jiao, X., Sun, Y. et al. The preparation and characterization of a novel sphingan WL from marine Sphingomonas sp. WG. Sci Rep 6, 37899 (2016). https://doi.org/10.1038/srep37899
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37899
This article is cited by
-
Preparation and properties of marine-derived sphingan WL gum-metal ion composite hydrogels
Colloid and Polymer Science (2023)
-
Cost-Efficient Production of the Sphingan WL Gum by Sphingomonas sp. WG Using Molasses and Sucrose as the Carbon Sources
Marine Biotechnology (2023)
-
Efficient biosynthesis of polysaccharide welan gum in heat shock protein-overproducing Sphingomonas sp. via temperature-dependent strategy
Bioprocess and Biosystems Engineering (2021)
-
The Function of β-1,4-Glucuronosyltransferase WelK in the Sphingan WL Gum Biosynthesis Process in Marine Sphingomonas sp. WG
Marine Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.