Abstract
Comparisons of soil respiration (RS) and its components of heterotrophic (RH) and rhizospheric (RR) respiration during daytime and nighttime, growing (GS) and dormant season (DS), have not being well studied and documented. In this study, we compared RS, RH, RR, and their responses to soil temperature (T5) and moisture (θ5) in daytime vs. nighttime and GS vs. DS in a subalpine forest in 2011. In GS, nighttime RS and RH rates were 30.5 ± 4.4% (mean ± SE) and 30.2 ± 6.5% lower than in daytime, while in DS, they were 35.5 ± 5.5% and 37.3 ± 8.5% lower, respectively. DS RS and RH accounted for 27.3 ± 2.5% and 27.6 ± 2.6% of GS RS and RH, respectively. The temperature sensitivities (Q10) of RS and RH were higher in nighttime than daytime, and in DS than GS, while they all decreased with increase of T5. Soil C fluxes were more responsive to θ5 in nighttime than daytime, and in DS than GS. Our results suggest that the DS and nighttime RS play an important role in regulating carbon cycle and its response to climate change in alpine forests, and therefore, they should be taken into consideration in order to make accurate predictions of RS and ecosystem carbon cycle under climate change scenarios.
Similar content being viewed by others
Introduction
As an important component of the terrestrial C cycle, soil respiration (RS) in forest ecosystems accounts for 30–90% of the total ecosystem respiration1,2. Thus, forest RS and its components, including heterotrophic respiration and autotrophic respiration, have been extensively studied over the past decades3,4. However, most previous studies on RS, heterotrophic respiration, autotrophic respiration and their responses to environmental changes were conducted during the growing season (GS) and during daytime, and few studies were executed at nighttime and during the dormant season (DS), due mainly to difficulty in field measurement at nighttime and during the DS, in particular, very cold and wet conditions at night time in winter season. Considering greater warming effects (temperature differences) at nighttime than in the daytime, and in winter season than in summer season in Tibetan Plateau5,6, it is essential to quantify soil CO2 fluxes and their temperature responses at nighttime and during the DS.
Soil CO2 efflux in the DS plays an important role in the regional, national, and the global carbon balance7,8, which accounts for 3–50% of annual soil CO2 efflux in forests8,9,10. Annual carbon sequestration would be largely overestimated if soil CO2 fluxes in the DS were not included10. Moreover, several studies by using different approaches including stable isotope methods11, modeling methods verified by eddy covariance system12, or by using soil respiration monitoring system13,14 indicated that soil CO2 fluxes differed largely between daytime and nighttime. Nevertheless, we have still known little about the proportions and variations of nighttime and the DS RS in annual soil C fluxes in specific ecosystems.
The temperature sensitivity (Q10) of RS is an important fundamental parameter in soil carbon cycle models15. It varies with environmental factors and with different components of RS, largely due to the fact that Q10 is regulated by various biotic and abiotic factors, such as soil temperature and soil water content, microbial biomass, substrate quality, and plant physiological activity13,15,16. However, estimates of Q10 are mostly based on measurements in daytime and during the GS, and few measurements were conducted in winter and at nighttime. It was reported that Q10 varied from 1.16 to 24.30 in growing season in sub-alpine forest ecosystem17. However, Wang et al. found that annual Q10 ranged from 3.10 to 4.69, indicating that Q10 varies between the GS and the DS8. Additionally, some studies reported that Q10 value of heterotrophic respiration was lower than that of autotrophic respiration18, while other studies showed the opposite result4,13. Because a slight deviation of Q10 may cause a huge bias in the estimate of RS15, a better understanding of Q10 of different RS components in different times can improve our understanding of the roles of forests in regulating carbon cycle and shaping its response to climate change.
The subalpine ecosystems in Tibetan Plateau are considered to be highly sensitive and prone to global warming impacts19. The changes in RS and its temperature sensitivity will have a significant influence on regional and global carbon cycle and their feedback on climate change20. Some studies on RS and heterotrophic respiration have been conducted in subalpine forest ecosystems9,21, but most of them were based on short term measurements in daytime and during the GS. The CO2 fluxes in the DS and at nighttime in the subalpine forests in Eastern Tibetan Plateau have not been fully determined, and the factors controlling the temperature sensitivity of the RS are even less understood. In the context of global climate change, the rising temperatures may result in greater CO2 emissions to the atmosphere from soils in these areas22,23. Therefore, it is very necessary to accurately estimate RS and to explore its controlling factors, especially in DS and at nighttime.
In this study, we measured spatial and temporal variations of RS in a typical oak forest in subalpine mountains and further examined their soil temperature and moisture sensitivity in daytime and at nighttime, and during the GS vs. the DS. We conducted a trenching experiment to partition soil respiration (RS) into rhizospheric (RR) and heterotrophic respiration (RH). The specific objectives of this study were to: (1) quantify the differences of RS, RH, RR and their responses to environmental factors between daytime and nighttime, and the GS vs. the DS; (2) examine the temperature sensitivity of RH and RS and their controlling factors during daytime vs. nighttime, and GS vs. DS.
Results
Variations of soil surface CO2 fluxes in different time and seasons
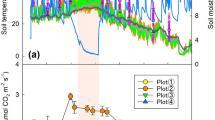
Seasonal dynamics of RH and RS showed the similar patterns with that of soil temperature (Fig. 1a and b). The maximum RS (daytime 4.15 ± 0.20 μmolm−2s−1; nighttime 3.04 ± 0.13 μmol m−2s−1) and RH (daytime 3.05 ± 0.16 μmol m−2s−1; nighttime 2.33 ± 0.11 μmol m−2s−1) occurred in August, while the minimum RS(daytime 0.47 ± 0.02 μmol m−2s−1; nighttime 0.30 ± 0.02 μmol m−2s−1) and RH (daytime 0.35 ± 0.02 μmol m−2s−1; nighttime 0.18 ± 0.01 μmol m−2s−1) occurred in January. Additionally, soil CO2 fluxes was obviously lower in trenched plots than in untrenched plots in the first 6 months after trenching (before December 2010), in order to minimize influence of decomposing roots on RS, we used the data (RS, RH, T5 and θ5) from 2011 to analyze the changes in soil surface CO2 fluxes.
Seasonal dynamics of the mean soil surface CO2 fluxes from the daytime (Day RS and RH) and nighttime (Night RS and RH), soil temperature (Day TUT and Night TT) and soil moisture (Day θUT and Night θT) from untrenched and trenched plots in 2010–2011.
Vertical bars represent the standard deviation of the mean (n = 9 for untrenched and trenched, respectively). *and **Denote significant difference at P = 0.05, P = 0.01, respectively. Red and black asterisk represent the significant difference of the day RS vs. day RH and night RS vs. night RH, respectively.
Both RS and RH were significantly higher in daytime than at nighttime (all P < 0.05) in all seasons (Table 1). On an average, nighttime RS accounted for 69.5 ± 4.4% (mean ± std err) and 64.5 ± 5.5% of daytime values in the GS and DS, respectively, while nighttime RH made up 69.8 ± 6.5% and 62.3 ± 8.5% of the daytime values, respectively. Although T5 and θ5 were higher in trenched than in untrenched plots throughout 2011 (Fig. 1), they were not significantly different (P > 0.05). Soils generally were drier in DS and wetter in GS (Fig. 1c). Moreover, either T5 or θ5 was not significantly different between daytime and nighttime (all P > 0.05, Table 2).
The mean RS rates and RH rates were significantly higher in the GS than in the DS (P < 0.05, Table 1). Overall, DS total mean RS and RH (daytime plus nighttime data, respectively, Table 1) account for 27.3 ± 2.5% and 27.6 ± 2.6% of GS total mean RS and RH, respectively. θ5 and T5 were also higher in the GS than in the DS in both daytime and nighttime (Table 2). On average, total mean TT5 (trenched)/TUT5 (untrenched) and θT5 (trenched)/θUT5 (untrenched) were 7.4/8.3 and 1.8/1.6 times larger in the GS than in the DS, respectively.
Factors controlling daytime and nighttime RS and its components
Both daytime and nighttime RS and its components followed exponential regression relationships with T5 (all P < 0.001, Fig. 2) and a power model relationship with θ5 across the seasons (Fig. 3). T5 alone explained approximately 42.8–90.8% and 49.5–86.7% of the variations in the daytime and nighttime soil C fluxes, respectively. Across all seasons, θ5 alone explained approximately 61.9–70.4% and 16.2–37.2% of the variations in the daytime and nighttime soil C fluxes, respectively. In DS, the variability in RS and its components can be explained alone by θ5 more in daytime than in nighttime. In the GS, we found that only nighttime RS had significant relationship with θ5 (Fig. 3d), suggesting that θ5 was not key factor controlling diurnal dynamics of soil respiration components during the GS.
Relationship of soil respiration and its components with soil temperature at 5 cm soil depth in the daytime (a–c) and nighttime (d–f). Each value represents the monthly mean of soil CO2 flux from the subplot collars measurements for three altitudinal gradients in different seasons (DS pink and GS grey, n = 18. All seasons, n = 36) during 2011. The solid line represents the respiration–temperature relationship estimated according to the model of Eqn (1). All bars indicate mean ± Std Dev (standard deviation). Pink blank circles and grey solid circles represent the monthly mean of soil CO2 flux rate in the DS and the GS, respectively.
Relationship of soil respiration and its components with soil moisture (volumetric, %) at 5 cm soil depth in the daytime (a–c) and nighttime (d–f); Each value represents the monthly mean of soil CO2 flux from the subplot collars measurements for three altitudinal gradients in different seasons (DS and GS grey, n = 18. All seasons, n = 36) during 2011. The solid line represents the respiration–moisture relationship estimated according to the model of Eqn (4). All bars indicate mean ± Std Dev (standard deviation). Pink blank circles and grey solid circles represent the monthly mean of soil CO2 flux rate in the DS and the GS, respectively.
The combined functions of T5 and θ5 (T-θ model, equation 4) can better explain the variability of RS, RH, and RR, indicating that soil respiration and its components were dominated by the interaction of T5 and θ5 rather than a single factor.
Variations in Q10 and its relationships with soil properties
The mean Q10 values of RS and RH were significantly lower in daytime than in nighttime either in the GS or in the DS (all P < 0.05, Table 3). Either in daytime or nighttime, we found that Q10 values of RS and RH were significantly higher in the DS than in the GS (all P < 0.05).
Q10 values of RS and RH in daytime and nighttime showed a significantly negative correlation with T5 (Fig. 4). θ5 had no significant correlation with Q10 of either RS (P = 0.077) or RH (P = 0.663) in daytime, while a strong positive/negative correlation was observed between nighttime Q10 of RS/RH and θ5. Either in daytime or nighttime, Q10 of RS and RH had positive correlation with TOC and MBC. TN had no effect on the Q10 of RS or RH in the daytime, but significant effect occurred at nighttime (Fig. 4).
The relationship of Q10 of RS and RH in the daytime (upper panels) and nighttime (lower panels) with soil temperature (a,f) and soil moisture (b,g), soil total nitrogen (c,h), soil total organic carbon (d,i), soil microbial biomass carbon and nitrogen stocks (e,j) (n = 27). Each dot represents a Q10 value that was calculated across seasons for each subplot during the daytime and at nighttime. *and **Denote significant difference at P = 0.05, P = 0.01, respectively.
Q10 of RSin the GS and the DS had significant correlations with T5 (Fig. 5). Q10 of RH had negative correlations with T5 in the DS but not in the GS. In contrast, θ5 showed significant relationship with Q10 of RS in the GS, but not in the DS. Additionally, in the GS, Q10 of RS had positive correlation with TOC and TN, but Q10 of RH was not affected by TOC and TN. In the DS, however, Q10 of both RSand RH was positively influenced by TOC and MBC (Fig. 5).
The relationship of Q10 of RS and RH in the GS (upper panels) and the DS (lower panels) with soil temperature (a,f) and soil moisture (b,g), soil total nitrogen (c,h), soil total organic carbon (d,i), soil microbial biomass carbon and nitrogen stocks (e,j) (n = 27). Each dot represents a Q10 value that was calculated across daytime and nighttime observations for each subplot in the GS and the DS. *and **Denote significant difference at P = 0.05, P = 0.01, respectively.
Discussion
Comparisons of soil respirations in daytime vs. nighttime and the GS vs. the DS
In this study, we found that the measured RS and RH was 30.5 ± 4.4% (mean ± SE) and 30.2 ± 6.5% lower at nighttime than in daytime during the GS, while they were 35.5 ± 5.5% and 37.3 ± 8.5% lower during the DS, respectively. The changes in soil C fluxes in daytime and at nighttime were due mainly to variations of soil temperature and moisture. Additionally, previous studies reported that the diurnal variability of soil CO2 efflux was affected by the turnover of recent photosynthate11, microbial growth24, plant biological activities25, and allocation of photosynthetic C11,26. In the daytime, favorable soil temperature and light condition can promote the enhancement of microbial metabolism. Moreover, higher daytime RS rates may result from greater translocation of high photosynthesis from the plant shoots to the roots during the daytime relative to at nighttime. The differences in the content and quality of SOC were also reported to determine high and low RS in previous studies27,28,29.
Previous studies shows that RH for a certain time after trenching treatment may be increased in trenched plots30, or kept almost unchanged, or decreased31. In this study, we observed that RH rates in trenched plots significantly lower than in untrenched plots for over 2 months after trenching (September 2010; Fig. 1). The results are consistent with that previously reported in different forest types21,32. In our study, we found that RH was the dominant component of RS during the DS and the GS in 2011. The RH accounted for 72.2 ± 1.2% (mean ± SE) of the whole year RS, with 73.9 ± 2.0% of RS in the DS and 71.7 ± 1.3% of RS in the GS, respectively (Table 1). Our findings are within the range of 16–80% of the contribution of RH to RS reported previously in temperate coniferous forests7,33. However, there were no significant differences in proportion of RH to RSbetween DS and GS. Higher rates of RH in DS were likely due to relatively high metabolic reaction of roots and soil microbial activity for maintaining respirations in the study stands in the winter. Our results suggest that RH was the dominant component of RS, indicating a dominant control of microorganism-associated respiration on RS in the subalpine forest. In the DS, we supposed that soil microbial activity is still functioning to generate CO2 fluxes by decomposing soil organic matters, while the root activity of plants is inhibited in winter because plants stop growing in cold temperature9, and consequently, ending up with a high proportion of RH to total RS (Table 1). Therefore, soil C fluxes in the DS must be taken into consideration when assessing the carbon sink/source patterns of the subalpine forests.
Environmental factors influencing soil CO2 flux
In this study, the daytime and nighttime RS and its components were significantly influenced by the interactions of T5 and θ5. Our results are similar to the findings observed in other forests32,34. In this study, however, a new finding is that T-θ model fits the observations better in the DS than the GS for the all components of RS, indicating that RS and its components can be better predicted through T5 and θ5 in the DS than in the GS. This provides a simple but effective basis for estimating soil respirations in winter.
We also found that θ5 alone didn’t impact either the daytime or the nighttime RH and RR in the GS but T5 did so, suggesting that T5 rather than θ5 is a main factor controlling RH and RR in the GS. Daytime RS had no relationship with θ5, but nighttime RS decreased linearly with increasing θ5 in the GS (Fig. 3d). This is because a high precipitation frequency at nighttime in the GS decreases T5 and consequently suppresses soil CO2 efflux at nighttime. The different relationships of RS and its components with θ5 between the GS and the DS (Fig. 3) suggest that the different roles of T5 and θ5 as independent environmental variables in GS vs. the DS should be specifically taken into consideration when we predict soil respirations.
Constraints on Q10
There have been inconsistent conclusions on Q10 values of RS and its components in previous studies35. Moreover, few studies have compared Q10 of RS and its components in the daytime vs. the nighttime and the GS vs. the DS, especially at the high altitude in subalpine regions. In this study, the RS and RH at nighttime and during the DS are apparently more sensitive to temperature than that during daytime and the GS (Table 3). Our result is in line with some of previous studies that Q10 values were higher at lower temperature17,36, but conflicts with other reports that Q10 was not characterized by a reflection of temperature change37. The high temperature sensitivity of RS and RH at low temperature environments may be due to a high rate of substrate utilization and changes in microbial populations and microbial activity aiming to maintain high decomposition rates37,38. This indicates that RS or RH at nighttime and during the DS might be more sensitive to temperature change than during daytime and the GS. In the GS, other parameters, such as substrate supply by roots and leaf area index in relation to phenology, may be more important than temperature in controlling the carbon flux rates14,39.
In this study, soil moisture did not influence Q10 values of RS and RH in the daytime and the DS, but we found that Q10 values of RS increased with increasing θ5 at nighttime and the GS, while Q10 of RH decreased with increasing θ5 at nighttime (Figs 4 and 5). The similar results were also reported in the sub-alpine forests of the Eastern Qinghai-Tibet Plateau17. Soil moisture may cause changes in microbial community structure and soil mineralization rate40,41, and furthermore affect sensitivities of their biotic and physicochemical processes to temperature42. In addition, plant-microbe-soil interactions and plant metabolism could contribute to Q10 values of RS and its components43.
The availability of soil substrates can influence microbial metabolism44. Therefore, increased availability of either soil carbon or nitrogen is expected to stimulate microbial growth and activity in soils45,46, leading to the subsequent changes in Q10 of RS and RH47. In this study, we found that TOC and MBC were key factors affecting the temperature sensitivities of both daytime RS and RH and nighttime RS and RH during DS (Figs 4 and 5). Therefore, in addition to soil temperature and moisture, the changes in TOC and MBC additionally explain the temperature sensitivity of soil C flux47. Our results suggest that if a constant seasonal Q10 is used in the models for estimating soil CO2 efflux, we cannot make accurate prediction on future soil CO2 losses15.
Uncertainty of trenched effects on soil respirations
The trenching method for root exclusion is generally used to estimate RH, especially in forest ecosystems although some uncertainties exist on its accuracy and interpretation of the results48,49. One possible uncertainty may originate from the change in soil environmental conditions caused by trenching50. In this study, we found a higher level of T5 and θ5 through 2011 (daytime: 9.0% and 7.6%, nighttime: 8.6% and 7.7%, respectively, absolute difference) in the trenched plots compared with the untrenched plots, which may lead to an overestimation of RH51. However, we did not find any significant trenching effect on T5 or θ5 in either the GS or the DS (Table 2). Therefore, the trench-induced changes in soil microclimate can be neglected in this study. Another possible impact of trenching on soil chemical parameters is that trenching can change the nutrient conditions and thus alter microbial activities52. In the present study, the soil carbon and nitrogen components were different degrees higher in untrenched plots than in trenched plots (Fig. 6), and suggested that the difference could be attributed to the suppression of photosynthetic products supply to the rhizosphere. Additionally, we used a linear regression model to determine this estimation error4. Our results showed that the trenched respiration contribution decreased by 4.3% and 3.9% after this correction in daytime and nighttime. We believe this process is unlikely to affect our estimation of RH due to its minor contribution (5–8%) to soil respirations in temperate forests4,18,53. Although some under- or over-estimation may be inevitable, we feel that trenching method is reasonable in this study.
Mean contents of inorganic nitrogen (IN, NH4++NO3−), soil microbial biomass carbon (MBC) and nitrogen (MBN), total nitrogen (TN), soil total organic carbon (TOC) in the soil of trenched and untrenched plots (n = 27), respectively.
Bars with the different lower-case letters indicate mean ± SE (standard error) within trenched and untrenched subplots that are significantly different (P < 0.05).
Previous studies suggested that the fine roots may quickly decompose after trenching and cause a high CO2 fluxes4,52. In this study, we found that the following third month after trenching, the RS was obviously higher in untrenched plots than in trenched plots (Fig. 1, P = 0.000). The similar phenomenon were also reported by Wang and Yang32 in the temperate deciduous and evergreen pine forest32. In fact, we used the measurement data after 6 months since the onset of trenching experiment in order to avoid the impact of rapid CO2 flush caused by trenching. Previous studies indicated that priming effects from dead roots after trenching can be avoided if it is properly managed4,54. The trenching had no significant influence on T5 and θ5 in this study, whereas controlling mechanisms on the temporal and spatial variation of RH were more complex. Advanced techniques (e.g. stable 14C and 13C isotope tracing) and laboratory incubation experiments based on plant physiology are needed to elucidate the variation mechanism of RH and rhizospheric respirations. Moreover, we observed in the study site that most roots (<5 mm) of Q. aquifolioides forest were distributed at the soil depth of 0–30 cm and few roots existed below the depth of 50 cm. Trenching down to the soil depth of 70 cm in this study should be deep enough to exclude the root impacts, and our results justified it to some extent.
Conclusion
In subalpine forests, RS and its components are obviously greater in the GS than in the DS and are higher during daytime than at nighttime. The temporal variations of daytime and nighttime RS and its components can be well explained by the interactions of soil temperature and moisture regardless of seasons. RS and its components are more sensitive to temperature in the DS than in the GS, and at nighttime than in daytime. Additionally, the substrate availabilities affected differently on Q10 of RS and RH on a daily and seasonal scale. Our findings suggest that soil CO2 emission throughout the DS and at nighttime in the high-altitude subalpine area plays a vital role in annual soil carbon budgets and their responses to climate change. Temporal variations of RS and its responses to environmental factors need to be considered in order to accurately predict changes in soil CO2 fluxes.
Materials and Methods
Site description
The research was conducted in Wolong Nature Reserve in Sichuan Province, China (102°58′–103°06′ E, 30°53′–58′ N), which is located at the Balang Mountain in the east branch of Qionglai Mountains, southeast of Tibetan Plateau. The brown mountainous soil (Chinese classification) is the main soil type across the whole study area of the Quercus aquifolioides distribution. The area is characterized by the typical Tibetan Plateau climate with distinct wet and dry seasons. At the 2700 m a.s.l., annual mean air temperature is about 8.4 °C, with the average minimum/maximum (January) and maximum/minimum (July) air temperature being −1.7/+5.1 and +17/6.3 °C, respectively. The annual precipitation is 861.8 mm, of which 68.1% occurs from May to September. According to the field observation, the average soil temperature at 5 cm soil depth in 2011 was 5.2 ± 4.2 °C (mean ± std. deviation) at 3549 m a.s.l., 4.8 ± 5.3 °C at 3091 m a.s.l., and 7.4 ± 5.3 °C at 2551 m a.s.l., with corresponding soil moisture values of 29.1 ± 10.3%, 30.4 ± 15.8% and 34.6 ± 11.1%, respectively. In the study site, Q. aquifolioides is the dominant species of this typical oak forest, while other species include Cotoneaster horizontalis, Daphne tangutica, Deyeuxia levipes, Oryzopsis munroi, Pedicularis davidii, Athyrium pachyphlebium.
Experiment design
Three Q. aquifolioides forest experimental sites were set up along the altitude gradient within its optimal spatial coverage (2551, 3091 and 3549 m, Table 4), on the southeast slope of Balang Mountain. At each site, three 20 m × 20 m replicate plots were established with a distance of 10 m between plots. In each plot, three 80 cm × 80 cm subplots were randomly deployed for trenching experiment and a buffer area was set with 10 m apart from each other. On 18 June 2010, at the outside edges of each subplot, a trench of 0.7 m deep (to the bedrock or below where few roots existed) was dug using a steel knife and shovel. On this study site, we observed that most roots (diameter <5 mm) were distributed in soil between 0 to 30 cm in depth in Q. aquifolioides forest, and few roots existed below the depth of 50 cm. To prevent the trenched plots from root encroachment, we lined the trenches with double-layer plastic sheets, and then refilled them carefully with the same soil. At each trenched subplot, one PVC (20 cm inside diameter × 8 cm in height) collar was installed into soil to a depth of 5 cm for CO2 efflux sampling (RH). Furthermore, all aboveground vegetation was carefully removed with minimal soil disturbance to keep a free of seedlings and herbaceous vegetation in these subplots throughout the course of experiment (from Sept, 2010 to Dec, 2011). Another three PVC collars were randomly inserted into the soil to a depth of 5 cm within 3 m around each trenched subplot (including the litter layer) to measure the RS, which was considered as the total soil respirations rate. Once inserted, the collars were left unmoved during the entire study period. Our first measurements of RS started two and half months later after the onset collar installation (mid-September 2010). Site characteristics are listed in Table 4 and Fig. 6.
Soil respirations, temperature and moisture measurements
In order to minimize the possible influence of live and dead roots in trenched plots decay, soil CO2 fluxes was measured 75 days later since the onset of trenching experiment32. In this study, we actually used the measurement data 6 months later since the onset of trenching for minimizing the effects of root decomposition in trenched plots. Therefore, soil CO2 flux in trenched plots can be regarded as the trench respiration (RH), while RR was the difference between RS and RH18,21. Soil respirations rates in the untrenched plots (RS) and the trenched (RH) were measured monthly from September 2010 to December 2011 (8th~18th per month except rainy days). At the time of RH and RS measurements, Soil temperature (T5) and moisture (volumetric water content, θ5) at 5 cm depth were measured automatically using the soil temperature probe and an attached Theta probe ML2x (Delta-T Devices, Cambridge, England) equipped with a Li-8100A soil CO2 flux system (LI-Cor Inc., Lincoln, NE, USA). In the daytime, respiration was measured between 9:00 and 16:00 hours (local time), while at nighttime it was measured between 20:00 and 4:00 hours55,56. The RS and RH were measured continuously in two cycles at each collar, 3 minutes per each cycle. The two measurements were averaged to produce the collar’s mean RS and RH rates and mean T5 or θ5. The average values of the 9 (3 subplots × 3 replicates) measurements at each altitude for RH, TT and θT (trenched), respectively, while the three collars’ measurements from the nearby trenched subplots were averaged to produce one mean value of RS, TUT and θUT (untrenched), respectively. The average values of the 27 (3 subplots × 3 replicates × 3 altitudes) measurements around each trenched subplot in each month for soil CO2 rates, T5and θ5 were used for data analysis.
Soil chemical property measurements
Soil was sampled at 0–30 cm depth with a sample ring kit with closed ring holder (Eijkelkamp 07.53.SC, Holland) in mid-April, mid-August and mid-November of 2011, respectively. In each subplot, near each PVC collar (within 80 cm) three soil cores were collected and mixed thoroughly into one sample. Altogether, nine samples from each plot were stored in a cooler before being carried back to the lab. Roots, gravel, and other miscellany of things in the samples were manually removed and the soil was sieved with a 2 mm sieve. Each sample was divided into two parts. One was stored at room temperature and was air-dried for analysis of soil chemical properties, the other was immediately deposited in a refrigerator at 4 °C for soil microbial biomass measurement that was conducted within 3–4 days.
The contents of soil NH4+ and NO3− were determined by using the indophenol blue colorimetric method and dual-wavelength spectrophotometry, respectively. Soil TOC was determined with the potassium dichromate oxidation heating method, and TN was determined with the semimicro Kjeldahl method using the air-dried soil. The measurements of soil microbial biomass carbon (MBC) and nitrogen (MBN) was performed by using the chloroform fumigation extraction method according to Vance et al.57.
Calculation of temperature sensitivity
Based on the measured data in 2011, an exponential equation (Van’t Hoff model, T-model; Eq. 1) was formulated to interpret the relationship between RS, RH or RR and soil temperature (T5) at 5 cm soil depth in three altitudinal plots. The temperature response of RS, RH or RR was estimated in this study according to the following equations Eqs 1 and 2, respectively.


Where RS, RH are the mean soil surface CO2 fluxes (μmolm−2s−1), a and b are fitted parameters. The b values were used to calculate the temperature sensitivity (Q10). We calculated daytime and nighttime respiration Q10 across seasons for each subplot. Additionally, we calculated the DS (early November through late April) and the GS (early May through late October, the growing season was determined by the phenological events of dominant Q. aquifolioides) Q10 values across daytime and nighttime measurements.
Relationship between RS (RH or RR) and soil moisture
A power equation (θ-model; Eq. 3) was used to describe the relationship between RS, RH or RR and soil moisture (θ5) at 5 cm soil depth at three altitudinal plots. A logarithm transformation was performed on RS and RH to achieve linearity and homogeneity of variance test, respectively, and was used as a function of T5 and θ54 (T-θ model; Eq. 4).


Where a, b and c are the fitted parameters.
Statistical analyses
A repeated measures analysis of variance (ANOVA) was used to test for differences of treatment (trenched and untrenched) on soil CO2 rates, T5 and θ5 based on the measurement data. A one-way ANOVA was used to compare the differences of soil CO2 flux, T5 and θ5 between daytime and nighttime in different seasons. Based on the monthly mean of soil CO2 fluxes at each altitudinal gradient, the dependence of RS, RH, and RR on T5 or θ5 was investigated using nonlinear and multiple linear regression models. A linear regression was used to analyze the relationship of Q10 with T5, θ5, and other soil properties in daytime/nighttime and seasons across 27 subplots (3 subplots × 3 replicates × 3 altitudes). The significant differences of Q10 values between daytime and nighttime, between the DS and the GS, were tested using the ANOVA at α = 0.05, respectively. In order to ensure the comparability of RH and eliminate the effects of trenching treatment on RH, measured data were corrected where T5 and θ5 diverged on trenched and untrenched plots throughout the measurements in 2011. We used a multiple linear regression function to evaluate the trenching effect on soil C fluxes. All statistical analyses were performed using SPSS (version 19.0 for Windows).
Additional Information
How to cite this article: Hu, Z. et al. Soil respiration and its environmental response varies by day/night and by growing/dormant season in a subalpine forest. Sci. Rep. 6, 37864; doi: 10.1038/srep37864 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Davidson, E. A., Richardson, A. D., Savage, K. E. & Hollinger, D. Y. A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Global Change Biology 12, 230–239 (2006).
Schimel, D. S. et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 414, 169–172 (2001).
Baggs, E. Partitioning the components of soil respiration: a research challenge. Plant and Soil 284, 1–5 (2006).
Luan, J., Liu, S., Wang, J., Zhu, X. & Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biology and Biochemistry 43, 503–512 (2011).
Li, L., Chen, X., Wang, Z., Xu, W. & Tang, H. Climate change and its regional differences over the Tibetan Plateau. Advances in Climate Change Research 6, 181–186 (2010).
Liu, X., Cheng, Z., Yan, L. & Yin, Z.-Y. Elevation dependency of recent and future minimum surface air temperature trends in the Tibetan Plateau and its surroundings. Global and Planetary Change 68, 164–174 (2009).
Subke, J. A., Inglima, I. & Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Global Change Biology 12, 921–943 (2006).
Wang, W., Peng, S., Wang, T. & Fang, J. Winter soil CO2 efflux and its contribution to annual soil respiration in different ecosystems of a forest-steppe ecotone, north China. Soil Biology and Biochemistry 42, 451–458 (2010).
Xu, Z., Zhou, F., Yin, H. & Liu, Q. Winter soil CO2 efflux in two contrasting forest ecosystems on the eastern Tibetan Plateau, China. Journal of Forestry Research 26, 679–686 (2015).
Brooks, P. D., McKnight, D. & Elder, K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Global Change Biology 11, 231–238 (2004).
Taneva, L. & Gonzalez-Meler, M. A. Distinct patterns in the diurnal and seasonal variability in four components of soil respiration in a temperate forest under free-air CO2 enrichment. Biogeosciences 8, 3077–3092 (2011).
Kutsch, W. et al. Heterotrophic soil respiration and soil carbon dynamics in the deciduous Hainich forest obtained by three approaches. Biogeochemistry 100, 167–183 (2010).
Song, W. et al. Simulated rain addition modifies diurnal patterns and temperature sensitivities of autotrophic and heterotrophic soil respiration in an arid desert ecosystem. Soil Biology and Biochemistry 82, 143–152 (2015).
Shi, P., Zhang, X., Zhong, Z. & Ouyang, H. Diurnal and seasonal variability of soil CO2 efflux in a cropland ecosystem on the Tibetan Plateau. Agricultural and Forest Meteorology 137, 220–233 (2006).
Xu, M. & Qi, Y. Spatial and seasonal variations of Q10 determined by soil respiration measurements at a Sierra Nevadan forest. Global Biogeochem. Cycles 15, 687–696 (2001).
Pavelka, M., Acosta, M., Marek, M., Kutsch, W. & Janous, D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant and Soil 292, 171–179 (2007).
Chen, B., Liu, S., Ge, J. & Chu, J. Annual and seasonal variations of Q10 soil respiration in the sub-alpine forests of the Eastern Qinghai-Tibet Plateau, China. Soil Biology and Biochemistry 42, 1735–1742 (2010).
Lee, N. Y., Koo, J. W., Noh, N., Kim, J. & Son, Y. Autotrophic and heterotrophic respiration in needle fir and Quercus-dominated stands in a cool-temperate forest, central Korea. Journal of Plant Research 123, 485–495 (2010).
Chen, H. et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Global Change Biology 19, 2940–2955 (2013).
Zhuang, Q. et al. Carbon dynamics of terrestrial ecosystems on the Tibetan Plateau during the 20th century: an analysis with a process-based biogeochemical model. Global Ecology and Biogeography 19, 649–662 (2010).
Liu, Q., Zhao, C., Cheng, X. & Yin, H. Soil respiration and carbon pools across a range of spruce stand ages, Eastern Tibetan Plateau. Soil Science and Plant Nutrition 61, 440–449 (2015).
Chen, H. et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai‐Tibetan Plateau. Global change biology 19, 2940–2955 (2013).
Lu, X. & Cheng, G. Climate change effects on soil carbon dynamics and greenhouse gas emissions in Abies fabri forest of subalpine, southwest China. Soil Biology and Biochemistry 41, 1015–1021 (2009).
Kuzyakov, Y. & Cheng, W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biology and Biochemistry 33, 1915–1925 (2001).
Vargas, R. et al. On the multi-temporal correlation between photosynthesis and soil CO2 efflux: reconciling lags and observations. New Phytologist 191, 1006–1017 (2011).
Peng, S. et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501, 88–92 (2013).
Chen, Y., Luo, J., Li, W., Yu, D. & She, J. Comparison of soil respiration among three different subalpine ecosystems on eastern Tibetan Plateau, China. Soil Science and Plant Nutrition 60, 1–11 (2014).
Scala, N. L., Marques, J., Pereira, G. T. & Corá, J. E. Carbon dioxide emission related to chemical properties of a tropical bare soil. Soil Biology & Biochemistry 32, 1469–1473 (2000).
Luan, J. W., Liu, S. R., Zhu, X. L. & Wang, J. X. Soil carbon stocks and fluxes in a warm-temperate oak chronosequence in China. Plant and Soil 347, 243–253 (2011).
Lee, M. S., Nakane, K., Nakatsubo, T. & Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant and Soil 255, 311–318 (2003).
Han, M.-G., Shi, B.-K. & Jin, G.-Z. Temporal variations of soil respiration at multiple timescales in a spruce-fir valley forest, northeastern China. Journal of Soils and Sediments 16, 1–10 (2016).
Wang, C. & Yang, J. Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Global change biology 13, 123–131 (2007).
Zhou, F., Lin, B., Liu, Q. & Li, W. Soil respiration of subalpine coniferous forest in winter in the east of the Qinghai-Tibet plateau, China. Chinese Journal of Applied and Environmental Biology (Chinese Version) 19, 761–767 (2009).
Beverly, D. & Franklin, S. Heterotrophic and autotrophic soil respiration under simulated dormancy conditions. Open Journal of Forestry 5, 274–286 (2015).
Högberg, P. Is tree root respiration more sensitive than heterotrophic respiration to changes in soil temperature? New Phytologist 188, 9–10 (2010).
Schipper, L. A., Hobbs, J. K., Rutledge, S. & Arcus, V. L. Thermodynamic theory explains the temperature optima of soil microbial processes and high Q10 values at low temperatures. Global Change Biology 20, 3578–3586 (2014).
Janssens, I. A. & Pilegaard, K. I. M. Large seasonal changes in Q10 of soil respiration in a beech forest. Global Change Biology 9, 911–918 (2003).
Monson, R. K. et al. Winter forest soil respiration controlled by climate and microbial community composition. Nature 439, 711–714 (2006).
Wei, H. et al. Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Scientific Reports 5, 1–11 (2015).
Schroll, R. et al. Quantifying the effect of soil moisture on the aerobic microbial mineralization of selected pesticides in different soils. Environmental Science & Technology 40, 3305–3312 (2006).
Giardina, C. P. & Ryan, M. G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404, 858–861 (2000).
Kruse, J., Simon, J. & Rennenberg, H. Soil respiration and soil organic matter decomposition in response to climate change. Dev. Environ. Sci 13, 131–149 (2013).
Bader, M. K. F. & KÖRner, C. No overall stimulation of soil respiration under mature deciduous forest trees after 7 years of CO2 enrichment. Global Change Biology 16, 2830–2843 (2010).
Zhou, Z. H. & Wang, C. K. Reviews and syntheses: Soil resources and climate jointly drive variations in microbial biomass carbon and nitrogen in China’s forest ecosystems. Biogeosciences 12, 6751–6760 (2015).
Schulz, H., Dunst, G. & Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 33, 817–827 (2013).
Fang, H. et al. Changes in soil heterotrophic respiration, carbon availability, and microbial function in seven forests along a climate gradient. Ecological Research 29, 1077–1086 (2014).
Kuzyakov, Y. & Gavrichkova, O. Review: Time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Global Change Biology 16, 3386–3406 (2010).
Bond-Lamberty, B., Bronson, D., Bladyka, E. & Gower, S. T. A comparison of trenched plot techniques for partitioning soil respiration. Soil Biology and Biochemistry 43, 2108–2114 (2011).
Huang, W., Han, T., Liu, J., Wang, G. & Zhou, G. Changes in soil respiration components and their specific respiration along three successional forests in the subtropics. Functional Ecology 97, 2895–2899 (2016).
Hopkins, F. et al. Ecosystem-level controls on root-rhizosphere respiration. New Phytol. 199, 339–351 (2013).
Yan, L. M., Chen, S. P., Huang, J. H. & Lin, G. H. Differential responses of auto- and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biology 16, 2345–2357 (2010).
Ngao, J., Longdoz, B., Granier, A. & Epron, D. Estimation of autotrophic and heterotrophic components of soil respiration by trenching is sensitive to corrections for root decomposition and changes in soil water content. Plant and Soil 301, 99–110 (2007).
Ohashi, M., Gyokusen, K. & Saito, A. Contribution of root respiration to total soil respiration in a Japanese cedar (Cryptomeria japonica D. Don) artificial forest. Ecological Research 15, 323–333 (2000).
Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biology and Biochemistry 38, 425–448 (2006).
Zribi, L., Mouillot, F., Gharbi, F., Ourcival, J.-M. & Hanchi, B. Warm and fertile sub-humid conditions enhance litterfall to sustain high soil respiration fluxes in a Mediterranean Cork Oak Forest. Forests 6, 2918–2940 (2015).
Suseela, V., Conant, R. T., Wallenstein, M. D. & Dukes, J. S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Global Change Biology 18, 336–348 (2012).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19, 703–707 (1987).
Acknowledgements
This work was financially supported by the Ministry of Science and Technology of China (Nos 2015DFA31440; 2012BAD22B01) and the China’s Ministry of Finance (Nos 201404201; 200804001; 201104006). We are grateful to the local residents in Wolong Nature Reserve in Sichuan Province and the staff in the Sichuan Provincial Wolong Ecological Environmental Monitoring Station for their generous assistance during the field measurement. We also thank the anonymous reviewers for their valuable suggestions.
Author information
Authors and Affiliations
Contributions
Z.H. was the principal investigator for this study. He undertook the main data analyses and wrote the manuscript. S.L. supervised the research project and contributed to layout and design of the experiment, and the manuscript writing and revision of earlier drafts until final approval. J.W. and K.L. contributed largely to data processing and statistical analysis, and X.L., L.F., X.H., Y.Z. and F.H. contributed to the interpretation and discussion of the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hu, Z., Liu, S., Liu, X. et al. Soil respiration and its environmental response varies by day/night and by growing/dormant season in a subalpine forest. Sci Rep 6, 37864 (2016). https://doi.org/10.1038/srep37864
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37864
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.