Abstract
The growing popularity of levonorgestrel (LNG)-releasing intra-uterine systems for long-acting reversible contraception provides strong impetus to define immunomodulatory properties of this exogenous progestin. In initial in vitro studies herein, we found LNG significantly impaired activation of human dendritic cell (DCs) and their capacity to promote allogeneic T cell proliferation. In follow-up studies in a murine model of intranasal Chlamydia trachomatis infection, we analogously found that LNG treatment prior to infection dramatically reduced CD40 expression in DCs isolated from draining lymph nodes at 2 days post infection (dpi). At 12 dpi, we also detected significantly fewer CD4+ and CD8+ T cells in the lungs of LNG-treated mice. This inhibition of DC activation and T cell expansion in LNG-treated mice also delayed chlamydial clearance and the resolution of pulmonary inflammation. Conversely, administering agonist anti-CD40 monoclonal antibody to LNG-treated mice at 1 dpi restored lung T cell numbers and chlamydial burden at 12 dpi to levels seen in infected controls. Together, these studies reveal that LNG suppresses DC activation and function, and inhibits formation of pathogen-specific T cell immunity. They also highlight the need for studies that define in vivo effects of LNG use on human host response to microbial pathogens.
Similar content being viewed by others
Introduction
Intra-uterine systems (IUSs) have become a popular choice for long-acting reversible contraception (LARC) worldwide1. While especially popular in Asia2, IUS use among contraceptors in the U.S. increased from 2.0% in 2002 to 10.3% in 2012 3. Among the 3 IUSs now approved in the U.S. for LARC, 2 release the exogenous progestin levonorgestrel (LNG). Expressly, Skyla® (13.5 mg LNG) and Mirena® (52 mg LNG) are approved for 3 and 5 years use, respectively4,5. Based on their effectiveness at preventing unintended pregnancy, The American College of Obstetricians and Gynecologists and The American Academy of Pediatrics identified LNG-IUSs as top-tier LARC choices for women and adolescents6,7. Despite the increasingly widespread LNG-IUS utilization, only a limited number of laboratory animal and clinical studies have explored the effects of LNG on mechanisms of anti-pathogen host defense.
As examples, LNG-treated mice showed greater genital mucosal permeability and susceptibility to virus infection8, while multiple clinical studies identified IUSs users as most likely to develop pelvic inflammatory disease (PID) during the first 3 weeks after IUS insertion9,10,11. Conversely, the incidence of acquiring sexually transmitted infection among women using LNG-IUS vs. no hormonal contraceptive is unexplored by prospective longitudinal study. Also underexplored are the in vivo effects of LNG on pathogen clearance. One retrospective study did observe reduced genital clearance of high-risk human papillomavirus (HPV) in women using LNG-IUS12, while Chlamydia trachomatis clearance was delayed in baboons infected subsequent to human-use LNG-IUS insertion13. Notably however, the immunomodulatory properties of LNG responsible for these experimental and clinical observations have not been defined.
Our laboratory previously reported dendritic cell (DC) activation and development of virus-specific immunological memory were inhibited in mice administered medroxyprogesterone acetate (MPA) prior to corneal infection with herpes simplex virus type 1 (HSV-1)14. MPA is the active component of the progestin-only, injectable hormonal contraceptive Depo-Provera. In the current study, we used in vitro assays with human DCs and a murine model of intranasal C. trachomatis infection to similarly explore the influence of LNG on early anti-pathogen immune responses. This animal model of respiratory infection represents an important complement to the mouse urogenital model, and can delineate chlamydial pathogenesis and host-chlamydia interactions, screen antimicrobials for anti-chlamydial activity, and gage efficacy of candidate Chlamydia vaccines15.
Results
LNG suppressed human DC activation and function
In prior studies, MPA modulated in vivo immune responses of mice to HSV-1 infection14, and inhibited human DC activation and function in vitro16. As MPA binds the glucocorticoid (GR) and progesterone receptors (PR)17, these findings may have been mediated by interactions of MPA with either receptor. While LNG more selectively binds the PR18, MPA and LNG did similarly increase mouse genital mucosal permeability and susceptibility to HSV-2 infection8. Therefore, we began the current study by positing LNG analogously inhibits DC activation and function. To first test this hypothesis, we isolated human primary DCs from the peripheral blood of 8 individuals by negative immunomagnetic selection. Selected cells were incubated for 24 h with vehicle alone or concentrations of LNG that ranged between 0.015 μM–4 μM. Cells were incubated an additional 24 h after adding the Toll-like receptor 3 (TLR3) agonist polyinosinic: polycytidylic (poly I:C) (1.5 μg/mL). Interrogation of these cells in flow cytometry-based studies revealed LNG did not alter myeloid DC (mDC) viability (Fig. S1), but that LNG concentrations > 0.250 μM inhibited expression of CD80 and CD86 by mDCs responding to poly I:C stimulation (Fig. 1a–c). Even more robust was LNG-mediated suppression of CD40 expression (Fig. 1d). This latter result was congruent with inhibition of CD40 expression in MPA-treated human DCs stimulated in vitro with poly I:C16, and reduced CD40 expression in DCs isolated from the draining lymph nodes (DLNs) of MPA-treated mice 2 days after corneal HSV-1 infection14.
LNG inhibits human DC activation.
Negatively selected human DCs were incubated for 24 h with indicated LNG concentrations or vehicle alone, then incubated for 24 h with poly I:C (1.5 μg/mL). DCs were stained with a live/dead near-IR dye and a panel of fluorescently-tagged mAbs to identify viable DC populations by flow cytometry (described in Materials and Methods). (a) Representative contour plots of CD40 and CD80 expression by untreated or LNG (4 μM)-treated mDCs stimulated with poly I:C; quadrant numbers denote percent expression. (b–d) mDC expression of (b) CD80, (c) CD86, and (d) CD40 after poly I:C stimulation. Data from 8 independent experiments with results normalized (i.e., by designating vehicle-only cultures as 100% activation) as detailed in Materials and Methods (bars denote means ± SD). Statistical analyses performed using 1-way ANOVA with Dunnett’s multiple comparisons test, *p < 0.05; ***p < 0.001.
Because LNG suppressed human DC activation in vitro, we posited it also inhibits DC function. To test this hypothesis, negatively selected human DCs from 6 individuals were sequentially administered vehicle alone or LNG for 24 h; poly I:C stimulated for 24 h; and incubated for 7 days with fluorescently-labeled naïve allogeneic T cells. Using flow cytometry, we detected significantly less CD4+ and CD8+ T cell proliferation in co-cultures that contained LNG-treated DCs (Fig. 2a–c). Of note, all but the lowest selected LNG concentration significantly inhibited this proliferation. Conversely, the stimulation of T cell-only cultures with anti-CD3 and anti-CD28 antibodies showed LNG treatment had no direct effects on T cell capacity to proliferate (Fig. 2d–f). Extending these results beyond DC response to TLR3 agonist stimulation, we also found that CD40 expression was significantly blunted when LNG-treated human DCs were stimulated in vitro with inactivated C. trachomatis (Fig. 3). Of note, stimulation with poly I:C or C. trachomatis produced a markedly different pattern of co-stimulatory molecule expression (Figs 1a and 3a), indicating that different DC activation pathways had been triggered by stimulation with this particular TLR3 agonist or Gram-negative bacterium.
LNG inhibits human DC function.
(a–c) Negatively selected human DCs were LNG-treated and poly I:C stimulated as described in Fig. 1, then co-cultured with CTV-labeled naïve allogeneic T cells. Co-cultures were maintained 7 days, then T cells immunostained for flow cytometric analysis of proliferation. (a) Representative contour plots of CD4+ T cell proliferation from co-cultures with untreated or LNG (4 μM)-treated DCs (numbers denote percentages of proliferating CD3ε+CD4+ T cells). (b–c) Proliferation of (b) CD4+ and (c) CD8+ T cells from co-cultures with untreated or LNG-treated DCs (data from 6 independent experiments normalized and analyzed as detailed in Materials and Methods); (bars indicate means ± SD). (d–f) CTV-labeled naïve allogeneic T cells were incubated with LNG or vehicle overnight, then stimulated with beads coated with anti-CD3 and anti-CD28 antibodies. T cell cultures were maintained 5 days, and cells immunostained for flow cytometric analysis of proliferation. (d) Representative contour plots of T cell proliferation (4 μM LNG); (numbers identify percentages of proliferating CD3ε+CD4+ T cells). Proliferation of (e) CD4+ and (f) CD8+ T cells in T cell-only cultures treated with vehicle or LNG (data from 6 independent experiments that were normalized and analyzed as defined in Materials and Methods); (bars denote means ± SD). All statistical analyses were performed using 1-way ANOVA with Dunnett’s multiple comparisons test, ****p < 0.0001.
LNG inhibits human DC response to C. trachomatis stimulation.
Negatively selected human DCs were incubated for 24 h with indicated LNG concentrations or vehicle, then incubated for 24 h with inactivated C. trachomatis (MOI = 0.1 prior to inactivation). To identify live DCs, cells were stained as described in Fig. 1. (a) Representative contour plots displaying CD40 and CD80 expression by untreated and LNG (4 μM)-treated mDCs stimulated with C. trachomatis; quadrant numbers indicate percent expression. (b–d) mDC expression of (b) CD80, (c) CD86, and (d) CD40 induced by C. trachomatis. Data from 8 independent experiments were normalized as described in Materials and Methods (i.e., by designating vehicle-only cultures as 100% proliferation); (bars denote means ± SD). Statistical analyses made using 1-way ANOVA with Dunnett’s multiple comparisons test, *p < 0.05; **p < 0.01; and ***p < 0.001.
LNG suppressed murine host DC response to C. trachomatis infection
Since LNG inhibited in vitro DC activation, we hypothesized that antecedent treatment of mice with LNG also reduces activation of DCs elicited by C. trachomatis infection. As an important experimental consideration, antecedent progestin treatment is exploited in numerous animal models of genital infection (including murine Chlamydia models that use intravaginal, trans-cervical, and direct ovarian bursal inoculation) to produce uniform susceptibility to infection8,19,20,21. We thus tested our hypothesis in a C. trachomatis respiratory infection model, a model in which uniform infection susceptibility is achieved without antecedent progestin treatment. In other words, the use of a non-genital infection route was the best way to prevent identification of between-group differences in DC function or T cell expansion spuriously created by a differential susceptibility of untreated and LNG-treated mice to genital infection. Using the respiratory model of infection, wild type Balb/cJ female mice were subcutaneously implanted with 21-d sustained release pellets containing 50 mg LNG or matching placebo pellet (Fig. 4a). With initial studies in this model, we collected blood 5 d after pellet insertion to quantify serum LNG concentrations (Fig. 4b). To assess LNG effects on DC activation, other mice were intranasally (i.n.) infected with C. trachomatis 5 days after LNG- or placebo-pellet insertion. Of note, we utilized live (not inactivated) Chlamydia in our exploration of this respiratory infection model, as only the former generated a mature DC phenotype that promoted pathogen-specific protective immunity22,23. Controls and LNG-pelleted mice were euthanized at 2 days post infection (dpi), and DLNs were processed for flow cytometric analysis of DC activation. Consistent with our human DC data, we found that antecedent LNG treatment of mice significantly reduced DC expression of CD40 and CD80 induced by C. trachomatis infection (Fig. 4c,d).
LNG reduces DC activation elicited by Chlamydia infection.
(a) LNG or matching placebo pellets were implanted into Balb/cJ female mice, and peripheral blood collected 5 days later to measure serum LNG concentrations. (b) Serum LNG levels; symbols represent values from individual mice and horizontal lines indicate means. (c–d) Other mice implanted with pellets as described in (a) were i.n. infected with 105 IFU of live C. trachomatis. Mice were euthanized at 2 dpi, and DLNs collected to assess DC activation by flow cytometry. Representative contour plots show (c) CD40 and CD80 expression in live CD11chiMHC-II+CD8α-CD11b+ cells (mDCs); numbers denote percent expression. (d) mDC expression of CD40 and CD80 from placebo- or LNG-pelleted mice from two independent experiments with 6 mice per condition; bars designate mean values ± S.D. Statistical analyses performed with 2-tailed unpaired Student t tests.
LNG dampened murine host T cell response to C. trachomatis infection
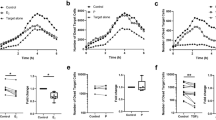
In separate experiments, mice received LNG or matching placebo pellet 5 days before infection, while other LNG-pelleted mice received agonist anti-CD40 monoclonal antibody 1 day after infection. Animals from all 3 groups were euthanized at 12 dpi, and flow cytometric analysis of lung tissue showed there were significantly fewer CD4+ and CD8+ T cells in mice receiving LNG pellet alone (Fig. 5a). LNG treatment was also associated with significantly less interferon (IFN)-γ secretion when lung-resident CD4+ and CD8+ T cells were stimulated ex vivo with Chlamydia-infected bone marrow-derived DCs (BMDCs) and significantly reduced CD8+ T cell levels of the serine protease granzyme B (GzmB) (Fig. 5b,c). Highlighting the importance of CD40-CD154 interactions for both T cell expansion and development of T cell effector function in this Chlamydia respiratory infection model, we conversely found comparable T cell numbers and T cell levels of IFN-γ and GzmB among LNG-pelleted mice administered agonist anti-CD40 antibody and mice treated with placebo pellet (Fig. 5a and c).
LNG impairs T cell expansion and pathogen-specific T cell effector function.
Mice were administered LNG or matching placebo pellet 5 days before i.n infection with 105 IFU of live C. trachomatis. Other LNG-pelleted mice received agonist anti-CD40 mAb injection 1 day after infection. (a) Mice were euthanized at 12 dpi, and lungs processed into single-cell suspensions to enumerate CD45+, CD90.2+, CD4+, and CD8+ cells by flow cytometry. (b-c) In separate studies, groups of mice were treated and infected identically as described in (a). Mice were euthanized at 12 dpi, and lungs processed into single-cell suspensions to quantify GrzB levels inside CD8+ T cells and define Chlamydia-specific CD4+ and CD8+ T cell effector function. For the latter, BMDCs were obtained from untreated, uninfected syngeneic mice as defined in Materials and Methods, and stimulated overnight with C. trachomatis (MOI = 0.1). The next day, Chlamydia-infected mice (i.e., at 12 dpi) were euthanized, and single-cell suspensions of lung tissue incubated 24 h with the Chlamydia-activated BMDCs to quantify T cell secretion of IFN-γ, TNF, and IL-17 by flow cytometry. (b) Representative contour plots display production of IFN-γ, TNF, and IL-17 by CD4+ T cells and GzmB levels inside CD8+ T cells. (c) Between group-differences in levels of GzmB (CD8+ T cells) or intracellular accumulation of IFN-γ, TNF, and IL-17 (CD4+ and CD8+ T cells) (bars indicate mean values ± S.D). Data in (a) and (c) are from 2 independent experiments with 6 mice per group. Statistical analyses were completed using 1-way ANOVA with Dunnett’s multiple comparisons test.
LNG impaired clearance of intranasal C. trachomatis infection
As previously established for mice, TH1 immunity is critical for control of C. trachomatis infection24. Upon observing diminished T cell expansion and TH1 effector function in LNG-treated mice infected with C. trachomatis, we posited that eradication of pulmonary infection is also impaired by LNG treatment. To explore this possibility, groups of mice received LNG or matching placebo pellet 5 days before infection. Other LNG-pelleted mice received agonist anti-CD40 antibody 1 day after infection, while other placebo-pelleted mice received CD4+ cell-depleting mAb 1 day prior to infection and for the duration of the study. Animals from each group were euthanized at 6 dpi or 12 dpi, and consistent with prior reports24,25,26, Chlamydia was effectively cleared from the lungs of placebo-pelleted mice between 6 and 12 dpi. In comparison, CD4+ T cell-depleted mice less successfully controlled infection (Fig. 6). Remarkably, clearance was similarly impaired in LNG-pelleted mice, whereas clearance in LNG-pelleted mice administered agonist anti-CD40 antibody was restored to levels detected in placebo-pelleted controls (Fig. 6). Congruent with delayed clearance in LNG-treated mice, we saw significantly more myeloid (CD45+CD11b+) mononuclear cell aggregates in the lungs of LNG-treated mice at 12 dpi compared to placebo-pelleted controls (Fig. 7). CD4+ cell depletion caused comparable increase in these myeloid cell aggregates, whereas treatment of LNG-pelleted mice with agonist anti-CD40 antibody reduced the inflammatory response to the levels seen in placebo-pelleted controls (Fig. 7). These studies thus revealed LNG treatment of mice prior to intranasal C. trachomatis infection delays both pathogen clearance and resolution of pulmonary inflammation.
LNG diminishes C. trachomatis clearance from pulmonary tissue.
Groups of mice were administered LNG or matching pellets 5 d prior to i.n infection with 105 IFU of live C. trachomatis. Other groups of LNG-pelleted mice received agonist anti-CD40 mAb 1 day after chlamydial infection or CD4+ cell-depleting mAb 1 d prior and every other day for study duration. Animals were euthanized at 6 dpi or 12 dpi, and both lung lobes excised to quantify chlamydial DNA by RT-qPCR; (bars indicate mean ± C.I); (data from 2 independent experiments with 6 animals per group). Chlamydia levels at each dpi were compared using the 2-tailed unpaired Student t test.
Greater chlamydial burden in LNG-treated mice elicits increased mononuclear cell inflammation.
In separate studies, groups of mice were treated and infected identically as described in Fig. 6. Mice were euthanized at 12 dpi, and pulmonary tissue processed to define inflammation by histology. (a) Representative results from hematoxylin and eosin, IHC, and IF staining depicts the increased mononuclear cell inflammation seen in the lungs of LNG-treated- and CD4+ cell-depleted mice; IF staining: CD11b+ (green), DAPI (blue); black and white bars denote 200 μm and 20 μm, respectively. (b) Quantification of pulmonary CD45+ inflammatory aggregates identified by IHC staining (5 high-power fields examined per section); (bars denote mean ± S.D.). Data are from 2 independent experiments with 6 mice per group. Statistical analyses were performed using 1-way ANOVA with Dunnett’s multiple comparisons test.
Discussion
A 2015 publication provided interesting first indication that LNG influences pathogen clearance. Among the 66 women in that study diagnosed with high-risk HPV genital infection prior to IUS insertion, 70% initiating copper-containing IUS use vs. 42% initiating LNG-IUS use cleared infection during the first year after insertion12. Such results implied LNG, and not the mere presence of an IUS, was the variable more closely associated with delayed HPV clearance. Of note, that study did not comparably assess HPV clearance in a group of women not using hormonal contraception. Moreover, while T cell responses are considered important for HPV eradication27, that study was not designed to determine if LNG-mediated effects on T cell immunity contributed to the greater persistence of HPV among LNG-IUS users.
Herein, we sought to define the impact of LNG on early adaptive immunity. In initial exploration of the in vitro effects of LNG on human DC activation and function, we saw LNG reduce CD40 expression in DCs responding to poly I:C and reduce DC capacity to induce T cell proliferation. The former observation likely predicted the latter, as CD40 signaling elicits changes that make DCs more effective promoters of T cell activation28,29,30,31. Remarkably, impaired DC capacity to promote T cell proliferation in our study was produced with LNG concentrations as low as 0.062 μM. Although peak and steady-state serum concentrations of LNG after LNG-IUS insertion are typically less than 1.2 nM32,33,34,35, systemic levels do not reflect the concentrations achieved in female genital mucosal tissue. Endometrial LNG tissue levels measured 1–2 months after LNG-IUS insertion ranged between 174 ng–660 ng36, and we approximated these tissue levels create LNG concentrations between 2.8 μM–10.6 μM in the typical 200 μl volume of uterine fluid37,38. These values indicate the LNG-mediated inhibition of human DC response to TLR3 agonist stimulation observed in our study occurred at pharmacologically relevant levels. Comparable levels of LNG likewise impaired in vitro activation of human DCs responding to C. trachomatis. Our results thus reveal LNG blunts human DC response to an immunostimulant used to mimic viral infection and a bacterial pathogen, and are congruent with studies reporting reduced in vitro activation and function of progesterone-treated mouse DCs and MPA-treated human DCs16,39. Though it was earlier postulated that greater PR specificity makes LNG less likely than MPA to impair DC function40, our work also newly indicates that suppression of DC activation and function are immunomodulatory properties shared by MPA and LNG.
Since LNG reduced CD40 expression in DCs responding to in vitro C. trachomatis stimulation, we explored effects of LNG on DC activation, T cell expansion, and T cell effector function in a murine model of Chlamydia respiratory infection. This model was chosen in general because CD40-CD154 interactions promote TH1-type immune responses needed to combat intracellular pathogens41,42, and specifically because CD40 knockout mice displayed prolonged infection courses after chlamydial infection43. Congruent with the reduced CD40 expression in LNG-treated human DCs, we saw significantly lower CD40 expression in DCs isolated from DLNs of LNG-treated mice at 2 dpi. Signifying this degree of inhibition of DC activation was a biologically relevant response, the impaired T cell expansion in lungs of LNG-treated mice was abrogated when LNG-treated mice also received agonist anti-CD40 antibody. Using this mouse model, we further showed that LNG impairs development of Chlamydia-specific TH1-type effector function. The latter finding result is compatible with prior studies reporting that DC CD40 expression is critical for Mycobacterium tuberculosis-specific T cell expansion44,45 and that CD40-CD154 interactions optimize formation of pathogen-specific T cell effector function46,47,48.
Consistent with impaired development of Chlamydia-specific TH1 immunity in LNG-treated mice was its association with delayed clearance of pulmonary infection. Remarkably, chlamydial load in LNG-treated mice was increased similarly to levels seen in mice depleted of CD4+ T cells. Moreover, delayed chlamydial clearance slowed resolution of the myeloid mononuclear cell-dominated pulmonary infiltrate. Conversely, administration of agonist anti-CD40 mAb to LNG-treated mice at dpi 1 produced Chlamydia burden and pulmonary inflammation at 12 dpi that closely resembled that seen in placebo-pelleted controls. Thus, while our use of sustained-release LNG pellets in a mouse model of respiratory C. trachomatis infection may not fully recapitulate the immunomodulatory effects of LNG-IUS use in the genital tract of women, the elevated chlamydial burden we detected in LNG-treated mice at 12 dpi is entirely consistent with the delayed eradication of genital HPV observed in women using LNG-IUS12.
In summary, this study newly identifies mechanisms by which LNG suppresses early adaptive immune responses and inhibits host ability to eradicate an intracellular bacterial pathogen from mucosal tissue. These findings may have specific clinical relevance, as any variable that delays pathogen clearance increases the chance for pathogen transmission from infected to uninfected individuals. Also of potential clinical relevance is the more exuberant inflammation found in the lungs of LNG-treated mice, as the inflammatory response elicited in women during a chronic genital C. trachomatis was identified as a risk factor for development of PID and tubal-factor infertility49,50. Our results thus ultimately serve to highlight the need for clinical studies that define formation development of pathogen-specific immune responses and rates of pathogen clearance among women using LNG-IUSs.
Methods
In vitro procedures
Buffy coats from de-identified individuals were provided by the Central-Southeast Ohio Region American Red Cross. Peripheral blood mononuclear cells (PBMCs) were isolated from these buffy coats by density gradient centrifugation (Ficoll-PaqueTM PLUS) (Healthcare Bio-Sciences AB, Uppsala, Sweden), and cryopreserved in 10% dimethyl sulfoxide (DMSO) (Mediatech, Manassas, VA, USA) and 90% fetal bovine serum (FBS) (Atlanta Biologicals, Flowery Branch, GA, USA). Thawed PBMCs were used to isolate primary DCs by negative immunomagnetic selection (EasySep™ Human Pan-DC Pre-Enrichment Kit) (StemCell Technologies, Vancouver, Canada). As indicated, allogeneic PBMC were labeled with CellTrace™ Violet Cell Proliferation Dye (CTV) (Invitrogen, Eugene, OR, USA), and naïve T cells were isolated using the Pan Naïve T Cell Isolation Kit (Miltenyi Biotec, San Diego, CA, USA). LNG (Sigma-Aldrich, St. Louis, MO) solubilized in DMSO was used to prepare 100 μM stock solutions. C. trachomatis serovar L2 (strain 434; ATCC® VR-902B) was grown on McCoy cells (ATCC® CRL-1696), and elementary bodies (EB) isolated and stored at −80 °C in sucrose-phosphate-glutamate buffer (SPG)51. Prior to experimental use, C. trachomatis inclusion-forming units (IFU) were enumerated as previously described52.
To assess DC activation, human primary DCs re-suspended in X-VIVO 20 (Lonza, Walkersville, MD, USA) containing 10% AB human serum (Atlanta Biologicals) were placed in individuals wells of 96-well, round bottom polypropylene plates (Corning Inc., New York, NY, USA) (5 × 104 DC/well). Cells were incubated 12 h at 37 °C in a 5% CO2 atmosphere using media + select LNG concentrations (final DMSO concentrations in untreated and LNG-treated wells were <0.001%). Cultures were administered vehicle or poly I:C (1.5 μg/mL) (InvivoGen, San Diego, CA, USA), and incubated an additional 24 h. Other vehicle-or LNG-treated DCs were stimulated with C. trachomatis (MOI = 0.1) previously inactivated by incubation at 56 °C for 15 min. DCs were harvested to evaluate co-stimulatory molecule expression by flow cytometry. To assess DC function, other negatively selected human DCs were sequentially plated (2.5 × 103 DC/well); vehicle- or LNG-treated; and poly I:C stimulated. Allogeneic, CTV-labeled naïve T cells (1 × 105 cells/well) were co-cultured with DCs for 7 d at 37 °C in 5% CO2 (with media replenished every third day), and T cell proliferation measured by flow cytometry. To evaluate the direct effects of LNG on T cell proliferation, CTV-labeled T cells (105 cells/well) were incubated for 12 h with vehicle or indicated LNG concentrations. Cells were stimulated with Dynabeads® Human T-Activator CD3/CD28 (Life Technologies, Oslo, Norway) (1:8 bead: cell ratio), and incubated 5 d (media was not replenished in this assay). T cell proliferation was assessed by flow cytometry.
In vivo and ex vivo procedures
Mouse studies were approved by The OSU Institutional Animal Care and Use Committee and performed in accordance with institutional welfare guidelines. For pellet insertion, 6- to 8-week-old wild type female Balb/cJ mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized by intraperitoneal (i.p.) injection of 0.18 mg xylazine (Lloyd Laboratories, Shenandoah IA, USA) and 1.8 mg ketamine hydrochloride (JHP Pharmaceuticals, LLC Rochester MI, USA). Sedated mice were surgically implanted14 with 21-d sustained release pellets containing 50 mg LNG or matching placebo pellets (Innovative Research of America, Sarasota, FL, USA). As indicated, mice were euthanized 5 d after pellet insertion to measure serum LNG levels via RIA according to manufacturer’s instructions (Immunometrics, London, U.K.). Other LNG- or placebo-pelleted mice were anesthetized for intranasal (i.n.) Chlamydia trachomatis infection (105 IFU). Where indicated, beginning 1 d before infection, mice received 100 μg i.p. injection of an anti-CD4 mAb (GK 1.5) (BioXCell, Lebanon, NH) every 48 h until study termination. Efficiency of CD4+ T cell depletion was evaluated in peripheral blood and lung tissue by flow cytometry (Fig. S3), and was routinely >97%. Also where indicated, LNG-pelleted mice were intravenously (i.v.) administered 100 μg agonist anti-CD40 mAb (FGK 4.5) (Bio X cell, Lebanon, USA).
To assess DC activation, mice were euthanized at 2 dpi, and cervical lymph nodes (i.e., DLNs) excised, digested with collagenase D (Roche, Indianapolis, IN, USA) and deoxyribonuclease I (Sigma-Aldrich) for 1 h at 37 °C, and processed into single-cell suspension for flow cytometric analysis. To assess T cell expansion elicited in the lungs after i.n. C. trachomatis infection, mice were euthanized at 12 dpi. Lungs were excised and processed for flow cytometry by incubation for 1 h at 37 °C in RPMI-1640 supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, non-essential amino acids, 50 μM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamycin (Mediatech) (hereafter termed complete media), 1 mg/mL collagenase D, and 0.25 mg/mL deoxyribonuclease I. As previously described14, GzmB levels in T cells isolated from the lungs of uninfected (Fig. S2) and Chlamydia-infected mice were evaluated by flow cytometry. To define the effects of LNG on Chlamydia-specific T cell effector function, BMDCs were generated using uninfected female Balb/cJ mouse. As described elsewhere53,54, BMDCs were incubated in complete media at 37 °C with recombinant murine GM-CSF for 3 d, then GM-CSF and recombinant murine IL-4 for 3 d (R&D Systems, Inc., Minneapolis, MN). BMDCs were stimulated with live C. trachomatis (MOI = 0.1) for 24 h, and mixed at a 1:1 ratio with single-cell suspensions from the lungs of uninfected and Chlamydia-infected mice. Co-cultures were incubated for 18 h, and GolgiPlug™ (BD Biosciences) added during the last 6 h. As previously described14, T cells were fixed and permeabilized to quantify the intracellular accumulation of IFN-γ, TNF, and IL-17 using flow cytometry. To measure effects of LNG on chlamydial clearance from the lungs, mice were euthanized at 6 and 12 dpi. Lung lobes were excised, and total DNA extracted using Genomic DNA buffer sets with Genomic-tip 100/G kits following manufacturer’s instructions (Qiagen, Hilden, Germany). DNA was quantified (260/280 and 260/230 ratios were >1.8). C. trachomatis DNA obtained from purified EBs with DNeasy Blood & Tissue kits (Qiagen), was used to generate standard curves that defined C. trachomatis DNA levels in infected lungs55. Where indicated, mice were euthanized at 12 dpi, and excised lungs fixed with 4% methanol-free formaldehyde (Thermo Scientific, Rockford, IL, USA). After 24 h, samples were paraffin embedded, sectioned, and hematoxylin and eosin stained. Where noted, paraffin-embedded sections underwent immunohistochemical (IHC) staining to identify CD45+ (clone 30-F11, BD Biosciences) cells. For immunofluorescence (IF) assays, 10 μm sections from paraffin-embedded lungs were mounted on glass slides, and de-paraffinized by sequential immersion in 100% xylene, 100% ethanol, 96% ethanol, and sterile DEPC-treated water. Antigen retrieval was performed using 10 mM sodium citrate buffer (pH 6.0) containing 0.05% Tween 20 (Sigma-Aldrich) (20 minutes at 95 °C). Treated sections were washed in PBS, and incubated for 12 h at 4 °C with 10% normal donkey serum; 1 h at ambient temperate with rabbit anti-CD11b (clone EPR1344); and 1 h with Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (Abcam, Cambridge MA, USA) (antibodies were diluted in PBS supplemented with 1% BSA and 0.05% Tween 20). Sections were counterstained with DAPI, and inflammatory infiltrates evaluated using the Olympus FV1000 spectral confocal microscope (Tokyo, Japan).
Flow cytometry
Where indicated, cells were first stained with Live/Dead Fixable near-IR (Invitrogen, Eugene, OR, USA). Human cells were stained with anti-HLA-DR FITC (G46-6), anti-CD11c PE (B-ly6), anti-CD80 PE-Cy7 (L307.4), anti-CD40 APC (5C3), anti-CD86 BV510 (FUN-1), anti-CD123 BV421 (9F5), CD3 FITC (UCHT1), anti-CD4 PE (RPA-T4), or CD8 PE-Cy7 (RPA-T8) (all BD Biosciences). Mouse cells were stained with anti-IL-17 A PE (TC11-18H10), anti-CD8a V450 (53-6.7) anti-CD40 FITC (3/23) anti-CD80 APC (16–10A1), anti-CD11b BV510 (M1/70) (all BD Biosciences); anti- IFN-γ APC (XM61.2), anti-CD11b PE-Cy7 (M1/70), anti-CD4 PE-Cy7 (RM4–5), anti-CD4 PE (RM4–4) was used when anti-CD4+ mAb was used to deplete CD4+ cell, anti-CD45 PerCP (30-F11), anti-CD90.2 PerCP (30-H12) (all BioLegend San Diego, CA); anti-TNF FITC (MP6-XT22), anti-CD90.2 FITC (54-2.1) anti-Granzyme B PE (NGZB), anti-B220 APC (RA3-6BC), anti-CD11c PE-Cy7 (N418), anti-CD90.2 PerCP-eF710 (30-H12), anti-CD11c PE (N418), or anti-MHC-II PE-Cy7 (M5/114.15.2) (all eBioscience, San Diego, CA). Samples were run on a FACSCanto II (BD Biosciences, San Jose, CA, USA), and analyzed using FACS Diva (BD Biosciences) and FlowJo software (Tree Star Inc., Ashland OR). Fluorescence minus one controls were used to define costimulatory molecule expression by DCs stimulated with poly I:C or chlamydial antigen.
Statistical considerations
Statistical analyses were performed using Prism 6 software (GraphPad, La Jolla, CA, USA). LNG-mediated effects on DC activation were defined by comparing percentages of activated DCs in vehicle-treated vs. LNG-treated cultures (data normalized by designating vehicle-only cultures as 100% activation); (T cell proliferation values were similarly normalized designating proliferation in vehicle-only controls as 100%). Normal distribution was tested by D’Agostino & Pearson omnibus test or evaluation of the residuals (when experimental sample numbers were <8). Differences among paired samples between 2 groups were compared by unpaired Student t or Mann–Whitney U tests (depending on data distribution). For multiple comparisons, 1-way ANOVA with Dunnett’s or Tukey’s post hoc test (parametric distribution) or Friedman test with Dunn’s or Kruskal-Wallis post hoc test (nonparametric distribution) were used. For all analyses, P values < 0.05 were designated as statistically significant.
Additional Information
How to cite this article: Quispe Calla, N. E. et al. Dendritic cell function and pathogen-specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection. Sci. Rep. 6, 37723; doi: 10.1038/srep37723 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Cleland, J. Contraception in historical and global perspective. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 165–176 (2009).
Clifton, D. & Kaneda, T. Family Planning Worldwide 2013 Data Sheet. Population Reference Bureau (date of access: 30/06/2016). http://www.prb.org/Publications/Datasheets/2013/family-planning-worldwide-2013.aspx.(2013)
Kavanaugh, M. L., Jerman, J. & Finer, L. B. Changes in Use of Long-Acting Reversible Contraceptive Methods Among U.S. Women, 2009-2012. Obstet. Gynecol. 126, 917–927 (2015).
Bayer Healthcare Pharmaceuticals. Skyla package insert. Wayne, NJ: Bayer Healthcare Pharmaceuticals (2016).
Bayer Healthcare Pharmaceuticals. Mirena package insert. Wayne, NJ: Bayer Healthcare Pharmaceuticals (2016).
Adolescents and long-acting reversible contraception: implants and intrauterine devices. Committee Opinion 539. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 120, 983–988 (2012).
Ott, M. A. & Sucato, G. S. Committee on Adolescence. Contraception for adolescents. Pediatrics 134, e1257–1281 (2014).
Quispe Calla, N. E. et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal. Immunol. 9, 1571–1583 (2016).
Farley, T. M., Rosenberg, M. J., Rowe, P. J., Chen, J. H. & Meirik, O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet 339, 785–788 (1992).
Grimes, D. A. Intrauterine device and upper-genital-tract infection. Lancet 356, 1013–1019 (2000).
Mohllajee, A. P., Curtis, K. M. & Peterson, H. B. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2, 145–153 (2006).
Lekovich, J. P. et al. Comparison of human papillomavirus infection and cervical cytology in women using copper-containing and levonorgestrel-containing intrauterine devices. Obstet. Gynecol. 125, 1101–1105 (2015).
Liechty, E. R. et al. The levonorgestrel-releasing intrauterine system is associated with delayed endocervical clearance of Chlamydia trachomatis without alterations in vaginal microbiota. Pathog. Dis. 73, ftv070 (2015).
Vicetti Miguel, R. D. et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J. Immunol. 189, 3449–3461 (2012).
Dutow, P. et al. An optimized, fast-to-perform mouse lung infection model with the human pathogen Chlamydia trachomatis for in vivo screening of antibiotics, vaccine candidates and modified host-pathogen interactions. Pathog. Dis. 74, ftv120 (2016).
Quispe Calla, N. E., Ghonime, M. G., Cherpes, T. L. & Vicetti Miguel, R. D. Medroxyprogesterone acetate impairs human dendritic cell activation and function. Hum. Reprod. 30, 1169–1177 (2015).
Africander, D., Verhoog, N. & Hapgood, J. P. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 76, 636–652 (2011).
Stanczyk, F. Z., Hapgood, J. P., Winer, S. & Mishell, D. R. Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr. Rev. 34, 171–208 (2013).
Marx, P. A. et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2, 1084–1089 (1996).
Cherpes, T. L. et al. Use of transcriptional profiling to delineate the initial response of mice to intravaginal herpes simplex virus type 2 infection. Viral Immunol. 26, 172–179 (2013).
Morrison, S. G. & Morrison, R. P. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175, 7536–7542 (2005).
Rey-Ladino, J., Koochesfahani, K. M., Zaharik, M. L., Shen, C. & Brunham, R. C. A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct. Infect. Immun. 73, 1568–1577 (2005).
Zaharik, M. L. et al. Genetic profiling of dendritic cells exposed to live- or ultraviolet-irradiated Chlamydia muridarum reveals marked differences in CXC chemokine profiles. Immunology 120, 160–172 (2007).
Nogueira, C. V., Zhang, X., Giovannone, N., Sennott, E. L. & Starnbach, M. N. Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae. J. Immunol. 194, 2319–2329 (2015).
Kuo, C. & Chen, W. J. A mouse model of Chlamydia trachomatis pneumonitis. J. Infect. Dis. 141, 198–202 (1980).
He, X. et al. Enhanced virulence of Chlamydia muridarum respiratory infections in the absence of TLR2 activation. PLoS One 6, e20846 (2011).
Steele, J. C. et al. T-cell responses to human papillomavirus type 16 among women with different grades of cervical neoplasia. Br. J. Cancer 93, 248–259 (2005).
Grewal, I. S. & Flavell, R. A. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 153, 85–106 (1996).
Armitage, R. J. et al. CD40L: a multi-functional ligand. Semin. Immunol. 5, 401–412 (1993).
Durie, F. H., Foy, T. M., Masters, S. R., Laman, J. D. & Noelle, R. J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol. Today 15, 406–411 (1994).
Ma, D. Y. & Clark, E. A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 21, 265–272 (2009).
Nilsson, C. G., Lahteenmaki, P., Robertson, D. N. & Luukkainen, T. Plasma concentrations of levonorgestrel as a function of the release rate of levonorgestrel from medicated intra-uterine devices. Acta. Endocrinol. (Copenh.) 93, 380–384 (1980).
Luukkainen, T., Lähteenmäki, P. & Toivonen, J. Levonorgestrel-releasing intrauterine device. Ann. Med. 22, 85–90 (1990).
Lahteenmaki, P., Rauramo, I. & Backman, T. The levonorgestrel intrauterine system in contraception. Steroids 65, 693–697 (2000).
Seeber, B. et al. Quantitative levonorgestrel plasma level measurements in patients with regular and prolonged use of the levonorgestrel-releasing intrauterine system. Contraception 86, 345–349 (2012).
Nilsson, C. G., Haukkamaa, M., Vierola, H. & Luukkainen, T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin. Endocrinol. (Oxf.) 17, 529–536 (1982).
Casslen, B. Uterine fluid volume. Cyclic variations and possible extrauterine contributions. J. Reprod. Med. 31, 506–510 (1986).
Maier, D. B. & Kuslis, S. T. Human uterine luminal fluid volumes and prolactin levels in normal menstrual cycles. Am. J. Obstet. Gynecol. 159, 434–439 (1988).
Xu, Y. et al. Immunosuppressive effect of progesterone on dendritic cells in mice. J. Reprod. Immunol. 91, 17–23 (2011).
Huijbregts, R. P., Michel, K. G. & Hel, Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception 90, 123–129 (2014).
Reichmann, G. et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68, 1312–1318 (2000).
Soong, L. et al. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4, 263–273 (1996).
Chen, L. et al. Distinct roles of CD28- and CD40 ligand-mediated costimulation in the development of protective immunity and pathology during Chlamydia muridarum urogenital infection in mice. Infect. Immun. 77, 3080–3089 (2009).
Lazarevic, V., Myers, A. J., Scanga, C. A. & Flynn, J. L. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19, 823–835 (2003).
Samten, B. et al. CD40 ligand trimer enhances the response of CD8+ T cells to Mycobacterium tuberculosis. J. Immunol. 170, 3180–3186 (2003).
Andreasen, S. O., Christensen, J. E., Marker, O. & Thomsen, A. R. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J. Immunol. 164, 3689–3697 (2000).
Bennett, S. R. et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393, 478–480 (1998).
Clarke, S. R. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J. Leukoc. Biol. 67, 607–614 (2000).
Brunham, R. C. & Rekart, M. L. Considerations on Chlamydia trachomatis disease expression. FEMS Immunol. Med. Microbiol. 55, 162–166 (2009).
Chavez, J. M., Vicetti Miguel, R. D. & Cherpes, T. L. Chlamydia trachomatis infection control programs: lessons learned and implications for vaccine development. Infect. Dis. Obstet. Gynecol. 2011, 754060 (2011).
Scidmore, M. A. Cultivation and laboratory maintenance of Chlamydia trachomatis. Curr. Protoc. Microbiol. Chapter 11, Unit 11 A.1, (2005).
Vicetti Miguel, R. D., Henschel, K. J., Dueñas Lopez, F. C., Quispe Calla, N. E. & Cherpes, T. L. Fluorescent labeling reliably identifies Chlamydia trachomatis in living human endometrial cells and rapidly and accurately quantifies chlamydial inclusion forming units. J. Microbiol. Methods 119, 79–82 (2015).
Yin, S. Y., Wang, C. Y. & Yang, N. S. Interleukin-4 enhances trafficking and functional activities of GM-CSF-stimulated mouse myeloid-derived dendritic cells at late differentiation stage. Exp. Cell Res. 317, 2210–2221 (2011).
Glennie, N. D. et al. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 212, 1405–1414 (2015).
Bernstein-Hanley, I. et al. Genetic analysis of susceptibility to Chlamydia trachomatis in mouse. Genes Immun. 7, 122–129 (2006).
Acknowledgements
Authors acknowledge the Central-Southeast Ohio Region American Red Cross, Kevin Henschel for technical assistance, Ann Thompson for empathetic discourse, Narender Kumar (Center for Biomedical Research, Population Council, New York, NY) for generously providing expertise for serum LNG quantification, and The Ohio State University’s Comparative Pathology and Mouse Phenotyping Shared Resource (NIH grant P30 CA016058), University Laboratory Animal Resources, and Campus Microscopy and Imaging Facility. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD072663) and The Ohio State University College of Medicine.
Author information
Authors and Affiliations
Contributions
Experimental design and data acquisition, analysis, and interpretation were done by N.E.Q.C, R.D.V.M, and T.L.C.; A.M., S.F, and J.R.G. performed some experiments and data analysis. Manuscript was drafted by N.E.Q.C., with all authors significantly contributing to its final form.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Quispe Calla, N., Vicetti Miguel, R., Mei, A. et al. Dendritic cell function and pathogen-specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection. Sci Rep 6, 37723 (2016). https://doi.org/10.1038/srep37723
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37723
This article is cited by
-
The contraceptive medroxyprogesterone acetate, unlike norethisterone, directly increases R5 HIV-1 infection in human cervical explant tissue at physiologically relevant concentrations
Scientific Reports (2019)
-
The Complexity of Interactions Between Female Sex Hormones and Chlamydia trachomatis Infections
Current Clinical Microbiology Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.