Abstract
Chalcogenide glass has been considered as a promising host for the potential laser gain and amplifier media operating in near- and mid-IR spectral region. In this work, the IR luminescence spectra of rare earth ions (Tm3+, Er3+, and Dy3+) doped 65GeS2–25In2S3–10CsI chalcogenide glasses were measured under the excitation of an 808 nm laser diode. To the best of our knowledge, it firstly provides the luminescence spectra of a full near- and mid-IR spectral range from 1 to 4 μm in rare earth ions doped chalcogenide glasses. The results of absorption spectra, luminescence spectra, and fluorescence decay curves were obtained in these samples with singly-, co- and triply-doping behaviors of Tm3+, Er3+, and Dy3+ ions. In order to search possible efficient IR emissions, the luminescence behavior was investigated specifically with the variation of doping behaviors and dopant ions, especially in the samples co- and triply-doped active ions. The results suggest that favorable near- and mid-IR luminescence of rare earth ions can be further modified in chalcogenide glasses through an elaborated design of doping behavior and optically active ions.
Similar content being viewed by others
Introduction
In order to obtain IR luminescence of new wavelengths or bandwidths, glasses doped with different rare earth ions (REIs), especially Dy3+, Er3+, and Tm3+, have been intensively investigated in the past decades. For instance, the 1.3 μm emission of Dy3+ ion has been well adopted for the applications of optical communication1. The broadband optical amplification beyond the conventional 1.54 μm window of Er3+-doped fiber amplifier is of great interests for the increasing demands of information traffic2. To further extend the bandwidth of the light sources, one of the effective approaches is to co-dope with other active ions, such as Tm3+, through the overlap of emission bands. The 3H4 → 3H6 transition of Tm3+ ions generates the emission around 1460 nm, which could provide an excellent complement for the 4I11/2 → 4I13/2 transition (1540 nm) of Er3+ and the 6F11/2 + 6H9/2 → 6H15/2 transition (1328 nm) of Dy3+ ions. Thus the Tm3+-Dy3+ co-doped chalcohalide glasses have been recognized as one of the promising candidate materials for fiber-amplifiers and IR laser devices3. Meanwhile, it has been shown that an Er3+-Tm3+ co-doped 20 m long silica fiber amplifier generates spontaneous emission with bandwidth over 90 nm (1460–1550 nm), if it is pumped at 980 nm4. Nevertheless, the radiative transition from 3H4 to 3H5 (∼4300 cm−1) of Tm3+ is absent in silica or silicate glasses due to their relatively high maximum phonon energy (MPE, ∼1100 cm−1)4. Thus in order to obtain the laser gain and amplifier media operating in near- and mid-IR spectral region, much efforts have been devoted into searching low MPE materials, such as chalcogenide glasses (<350 cm−1)5, fluoride glasses (∼550 cm−1)6, tellurite glasses (∼750 cm−1)7,8, and oxyfluoride glass-ceramics9. Among them, chalcogenide glass is distinguished due to its nature of the lowest MPE.
Since the rediscovery of the excellent mid-IR transmission property of As2S3 in 1950s10, chalcogenide glasses are well known because of their unique properties of broad IR transparency window, semiconductivity, photosensitivity, and fast ionic conduction. The IR transmission region extending from 12 to 20 μm, depending on the chalcogen elements of S, Se, and Te, makes them favorable in the applications of IR optics, e.g. the lens for thermal imaging. Besides, compared with that of oxide and fluoride glasses, they also have the lowest MPE, which plays an important role in bridging the small energy gaps for the radiative transitions11. It is of great significance to explore new photonic properties of active ions, especially for the IR luminescence12,13.
Among the numerous investigation of REIs doped chalcogenide glasses, GeS2–Ga2S3 glasses have been well studied because of the augmented REIs solubility, which is originated from the structural modification caused by gallium14. By considering the similarity of chemical properties between indium and gallium, GeS2–In2S3 glasses also have been evidenced to possess the similar property of high REI solubility akin to that of gallium glasses15,16. Here, GeS2–In2S3 based glasses were specially selected because of the other superior optical properties, e.g. high refractive index. Meanwhile, CsI was introduced for the following two reasons. First, good glass-forming ability could be achieved due to the large radius of cesium ion, which can stabilize the formed complex anion in the glassy network17. Second, the addition of iodine ions results in a broadening of optical bandgap and the consequent shift of the visible cut-off edge towards shorter wavelengths18, which would benefit for the selection of pump sources during luminescence measurements. Thus, in this report, chalcogenide glasses with the composition of GeS2–In2S3–CsI are selected as the hosts for doping three different REIs of Dy3+, Er3+, and Tm3+. The IR luminescence spectra ranging from 1 to 4 μm will be measured and the optical transitions of the singly-, co-, or triply-doping of REIs will also be discussed in detail. To the best of our knowledge, it might be the first work to present such full spectral region of near- and mid-IR luminescence in glassy materials.
Results and Discussion
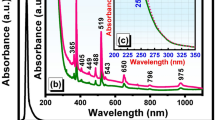
Figure 1 shows the absorption spectra of REIs singly-, co- and triply-doped 65GeS2–25In2S3–10CsI glass at room temperature in the wavelength region of 400–4000 nm. Some of the absorption bands in IR region are attributed to the impurities of O-H (~2800 nm), S-H (~3100 nm), and others (~3400 nm and ~3500 nm), respectively. Most of the excited states are labeled in Fig. 1, expect some of them with weak absorbance, e.g. 4I9/2 of Er3+ centered at 804 nm. For the Tm3+-doped glass sample, a strong absorption band of the excited state 3H4 locates around 798 nm. In the Er3+-Tm3+ co-doped sample, the absorption band centered at 806 nm is enhanced due to the very closer transitions of Er3+: 4I15/2 → 4I9/2 and Tm3+: 3H6 → 3H4. It indicates that the higher pumping efficiency could be realized compare to that of the Er3+ singly-doped glass sample. The absorption bands corresponding to internal 4f–4 f electronic transitions of Dy3+-doped are also indicated in the Fig. 1. The absorption peaks of around 914, 1114, 1298, 1724, and 2833 nm are obvious, whereas that of 808 nm is very weak, suggesting the pumping efficiency under the excitation of 808 nm LD will be very low in this sample. It is seen that all of the co- or triply-doped Ge-In-S-CsI glass show the combined absorption bands of the respective REIs in the spectra. The fluorescence spectra of the singly-, co-, or triply-doped samples will be displayed and analyzed in detail in the following.
Singly- and co-doping of Er3+ and Tm3+
Figure 2a shows the IR fluorescence spectra of the 65GeS2–25In2S3–10CsI glasses doped with Tm3+ and Er3+ in different doping behavior. There have 5 emission bands located at 1470, 1540, 1804, 2316 and 2721 nm, respectively. As indicated in Fig. 2b, the transitions of Tm3+: 3H4 → 3F4, Tm3+: 3H4 → 3H5 and Er3+: 4I11/2 → 4I13/2 contribute to the emissions located at 1470, 2316, and 2721 nm. The excited levels of Tm3+:3F4 and Er3+: 4I13/2 relaxes to the respective ground states of 3H6 and 4I15/2 generating the 1804 nm and 1540 nm emissions, respectively. Besides, in the Er3+ singly-doped samples, there has a band centered at 1700 nm which is originated from the transition of Er3+: 4I9/2 → 4I13/2. It is obvious that the 1540 nm emission of Er3+ singly-doped samples attributed to 4I13/2 → 4I15/2 transition has a full width at half-maximum (FWHM) of ∼80 nm. It can be further improved through the co-doping of Er3+-Tm3+, which shows an emission ranging from 1350 to 1600 nm with a FWHM of ∼140 nm, which is much broader than that of Er3+-Tm3+ co-doped silica fiber (90 nm)19.
As shown in Fig. 2b, the energy transfer between Tm3+ and Er3+ can be achieved through several shortcuts, which would lead to the variation of emission behavior. As shown in Fig. 2a, the intensity of 1804 nm emission is enhanced by co-doping with Er3+, whereas that of the 1470 nm and 2316 nm emissions are decreased. These can be ascribed to the small energy gap between Tm3+: 3H4 and Er3+: 4I9/2, which contributes to a resonant energy transfer for Er3+: 4I9/2 level by depopulating the Tm3+: 3H4 level. Although there exists energy transfer from Er3+: 4I13/2 to Tm3+: 3F4, the intensity of 1540 nm emission increases by 79% in Er3+-Tm3+ co-doped sample. It suggests that this co-doped sample has a better pumping efficiency and the sensitized effect of Tm3+ ions is stronger than the energy transfer process.
All emission lifetimes and the related parameters of the above-mentioned samples are calculated and collected in Table 1. The decay profiles were recorded by NIR-PMT (Hamamatsu R5509-72 Photomultiplier) and InSb detectors by monitoring of the emissions at 1–2 μm and 2–4 μm, respectively. The lifetime values are also related to the energy transfer processes, akin to the emission intensity. In Tm3+ singly and Tm3+-Er3+ co-doped samples, the emission lifetimes decrease from 172 to 156 μs for 1470 nm (3H4 → 3F4) and from 177 to 175 μs for 2316 nm (3H4 → 3H5), while it increases from 1189 to 1245 μs for 1804 nm (3F4 → 3H6). It is worthy to note that the emission at 1540 nm of the Er3+-doped sample become stronger by the co-doping with Tm3+ ions, indicating a higher pumping efficiency. The energy transfer efficiency of the studied samples are collected in Supplementary Table S1.
Figure 3 shows the typical fluorescence decay curves of Er3+-doped and Tm3+-Er3+ co-doped glasses, which were recorded by monitoring the transition of 4I13/2 → 4I15/2 (1540 nm) as displayed in Fig. 2b. The lifetime here is obtained according to the definition of the time where the emission intensity decreases to 1/e of its initial value. The lifetime of 1540 nm emission in co-doped sample decreases from 3523 to 2526 μs. The process is satisfied by the energy transfer from Er3+: 4I13/2 to Tm3+: 3F4. There is a particular shortcut that energy transfer from Er3+: 4I11/2 to Tm3+: 3H5. The lifetime of 2721 nm emission does not change significantly from 2159 to 2069 μs (within the error limit of ±5%). The variation of emission intensity and lifetime of 2721 nm are nearly consistent. Nevertheless, the effect of Tm3+ doping behavior on the 2721 nm emission of Er3+ should be further investigated and checked by the concentration variation.
The energy transfer efficiency from Er3+- to Tm3+-Er3+ co-doped can be calculated by the following equation20

where  and
and  are the lifetimes of 4I13/2 → 4I15/2 emission (1540 nm) in Er3+-doped and Tm3+-Er3+co-doped samples (the lifetimes in the absence and presence of acceptors), respectively. The lifetime values of 4I13/2 → 4I15/2 in these singly- and co-doped samples are marked in Fig. 3. The lifetime reduces strongly by co-doping with Tm3+, indicating the efficient energy transfer from Er3+ to Tm3+. In the co-doped sample, the energy transfer efficiency from Er3+ to Tm3+ is ~28.2%, which is different from that of ~80% reported in TeO2–WO3–PbO glass20 and tellurite fiber7,21. In this case, the low energy transfer efficiency is favorable to the interested broadband emission around 1540 nm.
are the lifetimes of 4I13/2 → 4I15/2 emission (1540 nm) in Er3+-doped and Tm3+-Er3+co-doped samples (the lifetimes in the absence and presence of acceptors), respectively. The lifetime values of 4I13/2 → 4I15/2 in these singly- and co-doped samples are marked in Fig. 3. The lifetime reduces strongly by co-doping with Tm3+, indicating the efficient energy transfer from Er3+ to Tm3+. In the co-doped sample, the energy transfer efficiency from Er3+ to Tm3+ is ~28.2%, which is different from that of ~80% reported in TeO2–WO3–PbO glass20 and tellurite fiber7,21. In this case, the low energy transfer efficiency is favorable to the interested broadband emission around 1540 nm.
In this doping behavior, for the near-IR emission of 1540 nm (Er3+: 4I13/2 → 4I15/2), the co-doping of Tm3+ can effectively extend its FWHM and intensity in the 65GeS2–25In2S3–10CsI chalcogenide glass. It is due to the significant energy transfer process between Tm3+ and Er3+. Thus, this Tm3+-Er3+co-doped sample has a broad bandwidth with a flat-gain spectrum for minimizing the channel-to-channel gain excursion and crosstalk in a high-speed network.
Singly- and co-doping of Dy3+ and Tm3+
Figure 4 shows the IR fluorescence spectra of Dy3+-doped and Tm3+-Dy3+ co-doped glasses in the ranges from 1200 to 3300 nm and also the corresponding energy-level diagram. In Dy3+-doped glass, three major emission bands are observed around 1328, 1752 and 2887 nm. As shown in Fig. 4b, they are ascribed to the transitions of 6F11/2 + 6H9/2 → 6H15/2, 6H11/2 → 6H15/2, and 6H13/2 → 6H15/2, respectively. As indicated in Fig. 4b, three shortcuts of the energy transfer are existed between Tm3+ and Dy3+. The first shortcut of the energy transfer from Tm3+: 3H4 to Dy3+: 6F5/2 leads to that the emission intensities and lifetimes of 1470 and 2316 nm decrease as displayed in Table 1. The energy transfer from Tm3+: 3H5 to Dy3+: 6F11/2 + 6H9/2 results in an overlapping emission from Dy3+ (1328 nm) to Tm3+ (1470 nm), a 17% increased emission at the 1328 nm (as shown in the inset of Fig. 4a), and a elongated lifetime from 41 to 68 μs. In addition, the two energy levels of Dy3+ (1752 nm, 6H11/2 → 6H15/2) and Tm3+ (1804 nm, 3F4 → 3H6) are very close, resulting in the energy transfer from Tm3+: 3F4 to Dy3+: 6H11/2. Thus the emission peak of the co-doped sample is shifted to longer wavelength (1794 nm), and the emission intensity enhances. Furthermore, the energy transfer from Tm3+: 3F4 to Dy3+: 6H11/2 can populate the initial state of the 2887 nm emission22, then contribute to the 18% increase of the 2887 nm emission intensity in Tm3+-Dy3+ co-doped sample.
The decay profiles of 6H13/2 → 6H15/2 transition in Dy3+-doped and Tm3+-Dy3+ co-doped samples are shown in Fig. 5. And the lifetime values are collected in Table 1. It is obvious that the intensities of 2887 nm emission vary slightly in singly- and co-doped samples, whereas their lifetimes change intensively from 2108 to 2829 μs.
In this doping behavior, the Dy3+ singly- and Dy3+-Tm3+ co-doped samples have three main peaks in near- and mid-IR spectral region. In particular, the 2887 nm emission always exists, which is better than that observed in Ge-Ga-S-CdI2 chalcohalide glasses3, indicating that the host glass is a good choice for doping Dy3+ ions. Furthermore, as indicated by the emission intensity and lifetime, we can see that the co-doping of Tm3+ ions is favorable to the emission of Dy3+ ions in the 2–4 μm region.
Singly- and co-doping of Er3+ and Dy3+
Figure 6a shows the fluorescence spectra of the (Er3+ and Dy3+) singly- and co-doped samples and the corresponding energy-level diagram. It is obvious that four main emission bands are located at 1540, 1752, 2721 and 2887 nm in the Dy3+ doped sample, and they decline with the co-doping of Er3+. It is in good accordance with the variation of the absorption bands in Fig. 1.
From the decay curve in Fig. 7, the lifetime of the 1540 nm emission in Er3+-Dy3+ co-doped sample is equal to 3218 μs (listed in Table 1). In comparison to the above results, it suggests that the energy transfer efficiency of Er3+-Dy3+ co-doped sample is far less than that of Tm3+-Er3+ and Tm3+-Dy3+ co-doped ones. The co-doping of these two REIs (Dy3+ and Er3+) are not suitable for the near- and mid-IR emissions in chalcogenide glasses, because the co-doped sample shows a weakening effect on each peak.
Singly-, co- and triply-doping of Er3+, Dy3+, and Tm3+
The fluorescence spectrum of Tm3+-Dy3+-Er3+ triply-doped 65GeS2–25In2S3–10CsI glass is shown in Fig. 8a. A broadband emission from 1300 nm to 1600 nm is observed, which includes the transitions of Dy3+ (1328 nm, 6F11/2 + 6H9/2 → 6H15/2), Tm3+ (1470 nm, 3H4 → 3F4) and Er3+ (1540 nm, 4I13/2 → 4I15/2). The emission intensity of the triply-doped sample is improved in comparison with that of Er3+-doped and Tm3+-Dy3+ co-doped samples.
Figure 9 shows the decay profiles of the 1540 nm emission (Er3+: 4I13/2 → 4I15/2) in Tm3+-Er3+ co-doped and Tm3+-Dy3+-Er3+ triply-doped samples, and the 1794 nm emission (Dy3+: 6H11/2 → 6H15/2 and Tm3+: 3F4 → 3H6) in Tm3+-Dy3+ co-doped and Tm3+-Dy3+-Er3+ triply-doped 65GeS2–25In2S3–10CsI glasses. The lifetime of Er3+: 4I13/2 → 4I15/2 transition (1540 nm) in Tm3+-Er3+-Dy3+ triply-doped sample is 2551 μs (listed in Table 1), which is close to that of Tm3+-Er3+ co-doped one as displayed in Fig. 9a. According to the lifetime values marked in Fig. 9a, the energy transfer efficiency of the triply-doped sample is as high as 27.5%, it is compared to ~28.2% in Tm3+-Er3+ co-doped one. For the 1794 nm emission, there has a special shortcut of the energy transfer from Er3+: 4I13/2 to Tm3+: 3F4 and Dy3+: 6H11/2 as indicated in Fig. 8b. Finally, the reduction and improvement of the studied emissions are concluded in Supplementary Table S2.
In this work, the triple doping in 65GeS2–25In2S3–10CsI glass is of a certain research value, not only because it remains a appropriate energy transfer efficiency, but also it is beneficial to the IR emissions at desirable wavelengths through the energy transfer process. To the best of our knowledge, this work is the first one to present such broad IR emission behavior in REIs doped chalcogenide glasses.
Conclusions
Near- and mid-IR luminescence spectra of Er3+, Tm3+, and Dy3+ ions was recorded in 65GeS2–25In2S3–10CsI glasses ranging from 1 to 4 μm with different doping behavior, e.g. singly-, co-, and triply-doping. The experimental results show that the co-doping of Tm3+ could greatly improve the pump efficiency of Dy3+ and Er3+ ions, and their fluorescence intensities. The Tm3+-Er3+ and Tm3+-Dy3+ co-doped samples present superior spectroscopic properties that is beneficial for the interested wavelength within 1–4 μm region. In addition, it provides the first luminescence investigation in Er3+-Tm3+-Dy3+ triply doped chalcogenide glasses. The energy transfer efficiency of the triple-doped sample is as high as Tm3+-Er3+ co-doped one, due to the energy transfer from Er3+ ions to Tm3+-Dy3+ ion. Although the efficient mid-IR laser is still absent in chalcogenide glasses, this work provide a full set of IR luminescence data available for the REIs doped chalcogenide glasses. It would be of significant guidance for the future development in this research field.
Method
Glass samples of 65GeS2–25In2S3–10CsI singly-, co-, and triply-doped with 0.1 mol% REIs (Tm, Dy2S3, and Er) were synthesized by melting the mixtures of constituent elements (Ge, In, S, and CsI of 99.999%) in evacuated (~10−3 Pa) and flame-sealed silica ampoule. The batches were melted at 990 °C for 22 h, and then quenched in a saturated brine. The obtained glassy samples were annealed at 310 °C for 6 h. Glass rods were obtained by taking them out from the ampoules and finally cut into discs (Ø 9 mm × 2 mm), which were then polished for succeeding experiments.
The absorption spectra were recorded by a UV/VIS/NIR spectrophotometer (PERKIN-ELMER-LAMBDA 950) and FTIR spectroscopy (Thermo Scientific Nicolet 380) in the spectral ranges of 400–2500 nm and 2500–4000 nm, respectively. Excitation and emission spectra were measured by employing a fluorescence spectrometer (Edinburgh, ENGLAND FLS980). Fluorescence spectra were obtained using 808 nm excitation from a laser diode (LD) (the average power is 1 W). NIR-PMT and InSb detectors cooled with liquid nitrogen were employed to record the near- and mid-IR fluorescence from 1100 to 3300 nm. The fluorescence decay curves were recorded with InSb detector by monitoring the interested IR emissions. To make the results comparable, a fixed configuration between the sample, laser, and detector is employed and carefully used. By checking the variation of emission data in several measurements for the same sample, the error of these results are within ±5%. All the measurements were performed at room temperature.
Additional Information
How to cite this article: Li, L. et al. GeS2–In2S3–CsI Chalcogenide Glasses Doped with Rare-Earth Ions for Near- and Mid-IR Luminescence. Sci. Rep. 6, 37577; doi: 10.1038/srep37577 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wei, K., Machewirth, D., Wenzel, J., Snitzer, E. & Sigel, G. Spectroscopy of Dy3+ in Ge–Ga–S glass and its suitability for 1.3 μm fiber-optical amplifier applications. Optics Letters 19, 904–906 (1994).
Jeong, H., Oh, K., Han, S. & Morse, T. Characterization of broadband amplified spontaneous emission from an Er3+–Tm3+ co-doped silica fiber. Chemical Physics Letters 367, 507–511 (2003).
Guo, H. et al. Host dependence of spectroscopic properties of Dy3+-doped and Dy3+, Tm3+-codped Ge-Ga-S-CdI2 chalcohalide glasses. Optics express 17, 15350–15358 (2009).
Seo, S.-Y. et al. Erbium–thulium interaction in broadband infrared luminescent silicon-rich silicon oxide. Applied Physics Letters 82, 3445–3447 (2003).
Xu, Y. et al. Broadband near-infrared emission in Er3+-Tm3+ codoped chalcohalide glasses. Optics Letters 33, 2293–2295 (2008).
Semenkoff, M., Guibert, M., Ronarc’h, D., Sorel, Y. & Kerdiles, J. Improvement of gain flatness of optical fluoride fiber amplifiers for multiwavelength transmission. Journal of Non-Crystalline Solids 184, 240–243 (1995).
Huang, L., Jha, A., Shen, S. & Liu, X. Broadband emission in Er3+-Tm3+ codoped tellurite fibre. Optics Express 12, 2429–2434 (2004).
Zhou, D., Song, Z., Chi, G. & Qiu, J. NIR broadband luminescence and energy transfer in Er3+–Tm3+-co-doped tellurite glasses. Journal of Alloys and Compounds 481, 881–884 (2009).
Lin, C. et al. Oxyfluoride Glass-Ceramics for Transition Metal Ion Based Photonics: Broadband Near-IR Luminescence of Nickel Ion Dopant and Nanocrystallization Mechanism. The Journal of Physical Chemistry C 120, 4556–4563 (2016).
Frerichs, R. New optical glasses with good transparency in the infrared. JOSA 43, 1153–1157 (1953).
Chen, D., Wang, Y., Bao, F. & Yu, Y. Broadband near-infrared emission from Tm3+/Er3+ co-doped nanostructured glass ceramics. Journal of Applied Physics 101, 113511–113511 (2007).
Zhou, B. & Pun, E. Y.-B. Broadband near-infrared photoluminescence and energy transfer in Tm3+/Er3+-codoped low phonon energy gallate bismuth lead glasses. Journal of Physics D: Applied Physics 44, 285404 (2011).
Shixun, D. et al. The near-and mid-infrared emission properties of Tm3+-doped GeGaS–CsI chalcogenide glasses. Journal of Non-Crystalline Solids 356, 2424–2428 (2010).
Weber, M. J. Luminescence decay by energy migration and transfer: observation of diffusion-limited relaxation. Physical Review B 4, 2932 (1971).
Abe, K., Takebe, H. & Morinaga, K. Preparation and properties of Ge-Ga-S glasses for laser hosts. Journal of Non-Crystalline Solids 212, 143–150 (1997).
Li, Z., Lin, C., Nie, Q. & Dai, S. Controlled crystallization of β-In2S3 in 65GeS2⋅ 25In2S3⋅ 10CsCl chalcohalide glass. Applied Physics A 112, 939–946 (2013).
Li, Z., Lin, C., Nie, Q. & Dai, S. Competitive Phase Separation to Controllable Crystallization in 80GeS2· 20In2S3 Chalcogenide Glass. Journal of the American Ceramic Society 96, 125–129 (2013).
Tver’yanovich, Y. S. et al. Chalcogenide glasses containing metal chlorides. Glass Physics and Chemistry 22, 9–14 (1996).
Jeong, H., Oh, K., Han, S. & Morse, T. Broadband amplified spontaneous emission from an Er3+–Tm3+-codoped silica fiber. Optics Letters 28, 161–163 (2003).
Balda, R., Fernández, J. & Fernández-Navarro, J. M. Study of broadband near-infrared emission in Tm3+-Er3+ codoped TeO2-WO3-PbO glasses. Optics Express 17, 8781–8788 (2009).
Li, K. et al. Broadband near-infrared emission in Er3+–Tm3+ co-doped bismuthate glasses. Journal of Alloys and Compounds 509, 3070–3073 (2011).
Heo, J., Cho, W. Y. & Chung, W. J. Sensitizing effect of Tm3+ on 2.9 μm emission from Dy3+-doped Ge25Ga5S70 glass. Journal of Non-Crystalline Solids 212, 151–156 (1997).
Acknowledgements
This work was partially supported by the Natural Science Foundation of Zhejiang Province (grant no. LY14E020001). And it was also sponsored by the K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Contributions
Legang Li prepared the samples and wrote this report. Junyi Bian and Zijun Liu tested all of the samples. Qing Jiao and Shixun Dai help the analysis of experimental results. Changgui Lin provided the idea of this work and revised this paper. All the authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, L., Bian, J., Jiao, Q. et al. GeS2–In2S3–CsI Chalcogenide Glasses Doped with Rare Earth Ions for Near- and Mid-IR Luminescence. Sci Rep 6, 37577 (2016). https://doi.org/10.1038/srep37577
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37577
This article is cited by
-
Crystal structure, infrared luminescence and magnetic properties of Tm3+-doped and Tm3+-, Dy3+-codoped BaY2Ge3O10 germanates
Journal of Materials Science: Materials in Electronics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.