Abstract
Distant metastasis is the primary failure pattern of nasopharyngeal carcinoma(NPC) in intensity-modulated radiation therapy(IMRT) era. This study was conducted to find the impact of genetic variations in the phosphatidylinositol 3-kinase(PI3K)/phosphatase and tensin homologue(PTEN)/v-akt murine thymoma viral oncogene homologue(AKT)/mammalian target of rapamycin(mTOR) pathway on the risk of distant metastasis in NPC. We genotyped 16 single-nucleotide polymorphisms(SNPs) in five core genes in this pathway from 496 patients treated by IMRT with or without chemotherapy. The relationships between genetic polymorphisms and distant progression were evaluated. We observed that two loci in the AKT1 gene(rs3803300 and rs2494738 alone or combined) were associated with prognosis, with patients carrying at least one variant allele had significantly reduced risk of distant failure, especially in N2-3 group. In addition, we found that genetic variation may had some joint effect with N classification in recursive-partitioning analysis(RPA) analysis, with which patients were stratified into four different risk subgroups (RPA model): RPA1(low risk), RPA2(moderate risk), RPA3(high risk) and RPA4(highest risk). Our findings suggested that genetic variations within the PI3K signaling pathway modulate the development and invasion of NPC patients. Further research is needed to replicate the study in other centers and races, and to unravel the functional significance of these polymorphisms.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease in Southeast Asia and southern China1. The application of chemotherapy and intensity-modulated radiation therapy(IMRT) have significantly improved the treatment outcomes. Even with the best available treatment in modern practice, retrospective reports of patients treated with IMRT over the last decade have revealed that 15% to 30% will experience failure at distant sites2. Tumor-nodal-metastasis (TNM) system is critical in predicting prognosis and facilitating treatment planning. However, a significant heterogeneity in treatment outcomes is observed for patients within the same clinical stages and a subset of patients are considered to be at higher risk of tumor progression and distant metastasis. Thus, it would be of clinical interest to identify prognostic factors for distant metastasis or tumor progression that would enable physicians to identify subgroups of patients who may benefit from more aggressive individualized therapy.
The PI3K/PTEN/AKT/mTOR pathway, which consists of phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog (PTEN), v-akt murine thymoma viral oncogene homolog (AKT), and mammalian target of rapamycin (mTOR), has been implicated in the regulation of angiogenesis and metastasis – both important processes in cancer development and progression3,4. Several literatures have been reported that genetic variations in this pathway are associated with clinical outcomes, invasion property, drug resistance to chemotherapy and treatment complications, including head and neck squamous cell carcinoma, esophageal cancer, cervical cancer, gastric cancer, colorectal carcinoma, lung cancer and bladder cancer5,6,7,8,9,10,11,12,13,14,15,16,17. Although the involvement of this signaling pathway in the development and invasion of NPC have been addressed in many literatures4,18,19,20,21,22, the clinical significance of genetic variations in this pathway remains unclear in NPC. Herein, we performed this study, which enrolled 496 NPC patients treated by IMRT with or without chemotherapy, aimed to identify the potential associations between genetic variations in PI3K/PTEN/AKT/mTOR pathway and the occurrence of distant metastasis in patients with NPC.
Materials and Methods
Ethical statement
This retrospective study was conducted in compliance with the policy of Fujian Provincial Cancer Hospital to protect the private information of patients enrolled. All methods were performed in accordance with the relevant guidelines and regulations of Fujian Provincial Cancer Hospital and was approved by its ethical committee. All subjects and/or guardians received and signed informed consent.
Patients’ characteristics
This study included 496 patients with histologically diagnosed non-metastatic NPC who were recruited between January 2012 and May 2013 at Fujian Provincial Cancer Hospital and had blood samples available for analysis. None had history of previous treatment or prior malignancy. All of them completed a pretreatment evaluation according to our institutional protocol23 and staged according to the 7th AJCC staging system. Peripheral blood specimens for genetic analysis were collected from each patient at the time of diagnosis prior to any treatment. They were pathologically confirmed, with 456(91.9%), 31(6.3%) and 9(1.8%) patients be classified as World Health Organization (WHO) type III, II, and I, respectively. Other clinical characteristics were listed in Table 1.
Treatment
All the included patients received IMRT with or without chemotherapy. A detailed description of the IMRT had been published previously23. Of the 472 patients with Stages II–IVB disease, 459(97.2%) were given platinum-based chemotherapy, the sequence used were induction in 71(15.9%), induction-concurrent 139(30.2%), induction-adjuvant 76(16.5%), induction-concurrent-adjuvant 151(32.9%), concurrent 18(3.9%) and adjuvant 2(0.4%), Whenever possible, salvage treatments (including intracavitary brachytherapy, surgery, chemotherapy and boost radiotherapy) were provided for those who developed relapse or persistent disease.
Single-nucleotide polymorphisms(SNPs) selection and genotyping
Genomic DNA was extracted from whole blood, using the Qiagen DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol, and stored at −80 °C until use. The DNA purity and concentration were determined by spectrophotometric measurement of absorbance at 260 and 280 nm.
Tagging SNPs were selected from 5-kb flanking and within the gene regions of five genes: PIK3CA, AKT1, AKT2, PTEN, and FRAP1 (mTOR) by using the tagger algorithm24, and then identified with a cut-off value of r2 = 0.8 and a minor allele frequency greater than 0.05 in the Chinese population by Haploview software, based on data from the HapMap Project (www.hapmap.org). Finally, a total of 16 SNPs, including haplotype-tagging SNPs and potential functional SNPs, were selected for genotyping (Table 2).
Among them, 14 SNPs were genotyped by using matrix-assisted laser desorption/ionization-time of flight mass spectrophotometry with the MassARRAY platform (Sequenom, Inc.). Assay data were analyzed using Sequenom TYPER software (version 4.0). Most of the SNPs had a call rate of at least 95%, except two SNPs, with rs2494738 and rs892119 shown to be 93.1% and 94.0%, respectively. Another two SNPs(rs2494732 and rs3803300) were genotyped by TaqMan assay. Randomly repeated assays were used for genotyping quality control.
Follow-up and statistical analyses
All patients were assessed weekly for treatment response and toxicity during treatment. After the completion of radiotherapy, they were required to be followed-up every three months for the first two years and every 3–6 months during years 3–5. Our study end points were distant metastasis and survival. The overall survival (OS) and distant metastasis-free survival(DMFS) were measured and calculated from the first day of diagnosis to death and distant failure. Data were analyzed using SPSS version 22.0. The Hardy-Weinberg equilibrium of the mutation was determined by χ2 test. Univariate and multivariate analyses were performed to define the association between each SNPs and the risk of distant metastasis and death. We also evaluated the combined effects by the number of unfavorable genotypes identified from the main effects analysis of single SNPs. Survival tree analyses were used to derive prognostic groups that combined unfavorable genotypes with N category. Survival tree analysis was performed using the STREE program (http://masal.med.yale.edu/stree/) which uses recursive-partitioning analysis(RPA) to identify subgroups of individuals at higher risk. A two-sided P-value of ≤0.05 was considered as statistically significant.
The performance of RPA model was compared with N category and clinical stage, by using Akaike information criterion(AIC)25 and Harrell’s concordance index(c-index)26. The AIC and c-index were both calculated for the Cox proportional hazards regression model. The AIC refers to the information loss of the selected prognostic model, a smaller AIC value suggests a better goodness of fit of the model. The c-index measures the ability to predict the outcomes, a higher c-index suggests a better discriminatory power of the model. All statistical analyses were conducted with SPSS 22.0 and R3.13.
Results
Survival and prognostic analysis
The median follow-up time was 40 months (range 4–51 months) for the whole cohort, with the 3-year OS and DMFS shown to be 91.8% and 85.1%, respectively. At the time of censorship, distant metastasis had developed in 74 patients. The sites of metastases included bone only (n = 21), lung only (n = 14), liver only (n = 20), and other unspecified sites (n = 2), or multiple sites (n = 17). The median time from NPC diagnosis to detection of distant metastasis was 16 months (range 4–47months).
Potential associations between patient-, tumor-, therapy-related characteristics and distant metastasis by univariate and multivariate analyses were tested, as shown in Table 3. We found that N classification was significantly associated with distant metastasis, with patients having advanced N category at higher risk of distant failure (P < 0.001). Neither T classification nor treatment factors were associated with distant metastasis in this population. This effect was virtually unchanged after adjustment for gender, age, T classification and cycles of chemotherapy in the Cox model (Table 3).
Associations between SNPs and survival
Statistical results of χ2 test indicated that the allele frequencies of the enrolled SNPs all fit with Hardy-Weinberg equilibrium (P > 0.05, data not shown). Variant genotypes of each of the 16 individual SNP were evaluated for association with DMFS after definitive chemo-radiotherapy, by using univariate and multivariate analysis.
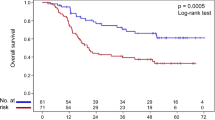
Of the 16 SNPs, only two SNPs- AKT1: rs3803300 and AKT1:rs2494738-were found to be significantly associated with DMFS after MVA (Table 4). AKT1: rs3803300, as detailed in Table 4, resulted in significantly inferior DMFS for patients with wild-type genotype compared with those with one or two variant alleles (Fig. 1) (74.5% vs. 86.7%, P = 0.025). The same result was verified after stratifying by patient-, tumor- and treatment-related factors (HR = 0.536; 95% CI = 0.292–0.986; P = 0.045). Patients carrying at least one variant allele for AKT1: rs2494738 had a better DMFS than those with wile-type genotype (86.9% vs. 80.5%, P = 0.106) (Fig. 1), this trend became significant after adjusting by gender, age, treatment, T- and N-category (HR = 0.530; 95% CI = 0.302–0.929; P = 0.027).
As showed in MVA, another two SNPs-AKT1:rs1130214, and mTOR:rs11121704 showed an obvious trend toward inferior DMFS in patients with one or two variant alleles (AKT1:rs1130214 HR = 1.566, 95% CI = 0.956–2.567, P = 0.075; mTOR:rs11121704 HR = 1.655, 95% CI = 0.933–2.935, P = 0.085). While AKT2: rs892119 indicated a clear trend to have reduced risk of distant failure when patients carried at least one variant allele (HR = 0.579, 95% CI = 0.317–1.057, P = 0.075). Similar analyses of the other 11 SNPs showed no associations between different genotypes and incidence of distant failure (Table 4).
Combined effect of SNPs on risk of distant metastasis
As these two AKT1 SNPs(AKT1: rs3803300 and AKT1:rs2494738) were consistently associated with risk of distant metastasis, we tried to perform an unfavorable genotype analysis to determine the effect of having one or both of these SNPs. For the 446 patients who had successfully genotyped for these two SNPs, 21, 83 and 342 cases were indicated to carry two, one and none of the unfavorable genotype, respectively. Because of the small number of patients who had two unfavorable genotypes, patients had one or two unfavorable genotype were combined as one group (n = 104), which was indicated to have significant lower DMFS than the other group which had no unfavorable genotype (78.0% vs. 88.3%, P = 0.009) (Fig. 1). Multiple Cox proportional hazard analysis with adjustments for T classification, N-classification, gender, age and treatment further confirmed this result (HR = 0.443; 95% CI = 0.264–0.744; P = 0.002).
Further subgroup analysis was performed to find out whether the unfavorable genotype had different prognostic role in N0-1 and N2-3 patients. Of the whole group (446 cases), there were 190 patients be classified as N2-3 stage, the MVA analysis indicated that unfavorable genotype was significantly associated with the risk of distant metastasis (HR = 0.456; 95% CI = 0.241–0.863; P = 0.016). However, for the 256 patients with N0-1 category, unfavorable genotype seems to had no significant effect on risk of distant failure (HR = 0.058; 95% CI = 0.210–1.228; P = 0.133).
Joint effect of SNPs and other prognostic factors
In current series, only N classification and unfavorable genotype were identified as factors strongly related to risk of distant failure. RPA for DMFS was conducted with these two factors to derive RPA groups objectively. The RPA algorithm finally classified these patients into four RPA groups: RPA1 (low risk: N0-1, without unfavorable genotype), RPA2 (moderate risk: N0-1, with at least one unfavorable genotype), RPA3 (high risk: N2-3, without unfavorable genotype) and RPA4 (highest risk: N2-3, with at least one unfavorable genotype), with patients in RPA 4 had the highest risk of distant failure (Fig. 2). Multivariate analysis, adjusted for gender, age, T classification and treatment confirmed that higher RPA group conferred an increased distant metastatic risk (HR = 1.783; 95% CI = 1.364–2.331; P < 0.001).
In fact, besides RPA model and N category, clinical stage was another significant prognostic factor for DMFS as calculated by MVA, adjusted for gender, age and treatment (HR = 1.438; 95% CI = 1.042–1.982; P = 0.027). In order to compare the performance of the RPA model and both N category and clinical stage, the AIC and c-index were introduced in this study. As detailed in Table 5, in comparison with the N category and clinical stage, the RPA model presented with the lowest AIC and the highest c-index. This suggested that the RPA model was the best model in predicting the risk of distant failure, when compared with N category and clinical stage.
Discussion
There is a growing realization that genetic polymorphisms not only influence the development of cancer, but also the progression of cancer and prognosis27. Better understanding of the influence of genetic variations on the clinical outcome of patients may in fact provide additional biomarkers for individualized treatment for NPC. In this study, we determined whether genetic variations in the PI3K/PTEN/AKT/mTOR pathway were associated with risk of distant failure in NPC patients. To our knowledge, this is the first report to apply a tagging SNP approach to evaluating the role of this pathway in clinical outcome for NPC.
We found that SNPs in AKT1: rs3803300 and AKT1: rs2494738 were strongly correlated to risk of distant failure. Patients carried at least one of these two unfavorable genotypes have significant lower DMFS than those had no unfavorable genotype, especially in N2-3 group. Furthermore, we performed RPA for DMFS to derive different prognostic groups that combined SNPs and N classification, which was the only predictor identified to be significant from MVA, especially in patients with N2-3 stage. That was RPA1 (low risk: N0-1, without unfavorable genotype), RPA2 (moderate risk: N0-1, with at least one unfavorable genotype), RPA3 (high risk: N2-3, without unfavorable genotype) and RPA4 (highest risk: N2-3, with at least one unfavorable genotype), with patients in RPA 4 had the highest risk of distant failure. This RPA group was found to be the most optimal model in predicting the risk of distant metastasis, when compared with N category and clinical stage. Our results suggesting that one’s genetic background would be an effective complementary for N classification to evaluate the risk of distant progression.
AKT1, which is the central node of PI3K signaling pathway, has been implicated in the regulation of angiogenesis and metastasis-both important processes in cancer development and progression28,29. Recent studies have identified that SNPs and their haplotypes of AKT1 were linked with AKT1 protein expression level and with apoptotic capacity30,31. The most interesting finding of current series was that two SNPs in this gene, AKT1:rs3803300 and AKT: rs2494738 alone or combined, were significantly associated with DMFS in NPC patients. The first SNP AKT1:rs3803300 is located in the 3′ untranslated region of AKT1 and may affect gene expression through changes in transcription factor-binding sites (i.e. RelA and YY1 detected in TRANSFAC programs), microRNA target sequences (i.e. miR-4270 predicted in http://www.bioguo.org/miRNASNP/), and/or splicing variants. Similar results for AKT1: rs3803300 have been observed in other cancers. In a Korea report initiated by Kim MJ et al.15, the impact of polymorphisms in the AKT1 gene on OS and disease-free survival(DFS) in 310 patients with surgically resected NSCLC were evaluated, and indicated that OS and DFS were significantly higher for patients with the AA genotype of AKT1: rs3803300 than those with GA/GG genotype. This result was then verified by the same Korea group in 814 NSCLC patients with pathologic stages I, II, or IIIA who underwent curative surgical resection32. Wang Y et al.8 from China also indicated that variant genotypes AG and GG of AKT1:rs3803300, especially the GG genotype, showed a strong association with higher Oral squamous cell carcinoma (OSCC) susceptibility than the wild-type genotype AA, but they did not find any association between OSCC progression and genotype distribution of this SNP.
As for AKT1: rs2494738, individuals who carrying at least one variant allele of this SNP had a better DMFS in our analysis. This SNP is located in the intron, when we detected it in http://snpinfo.niehs.nih.gov/, no any related function was found, this may suggested rs2494738 is not the functional variant but a surrogate marker for the underling genetic variation within that region on the genome. Additional studies will be required to identify the causative sequence variation and the mechanism(s) responsible for our observations. However, it is important to note that the AKT1: rs2494738 polymorphism was not found to be associated with prognosis in Caucasian patients with NSCLC and esophageal cancer11,13. Multiple explanations may underlie this disparity, including ethnicity and different cancer types.
The importance of these two SNPs in determining distant metastasis risk was further supported in a survival tree analysis. We indicated that gene polymorphisms had joint effect with anatomic factor in stratifying patients into different prognostic groups. The present data confirm that N classification is the primary factor that contributing to variation in different risk of distant failure, with this factor demonstrated to be the initial split on the survival tree. We found that the RPA dichotomized the N category into N0-1 versus N2-3. Unfavorable genotype, as has been showed above, permit additional discrimination in the model instead of using N category again and T category. This may implicate that once individuals were defined as N2-3, genetic variation would become the most important factor that influence their ability of distant metastasis. We found that patients in RPA4 (N2-3 with at least one unfavorable genotype) had significantly poorer DMFS rate than that in RPA3 (N2-3 without unfavorable genotype) (62.7% vs. 81.2%), even though it had fewer N3 patients than RPA3(22.0% vs. 32.9%). This observation highlighted the important role of genetic variation in modulating distant metastasis in NPC patients, which may provide additional biomarkers for individualized treatment.
Our results suggest that another SNP rs1130214 in AKT1 may modulate the invasion activity, as patients carrying at least one variant allele of rs1130214 in AKT1 had a clear trend toward higher risk of distant metastasis. This SNP is located in the 5′UTR of AKT1, and has been reported to be associated with the expression of PDK133, which is the phosphoinositide-dependent kinases responsible for phosphorylating AKT, resulting in AKT activation34. Similar influence of the SNP in clinical outcome has been reported in other cancers, including NSCLC (Chinese and Koreans), esophageal cancer (90% Caucasian) and prostate cancer (90% Caucasian)13,15,35,36. However, another NSCLC study conducted by Xia P et al.11 from USA observed a conflicting result in non-Hispanic Caucasian patients, they found that AKT1: rs1121304 resulting in significantly decreased risks of distant progression in patients carrying at least one variant allele. Racial difference maybe the primary reason responsible for the variation, different cancer types and sample sizes may contribute to this variation as well.
AKT2 is another pivotal player in the PI3K pathway. The current series found that rs892119 in AKT2 exhibited significant associations with the hazard of distant metastasis, patients who had at least one variant allele had significant reduction of distant failure. In accordance to our study, Hildebrandt MA et al.13 indicated that the rs892119 A allele was significantly associated with reduced risk of both recurrence and death, and was also found to be associated with a poorer response to therapy. However, in Wang LE et al.’s study37, the A allele was found to be linked with increase risk of death in endometrial cancer in Caucasian population.
mTOR is another critical regulator of the PI3K pathway, which was reported to be involved in several cellular processes, including carcinogenesis, proliferation, angiogenesis and metabolism38. The current study found that patients with the CT/CC genotype of mTOR: rs11121704 tended to have higher risk of distant failure than those with TT genotype. However, an American study conducted in Caucasians indicated that the TT genotype of rs11121704 was associated with poor survival and resistance to chemotherapy13. Ethnicity difference was considered as the main reason for this phenomenon, since this study exhibited an entirely different genotype distribution, higher proportion of TT genotype was noticed in our series based on Chinese Han population (85.1%) than the American study mainly based on Caucasian (8.2%). Different sample sizes and cancer types may be another issues that resulted in variant outcomes.
Several limitations should be addressed in our series. First of all, the retrospective nature of the current study certainly served as an inherited and fundamental pitfall. Secondly, although the five genes included in this analysis were the core functional components of the pathway, this pathway is complex, with several other genes warranting investigation, such as PDK1, PDK2, TSC1, and TSC2, as have been mentioned in other literatures13,14,16. This may contribute to additional variation in clinical outcome, especially in combination with genetically altered AKT. Moreover, our analysis was based on single institution and only in Chinese Han population, additional studies in other ethnicities and institutions are required to confirm our findings. Lastly, since the variants genotyped in this study were tagging SNPs, we are unable to identify all the causative SNP and mechanism responsible, future studies are clearly warranted in this regard.
In conclusion, polymorphisms in the PI3K/PTEN/AKT/mTOR pathway were found to be independent prognostic markers for NPC patients, especially in N2-3 patients. Consequently, in addition to anatomic factors, testing for the presence of these polymorphisms may help with identifying patient subgroups at high risk of distant failure. If the findings from the current study could be validated prospectively in multicenter and diverse ethnic populations, these results, in combination with clinical-pathologic data, could become the basis for selecting patient subgroups at high risk of distant metastasis, thereby helping to refine therapeutic decisions in the treatment of NPC.
Additional Information
How to cite this article: Guo, Q. et al. Genetic variations in the PI3K-PTEN-AKT-mTOR pathway are associated with distant metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Sci. Rep. 6, 37576; doi: 10.1038/srep37576 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wei, W. I. & Sham, J. S. Nasopharyngeal carcinoma. Lancet 365, 2041–2054 (2005).
Lee, A. W., Ma, B. B., Ng, W. T. & Chan, A. T. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J Clin Oncol 33, 3356–3364 (2015).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9, 550–562 (2009).
Chen, J. Roles of the PI3K/Akt pathway in Epstein-Barr virus-induced cancers and therapeutic implications. World J Virol 1, 154–161 (2012).
Pfisterer, K. et al. PI3K/PTEN/AKT/mTOR polymorphisms: association with clinical outcome in patients with head and neck squamous cell carcinoma receiving cetuximab-docetaxel. Head Neck 37, 471–478 (2015).
Guo, L. et al. Genetic variations in the PI3K/AKT pathway predict platinum-based neoadjuvant chemotherapeutic sensitivity in squamous cervical cancer. Life Sci 143, 217–224 (2015).
Tang, Y. et al. Genetic variants in PI3K/AKT pathway are associated with severe radiation pneumonitis in lung cancer patients treated with radiation therapy. Cancer Med 5, 24–32 (2016).
Wang, Y. et al. Genetic variants in AKT1 gene were associated with risk and survival of OSCC in Chinese Han Population. J Oral Pathol Med 44, 45–50 (2015).
Wang, X. et al. A GG allele of 3′-side AKT1 SNP is associated with decreased AKT1 activation and better prognosis of gastric cancer. J Cancer Res Clin Oncol 140, 1399–1411 (2014).
Li, Q. et al. Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clin Cancer Res 19, 6252–6260 (2013).
Pu, X. et al. PI3K/PTEN/AKT/mTOR pathway genetic variation predicts toxicity and distant progression in lung cancer patients receiving platinum-based chemotherapy. Lung Cancer 71, 82–88 (2011).
Chen, M. et al. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis 31, 1387–1391 (2010).
Hildebrandt, M. A. et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J Clin Oncol 27, 857–871 (2009).
Hildebrandt, M. A. et al. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict head and neck cancer patient second primary tumor/recurrence risk and response to retinoid chemoprevention. Clin Cancer Res 18, 3705–3713 (2012).
Kim, M. J. et al. AKT1 polymorphisms and survival of early stage non-small cell lung cancer. J Surg Oncol 105, 167–174 (2012).
Xu, J. L. et al. Genetic variants in the PI3K/PTEN/AKT/mTOR pathway predict platinum-based chemotherapy response of advanced non-small cell lung cancers in a Chinese population. Asian Pac J Cancer Prev 13, 2157–2162 (2012).
Lin, L., Zhang, Z., Zhang, W., Wang, L. & Wang, J. Roles of genetic variants in the PI3K/PTEN pathways in susceptibility to colorectal carcinoma and clinical outcomes treated with FOLFOX regimen. Int J Clin Exp Pathol 8, 13314–13322 (2015).
Morrison, J. A., Gulley, M. L., Pathmanathan, R. & Raab-Traub, N. Differential signaling pathways are activated in the Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res 64, 5251–5260 (2004).
Shair, K. H., Schnegg, C. I. & Raab-Traub, N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res 68, 6997–7005 (2008).
Cai, L. M. et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene 34, 2156–2166 (2015).
Cai, L. et al. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat Commun 6, 7353 (2015).
Jiang, H. et al. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep 32, 559–566 (2014).
Lin, S. et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 75, 1071–1078 (2009).
A haplotype map of the human genome. Nature 437, 1299–1320 (2005).
H, A. A new look at the statistical model identification. IEEE Trans Automat Contr 19, 716–723 (1974).
Harrell, F. E., Jr., Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. Jama 247, 2543–2546 (1982).
Loktionov, A. Common gene polymorphisms, cancer progression and prognosis. Cancer Lett 208, 1–33 (2004).
Dimmeler, S. et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (1999).
Grille, S. J. et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res 63, 2172–2178 (2003).
Harris, S. L. et al. Detection of functional single-nucleotide polymorphisms that affect apoptosis. Proc Natl Acad Sci USA 102, 16297–16302 (2005).
Emamian, E. S., Hall, D., Birnbaum, M. J., Karayiorgou, M. & Gogos, J. A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36, 131–137 (2004).
Lee, S. Y. et al. A Panel of Genetic Polymorphism for the Prediction of Prognosis in Patients with Early Stage Non-Small Cell Lung Cancer after Surgical Resection. PLoS One 10, e0140216 (2015).
Slattery, M. L., Lundgreen, A., Mullany, L. E., Penney, R. B. & Wolff, R. K. Influence of CHIEF pathway genes on gene expression: a pathway approach to functionality. Int J Mol Epidemiol Genet 5, 100–111 (2014).
Alessi, D. R. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7, 261–269 (1997).
Zhang, X. et al. Polymorphisms in epidermal growth factor receptor (EGFR) and AKT1 as possible predictors of clinical outcome in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Tumour Biol 37, 1061–1069 (2016).
Kwon, E. M. et al. Genetic polymorphisms in inflammation pathway genes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 20, 923–933 (2011).
Wang, L. E. et al. Roles of genetic variants in the PI3K and RAS/RAF pathways in susceptibility to endometrial cancer and clinical outcomes. J Cancer Res Clin Oncol 138, 377–385 (2012).
Strimpakos, A. S., Karapanagiotou, E. M., Saif, M. W. & Syrigos, K. N. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev 35, 148–159 (2009).
Acknowledgements
This work was sponsored by National Clinical Key Specialty Construction Program and Key Clinical Specialty Discipline Construction Program of Fujian, People’s Republic of China. This research is also supported by grants from the National Natural Science Foundation of China (grant No. 81341108 and No. 81470134), grant from the Fujian Provincial Natural Science Foundation of China (grant No. 2014J01406), grant from the Science and technology project of Fujian Province (grant No. 2015Y0051) and grant from the Fujian Provincial Health and Family Planning Commission (grant No. 2012-1-6). In addition, we wish to thank all of the patients for their blood, Dr. Minhua Guo for her work on sorting out blood specimens, Dr. Zhuhong Chen and Dr. Shenghua Zhan for their work on patients’ following up.
Author information
Authors and Affiliations
Contributions
Y.X., J.P. and Q.G. designed the research; Q.G. and L.T. performed the research; T.L., Y.C., Y.S., Y.Z., Z.C. and C.C. contributed the reagents/analytic tools; L.T. analyzed the data and performed patients’ following-up; L.S. contributed quality control of enrolled patients; Q.G. and L.T. wrote the manuscript. All the authors read and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, Q., Lu, T., Chen, Y. et al. Genetic variations in the PI3K-PTEN-AKT-mTOR pathway are associated with distant metastasis in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Sci Rep 6, 37576 (2016). https://doi.org/10.1038/srep37576
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37576
This article is cited by
-
ER resident protein 44 promotes malignant phenotype in nasopharyngeal carcinoma through the interaction with ATP citrate lyase
Journal of Translational Medicine (2021)
-
Targeting the signaling in Epstein–Barr virus-associated diseases: mechanism, regulation, and clinical study
Signal Transduction and Targeted Therapy (2021)
-
RETRACTED ARTICLE: MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2
Journal of Experimental & Clinical Cancer Research (2019)
-
Combining CDKN1A gene expression and genome-wide SNPs in a twin cohort to gain insight into the heritability of individual radiosensitivity
Functional & Integrative Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.