Abstract
Rapid and adequate mucosal healing is important for a remission of ulcerative colitis (UC) patients. Here, we examined whether Spred2, a member of the Sprouty-related EVH1-domain-containing proteins that inhibit the Ras/Raf/ERK pathway, plays a role in colonic mucosal homeostasis and inflammation by using Spred2 knockout (KO) mice. We first detected increased epithelial cell proliferation and cadherin 1 expression in the colon of naïve Spred2 KO mice compared to wild-type mice. Interestingly, Spred2 KO mice were resistant to dextran sulfate sodium (DSS)-induced acute colitis as indicated by lower levels of body weight loss and disease activity index. Histologically, epithelial cell injury and inflammation were milder in the colonic mucosa of Spred2 KO mice on day 3 and almost undetectable by day 8. Experiments with bone chimeric mice indicated that Spred2-deficiency in non-hematopoietic cells was responsible for the reduced sensitivity to DSS. Finally, Spred2 KO mice developed significantly fewer tumors in response to azoxymethane plus DSS. Taken together, our results demonstrate, for the first time, that Spred2 plays an important role in the regulation of colonic epithelial cell proliferation and inflammation by potentially down-regulating the activation of ERK. Thus, Spred2 may be a new therapeutic target for the treatment of UC.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC), one of the two major forms of the inflammatory bowel disease (IBD), is a chronic disease characterized by strong inflammatory responses and ulceration in the colon mucosa1. The incidence and prevalence of UC are increasing with time in many regions around the world2. The etiology of UC is believed to involve inappropriate host responses to the complex commensal microbial flora in the gut3,4,5. Intestinal barrier dysfunction is a main feature of UC6,7. Clinically, rapid and adequate mucosal healing after treatment is an important indicator for a long-term remission in UC patients and a goal of treatments8,9,10. However, achieving mucosal healing is difficult in some patients despite the latest advance in the treatments.
The intestinal epithelium is a single cell layer that forms the only barrier between the host and the luminal gastrointestinal contents. Maintenance of this cell layer, which turns over in every 3–5 days, is dependent on the tight regulation of cell proliferation and apoptosis11,12,13. The maintenance of intestinal homeostasis is regulated by the coordinated activation of signaling molecules. Various molecules, such as growth factors, inflammatory cytokines and toll-like receptor ligands, stimulate intestinal epithelial cell growth, proliferation, and differentiation14,15,16,17,18 via binding to their cognate transmembrane receptors. Thus, identifying factors that affect mucosal healing can greatly contribute to the advance of UC treatment.
Receptor tyrosine kinases transmit signals that regulate multiple cellular processes, including proliferation, differentiation, migration, survival and metabolism19,20. Activated receptors utilize signaling pathways, such as the Ras/Raf/ERK (extracellular signal-regulated kinase), the PI3K (phosphoinositide 3-kinase)-Akt, and the Signal Transducer and Activator of Transcription (STAT) pathways21,22,23. Epidermal growth factor (EGF) is one of the major growth-stimulating factors of intestinal epithelial cells24,25,26. A randomized, double-blind clinical trial of EGF enemas using patients with active left-sided UC indicated that EGF enemas were effective and caused no colonic dysplasia27. Hepatocyte growth factor (HGF) is also a major growth-stimulating factor28. Intraperitoneal administration of HGF facilitated colonic mucosal repair in a rat experimental colitis model29. EGF and HGF bind to their cognate receptor EGFR and c-MET, respectively30,31. A crosstalk between EGFR and c-MET can enhance epithelial cell proliferation and wound healing through the Ras/Raf/ERK and the PI3K-Akt pathways32,33,34.
Sprouty-related enabled/vasodilator-stimulated phosphoprotein homology 1 domain-containing protein (Spred)1, 2 and 3 are a family of proteins containing a cysteine-rich domain related to Sprouty of Drosophila at the carboxy terminus and inhibit, similar to Sprouty, the activation of MAP kinase by suppressing the phosphorylation and activation of Raf 35,36. Spred1 and Spred3 are preferentially expressed in the brain and cerebellum, whereas Spred2 is ubiquitously expressed in various tissues, including the colon37,38. Interestingly, mutations in the Spred1 gene are implicated in the pathogenesis of Legius syndrome, an autosomal dominant human disorder that resembles neurofibromatosis-139 and pediatric acute myeloblastic leukemia40. The results of genome-wide association studies suggest the Spred2 gene as a candidate gene on IBD susceptibility loci41; however, the physiological role of Spred2 remains unknown. Here, we demonstrate, for the first time, that Spred2 plays an important role in the regulation of mucosal epithelial cell homeostasis and inflammation in the colon.
Results
The proliferation of colonic epithelial cells and the expression of the cadherin 1 gene are increased in the colon of naïve Spred2 knockout (KO) mice
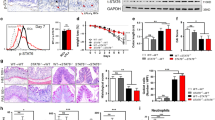
To examine the role of Spred2 in the colonic epithelial cell homeostasis and inflammation, we first examined the colon of naïve wild-type (WT) and Spred2 KO mice. Histologically, there was no notable difference between the colon of naïve WT and Spred2 KO mice by H&E staining, including the crypt height (Fig. 1a). However, interestingly, the incorporation of 5-Bromo-2′-deoxyuridine (BrdU) was significantly higher in the epithelial cells of Spred2 KO mice than those of WT mice (Fig. 1b,c), suggesting that Spred2 down-regulates the proliferation of colonic epithelial cells in vivo.
The incorporation of BrdU in the colon of naïve WT and Spred2 KO mice.
Colons were harvested from naïve WT or Spred2 KO mice 2 hours after intra-peritoneal injection of BrdU (50 μg/g body weight). (a) Photos of H&E staining of the distal portion of colon tissues from untreated WT or Spred2 KO mice are shown. (b) The incorporation of BrdU was examined by immunohistochemistry. Representative results from four animals are shown (original scale bar, 100 μm). (c) The incorporation of BrdU in colonic epithelial cells of WT and Spred2 KO mice was quantitated and presented as BrdU labeling index. Data is presented as the mean ± SEM. ***p < 0.0001. n = 4.
We next evaluated the effect of Spred2-deficiency on the integrity of the epithelial barrier functions by examining the expression of nine genes whose products are involved in tight junction formation, including the claudin (Cldn) 1, 2 and 4, mucin (Muc) 1 and 2, occludin (Ocln), trefoil factor 3 (Tff3), cadherin (Cdh) 1 and tight junction protein (Tjp) 1 gene14,42,43,44, by qRT-PCR. Among the genes, the expression of Cdh1, but not others, was significantly higher in the colon of naïve Spred2 KO mice than that of naïve WT mice (Fig. 2), suggesting that Spred2 may regulate the epithelial barrier functions in mice.
The expression of genes associated with mucosal barrier functions.
The expression of nine genes, including Cldn 1,2 and 4, Muc1 and 2, Ocln, Cdh 1, Tff3 and Tjp1 genes, in the colon of naïve WT or Spred2 KO mice was examined by qRT-PCR. Data is presented as the mean ± SEM. n = 4 for Muc2, n = 6 for the other 8 genes.
Spred2 KO mice are resistant to dextran sulfate sodium (DSS)-induced acute colitis
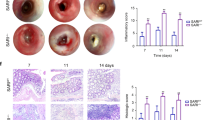
DSS-induced colitis is one of the most frequently used rodent models of UC45,46. It is widely accepted that DSS is toxic to colonic epithelial cells and DSS-induced breakdown of mucosal epithelial barrier function allows the entry of luminal antigens and microorganisms into the mucosa resulting in overwhelming inflammatory response47. To evaluate the role of Spred2 in colonic inflammation and tissue repair, WT and Spred2 KO mice were given 2% DSS in drinking water ad libitum, followed by water. As shown in Fig. 3a, WT mice began to lose weight on day 4, continued to lose weight until day 7, and slowly recovered thereafter. Disease activity index (DAI) also increased on day 4, peaked on day 5 and 6 and then gradually returned to the original level (Fig. 3b). In contrast to WT mice, Spred2 KO mice showed only a minor body weight loss and a minor increase in DAI (Fig. 3a,b), suggesting that Spred2 KO mice are resistant to DSS-induced tissue injury.
Spred2 KO mice are protected from DSS-induced acute colitis.
WT and Spred2 KO mice were given 2% of DSS in drinking water for 5 days, followed by regular water. Changes in body weight (a) and DAI (b) were examined. Data is presented as the mean ± SEM. **p < 0.01. n = 15. (c) Colons were harvested from mice on day 3 or 8 and processed for H&E staining. Representative results from five mice are shown (original scale bar, 100 μm). (d) The infiltration of Ly6G-positive cells (neutrophils) on day 6 was evaluated by immunohistochemistry. The number of positive cells was counted and presented as the mean ± SEM per HPF. n = 3 for WT and n = 4 for Spred2 KO mice.
Histologically, a significant level of epithelial cell damage and inflammatory cell infiltration was detected in the distal region of WT colon on day 3 and they became more severe by day 8 by H&E staining. In contrast, the colon of Spred2 KO mice showed only a low level of epithelial cell damage and inflammatory response on day 3 and returned to almost normal by day 8 (Fig. 3c). The infiltration of Ly6G-positive cells, most likely neutrophils, was markedly lower in the colon of Spred2 KO mice (Fig. 3d). Thus, Spred2 KO mice are resistant to DSS-induced tissue injury and inflammation.
Absence of Spred2 in non-hematopoietic cells attenuated DSS-induced colitis
As described above, Spred2 is ubiquitously expressed in various tissues37,38. To determine whether hematopoietic or non-hematopoietic Spred2 is associated with the decreased DSS sensitivity, we generated bone marrow (BM) chimeric mice. As shown in Fig. 4a and b, Spred2 KO mice which received Spred2 KO BM cells (KO BM → KO) lost less body weight than WT mice which received WT BM cells (WT BM → WT), and showed a lower DAI, consistent with our results with non-chimeric mice presented in Fig. 3. Transplantation of Spred2 KO BM cells into WT mice (KO BM → WT) or WT BM cells into Spred2 KO mice (WT BM → KO) did not alter the body weight loss or DAI observed in WT or Spred2 KO mice. By H&E staining, the presence of severe inflammatory responses and tissue damage were detected in WT mice that received WT BM cells or Spred2 KO BM cells but not in Spred2 KO mice that received WT BM cells or Spred2 KO BM cells on day 13 (Fig. 4c). These results indicated that the loss of Spred2 in non-hematopoietic cells, but not in hematopoietic cells, was responsible for the attenuated DSS-induced colitis detected in systemic Spred2 KO mice.
DSS-induced acute colitis in BM chimeric mice.
BM chimeric mice between WT and Spred2 KO mice were produced as described in the M&M section. Mice were given 2% DSS in drinking water for 5 days, followed by regular water. (a) Changes in body weight were examined. Data is presented as the mean ± SEM. *p < 0.05, **p < 0.01. WT BM → WT vs WT BM → Spred2 KO. #p < 0.05, **p < 0.01. Spred2 KO BM → WT vs Spred2 KO BM → Spred2 KO (n = 4). The cross indicates death to a mouse. (b) Disease activity index (DAI) was calculated as described in the M&M section. Data is presented as the mean ± SEM. *p < 0.05, **p < 0.01. WT BM → WT vs WT BM → Spred2 KO. #p < 0.05, ##p < 0.01. Spred2 KO BM → WT vs Spred2 KO BM → Spred2 KO (n = 4). The cross indicates death to a mouse. (c) Colons were harvested on day 13 and processed for H&E staining. Representative results from four animals are shown (original scale bar, 100 μm).
Spred2 KO mice develop significantly fewer tumors than WT mice in an azoxymethane (AOM)/DSS model
The AOM/DSS-induced colon cancer model is one of the most frequently used rodent colitis-associated cancer model48. To examine the role of Spred2 in the development of colitis-associated colon cancer, mice were treated with AOM, followed by three cycles of 2% DSS treatment and recovery (Fig. 5a). Sixty days after AOM injection, all mice were euthanized and the length of each colon and the number of tumors were examined. The length of colons of WT mice was significantly shorter than those of Spred2 KO mice (Fig. 5b). Furthermore, WT mice developed significantly higher number of tumors than Spred2 KO mice (7.5 vs 4.2 per colon) (Fig. 5c,d). The growth of epithelial cells was more prominent in tumors of WT mice than those of Spred2 KO mice (Fig. 5e) and tumors in the colon of WT mice appear to be larger than those of Spred2 KO mice. These results are likely due to more severe inflammatory responses in the colon of WT mice than Spred2 KO mice.
Tumor formation in WT and Spred2 KO mouse colon after treatment with AOM plus DSS.
(a) The experimental scheme of the colitis-associated cancer model used in the study. (b) Colons were harvested on day 60 from WT and Spred2 KO mice and colon length was measured. Data is presented as the mean ± SEM (n = 12 for WT mice and n = 13 for Spred2 KO mice). (c) The number of tumors whose diameter was more than 1 mm was counted. Data is presented as the mean ± SEM (n = 12 for WT mice and n = 13 for Spred2 KO mice). (d) Representative photos of tumors that arose in WT or Spred2 KO mice. (e) Photos of typical tumors developed in WT or Spred2 KO mice. H&E staining. The original scale bar was 200 μm.
Discussion
Under normal circumstances, IECs are constantly shed from the tips of villi after cell death. To maintain intestinal homeostasis, controlled proliferation of IECs is required to prevent loss of epithelial barrier function10. In this study, we examined a biological role of Spred2 in colonic epithelial homeostasis and inflammation using Spred2 KO mice. We found that the proliferation of epithelial cells and the expression of the Cdh1 gene were significantly increased in the colon of Spred2 KO mice compared to WT mice in a naïve state. Interestingly, Spred2 KO mice were less sensitive to DSS-induced acute colitis than WT mice and developed a fewer number of tumors in a chemically induced colon cancer level. Thus, we, for the first time, identified Spred2 as an important molecule that regulates the proliferation of colon epithelial cells and inflammation.
Previous studies have shown that the activation of the Ras/Raf/ERK pathway enhances colonic mucosal repair. Heparin-binding epidermal growth factor-like growth factor enhanced intestinal restitution after intestinal ischemia/reperfusion via PI3K/Akt and Ras/Raf/ERK activation49. Raf was shown to protect the mouse colon against epithelial injury and inflammation, and promote recovery from acute DSS-induced colitis by both Ras/Raf/ERK-dependent and -independent pathways50. Rebamipide, an amino acid derivative of 2(1 H)-quinoline used as a gastric mucosal protective and ulcer-healing agent, enhanced the migration of intestinal epithelial cells via Ras/Raf/ERK activation in a rat acute colitis model induced with trinitrobenzene sulfonic acid51. Oral administration of insulin stimulated intestinal epithelial cell turnover following massive small bowel resection in a rat and a cell culture model via PI3K/Akt and Ras/Raf/ERK activation52. Inhibition of Ras/Raf/ERK by U0126 significantly decreased cell proliferation and migration of rat intestinal epithelial cell line IEC-6 and worsened intestinal ischemia/reperfusion injury53. As described above, Spred proteins are negative feedback regulators of the ERK/MAPK pathway35, and Spred2 had the capacity to suppress VEGFR-3-mediated ERK activation in VEGFR-3-transfected 293 T cells54. Thus, Spred2 may play an important role in the proliferation and migration of colonic epithelial cells by regulating the activation of ERK.
In the present study, we demonstrated that Spred2 KO mice were resistant to DSS-induced acute colitis; however, it remains unclear how Spred2-deficincy results in the resistance to DSS-induced colitis. The incorporation of BrdU in the colonic epithelia and the expression of the Cdh1 gene in the colon tissue were significantly increased in Spred2 KO mice compared to WT mice, suggesting that increased epithelial restitution and barrier functions in Spred2 mice may account for our observation. The Raf/MEK/ERK pathway also regulates cell survival by controlling the activity or abundance of the members of the Bcl-protein family to promote cell survival55. Therefore, Spred2-deficiency might increase the survival of colonic epithelial cells, which could lead to the resistance to DSS-induced acute colitis.
The Ras/Raf/ERK pathway is shown to influence not only epithelial cells but also immune cells, such as T cells and macrophages. Pharmacologic inhibition of Ras/Raf/ERK was previously shown to enhance in vitro Th17 differentiation and increase the gene expression of il-17a, il-17f, il-21, il-22, and il-23r56. Th17 cells are now known to regulate the pathogenic mechanism of inflammatory bowel disease57,58. Intestinal microbiota also plays a critical role in the regulation of intestinal epithelial cell turnover and promotion of epithelial restitution43. Particularly, in DSS-induced colitis, bacteria that translocated to the mucosa interact with mucosal cells, such as macrophages, via toll-like receptors and promote the production of pro-inflammatory cytokines, resulting in overwhelming inflammatory responses48. We previously reported that Spred2-deficiency increased the production of pro-inflammatory cytokines by LPS-activated alveolar macrophages, and exacerbated LPS-induced lung injury59. In this study, however, the inflammatory response in the colonic mucosa of Spred2 mice was milder than WT mice, and only a small number of leukocytes infiltrated the mucosa of Spred2 KO mice; thus, the effect of Spred2-deficiency on macrophages cannot explain the decreased inflammatory responses detected in the colon of Spred2 KO mice. Furthermore, Spred2-deficiency in non-hematopoietic cells, but not hematopoietic cell, was responsible for the DSS-resistance in this study; therefore, Spred2 expressed in non-hematopoietic cells, such as epithelial cells, must be playing a critical role. Additional studies are necessary to identify the exact mechanism whereby Spred2-deficiency protects the colon from DSS-induced insults.
The Ras/Raf/ERK and the PI3K/Akt pathway also influence colon cancer growth60, suggesting that Spred2-deficiency may increase tumor development. However, Spred2 KO mice developed significantly fewer tumors than WT mice. Severity of colon inflammation is a risk factor for colorectal neoplasia in ulcerative colitis61,62. HGF ameliorates mucosal injuries leading to inhibition of colon cancer development in mice63. These reports suggest that chronic mucosal inflammation promotes colitis associated colon cancer growth. In Spred2 KO mice, the degree of the inflammatory response after DSS-treatment was clearly less severe than WT mice, providing the reason why fewer tumors developed in Spred2 KO mice in response to AOM/DSS-treatment.
To analyze the role of Spred2 expressed in colonic epithelial cells, we knocked down Spred2 expression in the human Caco-2 colon cancer cell line using siRNA and evaluated its effects in vitro. Interestingly, we detected a significant increase in the cell proliferation, migration and ERK phosphorylation in siRNA-transfected Caco-2 cells compared to non-transfected cells. However, the increase was very modest despite that the expression of Spred2 mRNA expression was reduced by 80%. Unexpectedly, about 50% of Spred2 protein still remained in the siRNA-transfected cells, potentially resulting in only small changes (Supplementary Figure 1 and 2). Thus, it will be necessary to use Spred2 knockout cells to obtain more definitive answers as to whether Spred2 regulates the proliferation and migration of colonic epithelial cells via the Ras/Raf/ERK pathway.
Rapid and adequate mucosal healing is one of the important prognostic factors for the long-term remission in UC patients. Our results obtained using Spred2 KO mice suggest that inhibition of Spred2 may be useful to prevent severe colitis and subsequent colon cancer; thus, Spred2 may be a new therapeutic target for the treatment of UC. Further investigation is needed to elucidate the mechanisms whereby Spred2 plays a role in colonic homeostasis, inflammatory responses and carcinogenesis.
Methods
Mice
The production of Spred2 KO mice was previously reported54,64. C57BL/6J mice were used as control. These mice were bred at the Department of Animal Resources, Okayama University, Okayama, Japan. Seven to 9-weeks old male mice were used in this study under a specific pathogen-free condition. Mice (5 per cage) were housed in a temperature-controlled environment and allowed free access to water and food. All animal protocols were approved by the Animal Care and Use Committee of the Okayama University, and all experiments were performed in accordance with relevant guidelines and regulations.
DSS-induced colitis
DSS (MW 36,000–50,000) was purchased from MP Biomedicals (Santa Ana, CA, USA). Spred2 KO mice and WT mice were administered 2% DSS in drinking water ad libitum followed by tap water. Mice were monitored every day and DAI was calculated as described before65. The values were based on the weight loss percentage (0 = <1%, 1 = 1–4.99%, 2 = 5–10%, and 3 = >10%), bleeding (0 = none, 1 = small spots of blood in stool: dry anal region, 2 = large spots of blood in stool: blood appears through anal orifice, and 3 = deep red stool: blood spreads largely around the anus) and stool consistency (0 = normal stools, 1 = soft pellets not adhering to the anus, 2 = very soft pellets adhering to the anus, and 3 = liquid stool on long streams: wet anus). The average of these three values constituted the DAI. Mice were euthanized at the indicated time intervals and colons were removed for macroscopic inspection and histological analysis.
In vivo BrdU labeling
BrdU (Sigma-Aldrich) was dissolved in PBS at 5 mg/ml. Mice were injected with the BrdU solution intraperitoneally at 50 μg/g body weight two hours before sacrifice. Colons were resected and fixed overnight with 10% buffered formalin and embedded in paraffin. Four-micrometer sections were cut and incubated with 1.25 μg/ml of rat anti-BrdU antibody (GeneTex, Inc., San Antonio, TX) overnight at 4 °C, followed by incubation with Histofine Simple Stain Mouse MAX PO (Rat) (Nacalai Tesque, Kyoto, Japan) for 45 minutes. Antigen was visualized with 3, 3′-diaminobenzidine substrate (Sigma-Aldrich). BrdU-labeling index was calculated as the percentage of BrdU−positive cells in 20 well-oriented crypts in 10 high power fields per colon section.
Bone marrow chimeras
To make BM chimeras, recipient WT C57BL/6 mice and Spred2 KO mice (5–7 week old) were irradiated at 11 Gy (divided into two irradiations at an interval of 2 hours). Donor BM was isolated from femurs and tibias of WT and Spred2 KO mice, and 2 × 106 BM cells in 100 μl RPMI 1640 were injected into recipient mice via tail vein. The following BM chimeras were created (donor → host): WT → WT, Spred2 KO → WT, Spred2 KO → Spred2 KO, and WT → Spred2 KO. Six weeks after BM reconstitution, 2% DSS in drinking water was administered to induce colitis. Mice were euthanized on day 13 and colons were dissected for macroscopic inspection and histological analysis.
Experimental models of colitis-associated cancer
AOM (Sigma-Aldrich) was dissolved in PBS at 1 mg/ml. Mice were injected intraperitoneally with the AOM solution (12 mg/kg body weight) or vehicle (PBS). Five days after AOM injection, mice were treated by 3 cycles of 2% DSS in the drinking water for 5 days, followed by regular water for 16 days66. All mice were euthanized on day 60 and colons were removed for macroscopic inspection and histological analysis. The number of tumors in the size of 1 mm or larger in diameter was counted.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using the High Pure RNA Isolation Kit (Roche Diagnostics, Basel, Switzerland). cDNA was then synthesized using the High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA) and 1000 ng of total RNA. qRT-PCR was performed using the StepOne Plus Real-Time PCR system (Life Technologies). The expression of the Spred2, Cldn1, 2, 4, Muc2 and Gapdh gene was analyzed by Taqman gene expression assays (Applied Biosystems). The expression of the Muc1, Ocln, Cdh1, Tff3, Tjp1 and Gapdh gene was analyzed by PrimeTime Mini qPCR assay (Integrated DNA Technologies). The expression level of each gene was normalized to that of the Gapdh gene and presented as fold change over the expression of control gene.
Statistics
Each experiment was repeated at least twice. The two-tailed unpaired Student’s t-test and the One-way analysis of variance (ANOVA) and Tukey’s test were performed using the GraphPad Prism version 5.0b for Mac (San Diego, CA, USA). p-values smaller than 0.05 were considered significant.
Additional Information
How to cite this article: Takahashi, S. et al. A Novel Role of Spred2 in the Colonic Epithelial Cell Homeostasis and Inflammation. Sci. Rep. 6, 37531; doi: 10.1038/srep37531 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Engel, M. A. & Neurath, M. F. New pathophysiological insights and modern treatment of IBD. J Gastroenterol 45, 571–583 (2010).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42; quiz e30 (2012).
Danese, S. & Fiocchi, C. Ulcerative colitis. N Engl J Med 365, 1713–1725 (2011).
Danese, S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61, 918–932 (2012).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Salim, S. Y. & Soderholm, J. D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 17, 362–381 (2011).
McGuckin, M. A., Eri, R., Simms, L. A., Florin, T. H. & Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis 15, 100–113 (2009).
Rutgeerts, P., Vermeire, S. & Van Assche, G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut 56, 453–455 (2007).
Froslie, K. F., Jahnsen, J., Moum, B. A. & Vatn, M. H. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133, 412–422 (2007).
Neurath, M. F. & Travis, S. P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61, 1619–1635 (2012).
Gunther, C., Neumann, H., Neurath, M. F. & Becker, C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62, 1062–1071 (2013).
Moore, K. A. & Lemischka, I. R. Stem cells and their niches. Science 311, 1880–1885 (2006).
van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71, 241–260 (2009).
Sturm, A. & Dignass, A. U. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 14, 348–353 (2008).
Noah, T. K., Donahue, B. & Shroyer, N. F. Intestinal development and differentiation. Exp Cell Res 317, 2702–2710 (2011).
Iizuka, M. & Konno, S. Wound healing of intestinal epithelial cells. World J Gastroenterol 17, 2161–2171 (2011).
Krishnan, K., Arnone, B. & Buchman, A. Intestinal growth factors: potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm Bowel Dis 17, 410–422 (2011).
Gyires, K., Toth, V. E. & Zadori, Z. S. Gut inflammation: current update on pathophysiology, molecular mechanism and pharmacological treatment modalities. Curr Pharm Des (2013).
McKay, M. M. & Morrison, D. K. Integrating signals from RTKs to ERK/MAPK. Oncogene 26, 3113–3121 (2007).
Raman, M., Chen, W. & Cobb, M. H. Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 (2007).
Rane, S. G. & Reddy, E. P. Janus kinases: components of multiple signaling pathways. Oncogene 19, 5662–5679 (2000).
Porter, A. C. & Vaillancourt, R. R. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene 17, 1343–1352 (1998).
Anderson, N. G., Maller, J. L., Tonks, N. K. & Sturgill, T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343, 651–653 (1990).
Berlanga-Acosta, J., Playford, R. J., Mandir, N. & Goodlad, R. A. Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut 48, 803–807 (2001).
Suzuki, A., Sekiya, S., Gunshima, E., Fujii, S. & Taniguchi, H. EGF signaling activates proliferation and blocks apoptosis of mouse and human intestinal stem/progenitor cells in long-term monolayer cell culture. Lab Invest 90, 1425–1436 (2010).
Biteau, B. & Jasper, H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045–1055 (2011).
Sinha, A., Nightingale, J., West, K. P., Berlanga-Acosta, J. & Playford, R. J. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 349, 350–357 (2003).
Gohda, E. et al. Purification and partial characterization of hepatocyte growth factor from plasma of a patient with fulminant hepatic failure. J Clin Invest 81, 414–419 (1988).
Tahara, Y. et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther 307, 146–151 (2003).
Fischer, O. M., Hart, S. & Ullrich, A. Dissecting the epidermal growth factor receptor signal transactivation pathway. Methods Mol Biol 327, 85–97 (2006).
Bottaro, D. P. et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251, 802–804 (1991).
Accornero, P., Miretti, S., Cucuzza, L. S., Martignani, E. & Baratta, M. Epidermal growth factor and hepatocyte growth factor cooperate to enhance cell proliferation, scatter, and invasion in murine mammary epithelial cells. J Mol Endocrinol 44, 115–125 (2010).
Xu, K. P. & Yu, F. S. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci 48, 2242–2248 (2007).
Jiang, H., Grenley, M. O., Bravo, M. J., Blumhagen, R. Z. & Edgar, B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011).
Wakioka, T. et al. Spred is a Sprouty-related suppressor of Ras signalling. Nature 412, 647–651 (2001).
Yoshimura, A. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med 58, 73–83 (2009).
Engelhardt, C. M. et al. Expression and subcellular localization of Spred proteins in mouse and human tissues. Histochem Cell Biol 122, 527–538 (2004).
Kato, R. et al. Molecular cloning of mammalian Spred-3 which suppresses tyrosine kinase-mediated Erk activation. Biochem Biophys Res Commun 302, 767–772 (2003).
Brems, H. & Legius, E. Legius syndrome, an Update. Molecular pathology of mutations in SPRED1. Keio J. Med. 62, 107–112 (2013).
Pasmant, E. et al. SPRED1, a RAS MAPK pathway inhibitor that causes Legius syndrome, is a tumour suppressor downregulated in paediatric acute myeloblastic leukaemia. Oncogene 34, 631–638 (2015).
Van Limbergen, J., Radford-Smith, G. & Satsangi, J. Advances in IBD genetics. Nat. Rev. Gastroenterol. Hepatol. 11, 372–385 (2014).
Yu, L. C., Wang, J. T., Wei, S. C. & Ni, Y. H. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J. Gastrointest. Pathophysiol. 3, 27–43 (2012).
Oshima, T. & Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 51, 768–7789 (2016).
Petersson, J. et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 300, G327 (2011).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Kishimoto, S. et al. Changes of colonic vasoactive intestinal peptide and cholinergic activity in rats with chemical colitis. Dig Dis Sci 37, 1729–1737 (1992).
Perše, M. & Cerar, A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012, 718617 (2012).
Neufert, C., Becker, C. & Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protocols 2, 1998–2004 (2007).
El-Assal, O. N. & Besner, G. E. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129, 609–625 (2005).
Edelblum, K. L. et al. Raf protects against colitis by promoting mouse colon epithelial cell survival through NF-kappaB. Gastroenterology 135, 539–551 (2008).
Takagi, T. et al. Rebamipide promotes healing of colonic ulceration through enhanced epithelial restitution. World J Gastroenterol 17, 3802–3809 (2011).
Ben Lulu, S. et al. Oral insulin stimulates intestinal epithelial cell turnover following massive small bowel resection in a rat and a cell culture model. Pediatr Surg Int 28, 179–187 (2012).
Ban, K., Peng, Z. & Kozar, R. A. Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One 8, e76790 (2013).
Taniguchi, K. et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol 27, 4541–4550 (2007).
Balmanno, K. & Cook, S. J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 16, 368–377 (2009).
Tan, A. H. & Lam, K. P. Pharmacologic inhibition of MEK-ERK signaling enhances Th17 differentiation. J Immunol 184, 1849–1857 (2010).
Strober, W. & Fuss, I. J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1756–1767 (2011).
Kamada, N. et al. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn’s disease. Inflamm Bowel Dis 16, 568–575 (2010).
Xu, Y. et al. Spred-2 deficiency exacerbates lipopolysaccharide-induced acute lung inflammation in mice. PLoS One 9, e108914 (2014).
Efferth, T. Signal transduction pathways of the epidermal growth factor receptor in colorectal cancer and their inhibition by small molecules. Curr Med Chem 19, 5735–5744 (2012).
Rubin, D. T. et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 11, 1601–8.e1 (2013).
Rutter, M. et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126, 451–459 (2004).
Yamaji, N. et al. Hepatocyte growth factor ameliorates mucosal injuries leading to inhibition of colon cancer development in mice. Oncol Rep 26, 335–341 (2011).
Nobuhisa, I. et al. Spred-2 suppresses aorta-gonad-mesonephros hematopoiesis by inhibiting MAP kinase activation. J Exp Med 199, 737–742 (2004).
Gommeaux, J. et al. Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Mol Cell Biol 27, 2215–2228 (2007).
Popivanova, B. K. et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118, 560–570 (2008).
Acknowledgements
We thank Dr. Akihiko Yoshimura for providing Spred2 KO mice. We also thank Hiroyuki Watanabe, Yasuharu Arashima, Miwa Sato, Megumi Mino and Yuki Nakashima for their excellent technical assistance. This work was supported in part by JSPS KAKENHI Grant number 25293095.
Author information
Authors and Affiliations
Contributions
S.T., T.Y. and A.M. planned experiments and wrote the main manuscript text. S.T., T.O., M.F., S.F., T.I., J.I. performed experiments and discussed the experimental findings and interpretation of results. S.H., H.O. and K.Y. discussed the experimental findings and interpretation of results. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Takahashi, S., Yoshimura, T., Ohkura, T. et al. A Novel Role of Spred2 in the Colonic Epithelial Cell Homeostasis and Inflammation. Sci Rep 6, 37531 (2016). https://doi.org/10.1038/srep37531
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37531
This article is cited by
-
Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis
Chinese Journal of Integrative Medicine (2023)
-
Activation of G protein coupled estrogen receptor prevents chemotherapy-induced intestinal mucositis by inhibiting the DNA damage in crypt cell in an extracellular signal-regulated kinase 1- and 2- dependent manner
Cell Death & Disease (2021)
-
Spred2-deficiency enhances the proliferation of lung epithelial cells and alleviates pulmonary fibrosis induced by bleomycin
Scientific Reports (2020)
-
Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway
Journal of Experimental & Clinical Cancer Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.