Abstract
Culex pipiens is the mosquito that vectors West Nile Virus and other human-pathogenic flavivruses in North America. In response to shortened day length and lower temperatures, female Cx. pipiense prepares for the diapause by actively feeding on carbohydrates to increase the biosynthesis of glycogen and lipid to store energy for overwintering. The effect of feeding different carbohydrates on glycogen and lipid biosynthesis in diapausing mosquitoes was investigated in vivo using 13C solid-state NMR. Diapause-destined adult females and nondiapausing counterparts after adult eclosion were fed with three different carbohydrate sources for 7 days: 1) 10% sucrose, 2) 10% D-[13C6]glucose, and 3) 1% D-[13C6]glucose co-provisioned with 10% sucrose. NMR measurements show that sucrose and glucose are metabolized differently in diapausing mosquitoes. Mosquitoes fed on sucrose primarily accumulate glycogen with increased branching structures, but less of lipids. In contrast, mosquitoes fed exclusively on glucose show accumulation of both glycogen and lipid with increased aliphatic chain length. Glucose is exclusively metabolized for the biosynthesis of triacylglyceride when mosquitoes were co-fed with sucrose. Our findings provide novel insights into the insect carbohydrate metabolism that governs glycogen and lipid biosynthesis during diapause, which is fundamental for the insect survival during inimical environments.
Similar content being viewed by others
Introduction
Diapause is a survival strategy that enables insects to cope with unfavorable environmental conditions or resource limitations1. Like many other temperate insect species, mosquito Culex pipiens enters an overwintering adult diapause in response to environmental cues such as shortened day length and decreasing temperatures. The timing of diapause is critical, as all the energy required for overwintering must be stored for the survival prior to entering diapause. Thus in preparation for winter, diapause-programmed females cease to seek blood meals and instead feed on nectar and other sugar sources2,3. This increased sugar uptake is converted into glycogen and lipid for energy storage4,5,6. Hypertrophy of adipocytes and elevation of stored fat start occurring within a week after adult eclosion in diapausing female, until the lipid reserves are two-fold higher than those found in their nondiapausing counterparts7. Figure 1 shows sucrose fed diapausing female with the enlarged abdomen with large fat storage cells. During winter, stored glycogen is utilized before lipids in fat body cells, and any remaining surplus lipids after overwintering are utilized for the production of eggs.
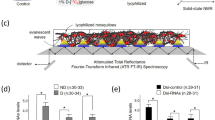
13C-Isotope labeling of Culex pipiens for solid-state NMR analysis.
(a) Female Cx. pipiens fed with 10% (10 g/100 mL) sucrose post 7-days adult eclosion in non-diapausing (left) and diapausing (right) states. Diapausing female post 7-days adult eclosion with a visibly enlarged abdomen (right). (b) Fluorescent microscope images of fat body cells. Fat body cells of diapausing female fed on 10% sucrose shows fat hypertrophy. Lipids in adipocytes were stained using BIODIPY 493/503 (green). Scale bars represent 50 μm. (c) 13C-isotope labeling scheme for solid-state NMR. Female Cx. pipiens after adult eclosion were fed for 7 days with 10% (m/m, 10 g/100 mL) unlabeled sucrose (natural abundance), 13C-isotope labeled 10% D-[13C6]glucose, or 10% sucrose (natural abundance) spiked with 1% D-[13C6]glucose. Intact whole organisms were lyophilized and packed into 7-mm zirconia rotor and spun at 5 kHz magic-angle spinning for 13C solid-state NMR analysis.
Since the uptake of carbohydrate is essential for the survival of diapausing mosquitos, different carbohydrate sources based on their habitats may contribute to variations in glycogen and lipid accumulation in diapausing mosquitoes8,9,10. The most common sugar source for mosquitoes is floral nectar, which consists primarily of three sugars: glucose, fructose, and sucrose11. Glucose and fructose are hexose monosaccharides, whereas sucrose is a non-reducible disaccharide of glucose and fructose (Fig. 1c). Relative amounts and concentrations of each carbohydrate can vary among species12. As these sugars are involved in signaling plant development13,14, the composition of carbohydrates in nectar can differ in response to the change in season and may pose a significant impact on diapausing mosquitoes. In response to temporal and dynamic changes in carbohydrate compositions of nectar, dipausing female mosquitoes are likely to have evolved to adopt a metabolic strategy that optimizes the utilization of most abundant carbohydrate for maximum nutrient (energy) storage, which increases the chance of survival for females through overwintering. Hence the underlying difference in metabolic requirements between males and females is reflected in their preference for different nectar sources they exhibit in the laboratory environment, where diapausing females tend to aggregate to flowers with nectar that are rich in monosaccharides (glucose and fructose)15 while males tend to favor flowers with high sucrose concentration nectar15,16. However, the effect of variation in the carbohydrate composition (glucose, fructose, and sucrose) of nectar on lipid and glycogen storage in diapausing mosquitoes has remained elusive17.
Here we investigate the effect of selective carbohydrate feeding by diapausing Cx. pipiens on glycogen and lipid accumulation. Diapause-destined adult females and their nondiapausing counterparts were 13C-isotope labeled (Fig. 1c) by feeding with three carbohydrate sources for 7 days after adult eclosion: 1) 10% (10 g/100 mL) sucrose (13C-natural abundance), 2) 10% D-[13C6]glucose, and 3) 1% D-[13C6]glucose co-provisioned with 10% sucrose. Accurate in situ quantifications of the glycogen and lipid accumulation in whole mosquitoes were performed by 13C solid-state NMR spectroscopy in order to determine effects of feeding different carbohydrates on glycogen and lipid accumulation. Our finding provides novel insights into the insect carbohydrate metabolism that governs glycogen and lipid biosynthesis during diapause, which is fundamental for the survival of insects in inimical conditions.
Results and Discussion
Adipocyte staining of diapausing Cx. pipiens
The short-day-reared diapausing Cx. pipiens females exhibit the typical enlarged abdomen compared to the long-day-reared nondiapausing females (Fig. 1a). The microscope image of BODIPY 493/503 stained adipocyte from the abdomen of diapausing female fed on 10% sucrose (10 g/100 mL) shows an increase in the total amount of lipids and number of lipid droplets found in fat bodies (Fig. 1b)7.
Sucrose metabolism in diapausing mosquitoes
Natural abundance 13C cross-polarization magic-angle spinning (CPMAS) spectra of nondiapausing (black) and diapausing (blue) female Cx. pipiens fed with 10% sucrose for 7-days post adult eclosion are superimposed and shown in Fig. 2c (bottom). Spectra are normalized to the 175-ppm intensity of peptidyl-carbonyl carbon of proteins and C7 of N-acetyl carbon in chitin (Fig. 2b)18. The general carbon chemical shift assignments are: i) carbonyl carbons at 170–180 ppm, ii) aromatic and ethylene carbons at 130 ppm, ii) anomeric carbons at 90–110 ppm, iii) O-alkyl carbons at 60–105 ppm, and iv) aliphatic carbons at 10–40 ppm. In diapausing mosquitoes, the O-alkyl carbon peak intensities at 61, 73, 82, 93, 99, and 104 ppm show a general increase. Chemical shift assignments for the O-alkyl carbons are shown in Fig. 2c where 61 ppm assigned to C6 of D-glucose (Fig. 2a) or N-acetylglucosamine (NAG) from chitin (Fig. 2b), and 73 ppm assigned to C2, C3, and C5 of D-glucose, or C3 and C5 of NAG. The C2 of NAG at 55 ppm is resolved from the C2 of D-glucose at 73 ppm, but overlaps with the Cα of proteins. The 82 ppm is assigned to C4 of both D-glucose and NAG19.
Natural abundance 13C-CPMAS spectra of female Cx. pipiens.
(a) Chemical structure of glycogen. Linearly polymerized D-glucoses are connected by α (1 → 4) glycosidic linkages (red) with a branch connection by an α(1 → 6) glycosidic bond (blue). (b) Chemical structure of chitin. (c) Bottom: 75-MHz natural abundance 13C-CPMAS spectra of female Cx. pipiens in nondiapausing (black) and diapausing (blue) states after exclusively being fed 10% (10 g/100 mL) sucrose for 7-days post eclosion. All spectra are normalized to 175-ppm peak of peptidyl-carbonyl carbons for proteins. O-alkyl carbons of glycogen are visible at 60–105 ppm, lipid peaks at 20–40 ppm, and aromatics and unsaturated carbons at 130 ppm. Diapausing mosquitoes show increased O-alkyl carbons at 72 and 61 ppm. The figure inset shows enlarged 90–110 ppm range of the spectrum with increased anomeric carbons in diapausing mosquitoes consistent with glycogen accumulation. Diapausing mosquitoes show an increase in 98-ppm intensity corresponding to C1 carbon in α(1 → 6) glycosidic linkage. These changes indicate glycogen accumulation accompanied with an increase in branching structure. Top: Difference spectrum by the subtraction of 13C-CPMAS spectrum of nondiapusing from diapausing mosquitoes. Diapausing mosquitoes show only a small increase in lipid (CH2 at 30 ppm) in contrast with large glycogen accumulation (60–105 ppm).
Chemical shift of the anomeric C1 carbon of D-glucose (Fig. 2c inset) is sensitive for different types of glycosidic linkages: i) from reducible sugars of monosaccharaides at 93 ppm, ii) branched D-glucose with α(1 → 6) linkage at 98 ppm, and iii) D-glucose connected by α(1 → 4) and β(1 → 4) at 104 ppm20. The increase in O-alkyl carbon intensities at 60–105 ppm in diapausing mosquitoes (Fig. 2c, blue) is due to the accumulation of glycogen and not from increase in chitin, as evidenced by absence of increase in acetyl-methyl carbon (C8) of NAG in chitin (Fig. 2b, yellow circles) resonating at 22 ppm18. As pupal cuticular proteins have been shown to increase in Cx. pipiens during the early stage of diapause21, cytoskeletonal reorganization may occur in preparation for overwintering. However, the net change in chitin biosynthesis in diapausing mosquitoes is small in comparison to the amount of large excess glycogen accumulation as observed by the increase in O-alkyl carbon intensities at 60–105 ppm. Figure 2c inset shows that diapausing mosquitoes have increased 99-ppm intensity corresponding to the branched C1 carbon with α(1 → 6) glycosidic linkage. Hence sucrose-fed diapausing mosquitoes store glycogen with increased branching structures. Glycogen accumulation is clearly visible in the difference spectrum (ΔS) obtained by subtracting the 13C-CPMAS spectrum of nondiapausing mosquitoes from diapausing ones (Fig. 2c, top). Compared to glycogen, aliphatic carbons at 30 and 33 ppm corresponding to CH2 in lipids show a smaller increase. These differential increases indicate that sucrose is preferentially routed to glycogen biosynthesis in diapausing mosquitoes.
Glucose metabolism in diapausing mosquitoes
13C-CPMAS spectra of nondiapausing (Fig. 3, lower left) and diapausing (Fig. 3, lower right) female Cx. pipiens fed exclusively on 10% D-[13C6]glucose for 7 days after adult eclosion demonstrate that glucose carbon is rapidly incorporated into both polysaccharides (60–110 ppm) and lipids (15–40 ppm). Unlike 13C-natural abundance spectra of sucrose-fed mosquitoes shown in Fig. 2c, 13C spectra of mosquitoes exclusively fed on 10% D-[13C6]glucose show increased CH2 intensities at 33 and 30 ppm (Fig. 3, dotted boxes) independently of diapause, indicating that glucose is preferentially metabolized for lipid biosynthesis in both diapausing and nondiapausing female Cx. pipiens. The relative peak-intensity ratio of glycogen at 76 ppm (dotted line) to lipid (30 ppm) in 10% D-[13C6]glucose-fed mosquitoes is approximately 1:1, whereas it is 2:1 in sucrose-fed mosquitoes shown in Fig. 2c. Therefore, mosquitoes exclusively fed on D-glucose show increased accumulations of both glycogen and lipid, while sucrose-fed mosquitoes show primarily glycogen accumulation.
13C-CPMAS spectra of 10% D-[13C6]glucose fed female Cx. pipiens.
Bottom) 75-MHz 13C-CPMAS spectra of nondiapausing (left) and diapausing (right) female Cx. pipiens fed with 10% D-[13C6]glucose as the sole-carbon source for 7-days post adult eclosion. The sample weights for D-[13C6]glucose-labeled nondiapausing mosquitos was 29.9 mg, and 39.4 mg for diapausing mosquitoes. The spectrum of nondiapausing mosquitos (left) was a result of 10000 acquisitions, and for diapausing mosquitos 10240 acquisitions (right). Middle) Difference spectra (ΔS) for nondiapausing (left) and diapausing (right) mosquitoes are obtained by subtracting the natural abundance CPMAS spectrum of nondiapausing mosquitoes from 10% D-[13C6]glucose-labeled CPMAS spectra. Top) Double-difference spectrum (ΔΔS) is obtained by subtracting the nondiapausing ΔS spectrum from the diapausing ΔS spectrum. Increased glycogen and lipid accumulations in diapausing mosquitoes are clearly visible in the ΔΔS spectrum.
Mosquitoes fed exclusively on 10% D-[13C6]glucose display a sharp ethylene carbon (-HC=CH-) resonance at 130 ppm. A sharp resonance of highly mobile olefin carbons is indicative of unsaturated lipids in the lipid bodies of adipocytes. A carboxyl-carbon resonance at 180 ppm, which appears as the shoulder to 175-ppm peak, is consistent with the carboxyl-carbon of fatty acid as D-[13C6]glucose is metabolized for lipid biosynthesis. In both diapausing and nondiapausing mosquitoes, D-glucose is metabolized for the biosynthesis of both lipid and glycogen.
Both nondiapausing and diapausing mosquitoes fed with 10% D-[13C6]glucose have a different aliphatic lipid composition than sucrose-fed mosquitoes (Fig. 3, bottom inset). The relative CH2 peak-intensity ratio of 33 to 30 ppm for nondiapausing mosquitoes (black) is 1.7 and for diapausing (blue) 1.1. In diapausing mosquitoes, 33-ppm peak intensity decreases concomitantly with 30 ppm peak intensity increase, whereas for mosquitoes exclusively fed on 10% sucrose, the 33 to 30 ppm ratio is constant at 0.6 for both diapausing and nondiapausing mosquitoes.
Difference spectra (ΔS) shown in Fig. 3 (middle) are the result of spectral subtraction of 13C-natural abundance CPMAS spectrum of 10% sucrose-fed nondiapausing mosquitoes (Fig. 2c, black) from the 13C spectrum of 10% D-[13C6]glucose-fed mosquitoes. Spectra are normalized to 175-ppm intensity prior to the subtraction. The spectral subtraction removes contributions from natural-abundance 13C, revealing the 13C distribution pattern solely from D-[13C6]glucose metabolism. Chitin biosynthesis is not involved in the metabolism of D-[13C6]glucose as evidenced by the absence of 55 ppm for C2 NAG in ΔS spectra. Differences between the ΔS of nondiapausing and diapausing mosquitoes are: i) more glycogen is accumulated in diapausing mosquitoes, ii) the olefin carbon of triacylglycerides (TAG) at 130 ppm is only visible in the ΔS of diapausing mosquitoes, and iii) diapausing mosquitoes show simultaneous increase in aliphatic CH2 carbon intensity at 30 ppm and decrease in 33-ppm intensity, which suggests an increase of lipid chain length in diapausing mosquitoes.
The difference in D-[13C6]glucose metabolism between diapausing and nondiapausing mosquitoes is further highlighted in the double-difference spectrum (ΔΔS) of Fig. 3 (top). The ΔΔS is obtained by subtracting the ΔS of nondiapausing from ΔS of diapausing mosquitoes. The ΔΔS spectrum shows that diapausing mosquitoes have increased intensities of O-alkyl carbon at 73 ppm, CH3 at 15 ppm, CH2 at 30 ppm, and olefin carbon at 130 ppm. The only peak decreases in intensity is the 33 ppm of CH2 positioned adjacent to either carboxyl carbons of lipid head group or olefin carbons in unsaturated lipids. Such changes indicate that D-[13 C6]glucose is metabolically routed preferentially to unsaturated aliphatic carbons during lipid biosynthesis. The absence of 180-ppm peak in ΔΔS suggests that the number of carboxyl-carbons in fatty acids are not increasing during diapause. This increase only in CH2 without a change in carboxyl-carbons of fatty acid is consistent with increased lipid chain length in diapausing mosquitoes.
Glucose metabolism in the presence of sucrose in diapausing mosquitoes
Figure 4 (right) shows 75-MHz 13C-CPMAS spectra of diapausing (bottom) and nondiapausing (top) female Cx. pipiens fed on a sugar mixture containing 1% uniformly 13C-isotope labeled D-[13C6]glucose and 10% unlabeled sucrose (blue) for 7 days post adult eclosion. These spectra are overlaid with spectra from mosquitoes exclusively fed on 10% D-[13C6]glucose (black) as comparison. All spectra are normalized to 175-ppm intensity. Although all spectra result from the metabolism of same isotope-labeled D-[13C6]glucose, 13C-CPMAS spectra of mosquitoes fed on 1% D-[13C6]glucose in presence of 10% sucrose (blue) show a distinct 13C-spectral profile different from ones fed exclusively on 10% D-[13C6]glucose (black). Changes in the metabolic profile of D-[13C6]glucose utilization are thus being observed through differences in 13C-CPMAS spectra between diapausing and nondiapausing mosquitoes.
13C-CPMAS spectra of 1% D-[13C6]glucose and 10% sucrose fed female Cx. pipiens.
75-MHz 13C-CPMAS spectra of nondiapausing (top) and diapausing (bottom) female Cx. pipiens fed with the mixture of 10% sucrose with 1% 13C-isotope labeled D-[13C6]glucose (blue) for 7-days post adult eclosion. Spectra are superimposed with 13C-CPMAS spectra of mosquitoes fed 10% sucrose (black). All spectra are normalized to 175-ppm intensity. Spectra of mosquitoes fed with the mixture shows D-[13C6]glucose was preferentially metabolized for the biosynthesis of triacylglycerol without glycogen accumulation.
The first major difference is found in the lipid profile. Enlarged lipid region (Fig. 4 insets) shows Cx. pipiens fed exclusively on D-[13C6]glucose (black) have CH2 resonances at 33, 30, and 25 ppm, whereas mosquitoes fed mixed carbohydrates (1% D-[13C6]glucose with 10% sucrose) show primarily 30 ppm (blue). Chemical shift assignments for saturated CH2 is 30 ppm, CH2 positioned next to the methyl-terminal end (ω-1) is 25 ppm, and CH2 adjacent to the ethylene carbon is 33 ppm. Therefore mosquitos that were fed exclusively 10% D-[13C6]glucose (without sucrose) demonstrate that D-[13C6]glucose is routed to biosynthesis of both saturated and unsaturated lipid. In contrast, mosquitos fed mixed carbohydrates preferentially routed 1% D-[13C6]glucose to saturated lipid biosynthesis.
The second major difference is that mosquitos fed mixed carbohydrates (1% D-[13C6]glucose with 10% sucrose) did not allocate D-[13C6]glucose to glycogen storage. Lack of glycogen biosynthesis is evidenced by absence of anomeric carbon intensities at 100 ppm (Fig. 4, star) despite the visible accumulation of O-alkyl carbons at 73 and 65 ppm. These O-alkyl carbons are not from glycogen as they have the intensity ratio of 1:2 consistent with the glycerol accumulation. On the other hand, mosquitoes exclusively fed on 10% D-[13C6]glucose have 73 to 65-ppm intensity ratio of 2:1 indicative of glycogen accumulation. Thus, mosquitos that were fed mixed carbohydrates show that D-[13C6]glucose is preferentially metabolized to i) mobile olefin carbons at 130 ppm, ii) saturated aliphatic carbons at 30 ppm, and iii) glycerol carbons at 73 and 65 ppm. These changes are in agreement with the exclusive routing of D-[13C6]glucose for the biosynthesis of TAG when co-provisioned with 10% sucrose in both diapausing and nondiapausing mosquitoes but not with glycogen biosynthesis. Thus female Cx. pipiens, when fed mixed sugars, preferentially utilizes sucrose for glycogen and glucose exclusively for the lipid biosynthesis.
Total glycogen and lipid quantifications in diapausing mosquitoes fed exclusively sucrose or glucose
The total lipid and glycogen in individual diapausing females Cx. pipiens fed exclusively on 10% (10 g/100 mL) sucrose or 10% glucose for 7 days after adult eclosion were determined (Fig. 5a) using modified methods described by Van Handel22,23. The total lipid was estimated based on quantifying the reaction of phosphor-vanillin with unsaturated aliphatic chains primarily found in lipids by measuring the optical density (OD) at 525 nm. The total glycogen was estimated following acid hydrolysis of hexose by quantifying the hydroxy-methyl-furfural reaction through measuring the concentration of hydroxy-methyl-furfurylidene-anthrone with OD at 625 nm. Relative amounts of glycogen (gray) and lipid (green) in mosquitoes fed with 10% sucrose were normalized to compare against amounts found in diapausing mosquitoes exclusively fed 10% glucose. The amounts of glycogen in mosquitoes fed with sucrose or glucose were comparable, but the total amount of lipids nearly doubled in mosquitoes fed exclusively on glucose. Thus feeding of glucose increases the lipid biosynthesis in diapausing female Cx. Pipiens.
Lipid and glycogen quantifications of 10% sucrose and 10% glucose fed female Cx. pipiens.
(a) Estimated total lipid and glycogen contents of individual diapausing females Cx. pipiens fed exclusively on 10% sucrose (left) and 10% glucose (right) for 7 days after adult eclosion as determined using methods described by Van Handel22,23. Total amounts of lipid (gray) and glycogen (green) per individual mosquitoes fed on 10% sucrose were normalized (dotted line) then compared with mosquitoes fed on 10% glucose. Error bars indicate standard error of mean. (b) Intensities of peaks at 70 and 33 ppm in 13C-CPMAS spectra of diapausing mosquitos fed exclusively on 10% sucrose (left) from Fig. 2, and on 10% D-[13C6]glucose (right) from Fig. 3. The 70-ppm intensity is proportional to the total carbohydrates (glycogen), and the 33-ppm intensity is proportional to the lipids in mosquitoes. The 70-ppm intensity (gray) and 33-ppm intensity (green) in mosquitoes fed with 10% sucrose were normalized. Feeding of different carbohydrate has a profound impact on the nutrient storage in diapausing mosquitoes. Mosquitoes fed exclusively on glucose show near doubling of lipid in comparison to mosquitoes fed solely on sucrose.
Total lipid and glycogen quantifications using methods described by Van Handel are in excellent agreement with the solid-state NMR result. Figure 5b shows the bar graph of 70 and 33 ppm intensities from 13C-CPMAS spectra of diapausing mosquitos that were exclusively fed 10% sucrose (left) and 10% D-[13C6]glucose (right). The 70-ppm intensity is directly proportional to the total carbohydrates (glycogen), and 33-ppm to lipids in the mosquito. The 70-ppm intensity (gray) and 33-ppm intensity (green) in mosquitoes fed with 10% sucrose were normalized. Mosquitoes that were fed exclusively on D-[13C6]glucose show the doubling of in lipids while the total glycogen remains unchanged.
Differential carbohydrate utilization and overwinter survival
We found that glucose and sucrose are metabolized differently in diapausing mosquitoes. This observation is surprising because sucrose is not taken up directly to hemolymph in the form of a disaccharide following the ingestion, but hydrolyzed into fructose and glucose in the crop of foregut by α-glucosidases secreted from salivary glands24,25. Cleaved monosaccharides are then absorbed in the midgut and transported to the fat body for long-term storage, and the absorption of glucose at midgut does not distinguish glucose based on its origin. Hence, all glucose should share same metabolic fate whether it originated from sucrose or by direct ingestion. However, solid-state NMR results clearly show that glucose is metabolically discriminated based on its origin based on following observations: (i) diapausing mosquitoes exclusively fed on sucrose show excess accumulation in glycogen with increasing branching structure, but no significant lipid accumulation (Fig. 2), (ii) diapausing mosquitoes exclusively fed on glucose increase biosynthesis of both glycogen and lipids with longer fatty acid chain length (Fig. 3), and (iii) diapausing mosquitoes co-fed with 1% glucose in presence of 10% sucrose utilize glucose exclusively for TAG biosynthesis (Fig. 4).
Differential utilization of glucose and sucrose by Cx. pipiens provides an intriguing insight into fundamental mechanisms of carbohydrate metabolism in insects. Although the mechanism that governs differential carbohydrate utilization is unknown, we speculate that it is closely related with insect detecting the carbohydrate composition in the feed. Based on the composition, activation of insulin and adipokinetic hormones can elicit changes to metabolic homeostasis. For example, in Drosophila the gustatory receptor in brain can sense fructose in hemolymph and trigger the production of insulin-like peptides to regulate the glucose homeostatsis26. Similarly, sensing of glucose in the brain of Drosophila increases the activity of insulin producing cells27. Relationships between carbohydrate composition of the feed and induced insulin and adipokinetic hormone levels, as well as cascading events by the effector molecules on insect metabolic homeostasis, remain to be determined.
We propose that differential carbohydrate utilization in diapausing mosquito is the result of co-evolution with plants in response to seasonal changes in carbohydrate compositions of flower nectar. Flower nectar is one of the main carbon sources utilized by diapausing mosquitoes in preparation for winter. The nectar consists primarily of sucrose, fructose, and glucose; relative amounts of each carbohydrate can vary depending on the flower and seasonal conditions. Hence, female mosquitoes may have adopted a feeding strategy that efficiently utilizes abundant carbohydrates to maximize the long-term nutrient storage. Selectively feeding on nectar with the desired carbohydrate composition can have a profound impact on the long-term nutrient storage and survival. Consequences of this metabolism switching are reflected by diapausing female mosquitoes exhibiting preferential feeding of flower nectars that are rich in glucose and fructose15. In contrast, the males that do not undergo diapause preferentially feed on flower nectars rich in sucrose and thus do not compete against the females for same carbon source. This preferential feeding enables boost in lipid accumulation for diapausing females. Figures 4 and 5 show that female mosquitoes exclusively fed on glucose, as compared to mosquitoes fed exclusively on sucrose, gain approximately two-fold increase in lipid storage. The amounts of glycogen stored in both glucose and sucrose-fed mosquitoes were identical. Large excess lipid accumulation in glucose-fed mosquitoes likely increases their survival rate over sucrose-fed mosquitoes during prolonged overwinter. This advantage in survival is due to the stored glycogen being readily utilized during the first month of overwinter in diapausing mosquito28. Following the glycogen exhaustion, stored TAG in fat body is converted into free fatty acids and transported to mitochondria or peroxisomes for the ATP generation through beta-oxidation, and the lipid in fat body becomes the sole energy source for the remaining duration of diapause. Hence increased lipid accumulation in glucose-fed mosquitoes ensures greater chance of survival for diapausing mosquitoes during overwinter and surplus lipid for the egg production in spring.
Materials and Methods
Insect rearing and fat body staining
The stock colony of Cx. pipiens was reared at 25 °C and 75% relative humidity under a 15-h light:9-h dark (L:D) photoperiod as previously described28. When larvae reached the second instar, rearing containers were placed under one of two environmental conditions: nondiapausing females were generated by rearing at 18 °C, 75% relative humidity, and 15:9 L:D. To induce diapause, mosquitoes were reared at 18 °C, 75% relative humidity, and 9:15 L:D. To confirm diapause status, primary follicle and germarium lengths were measured, and the stage of ovarian development was determined according to the methods described by Christophers29.
Diapausing and nondiapausing adult females and their fat bodies were collected from 7-days post adult eclosion. Fat body cells were examined by staining fixed tissues with BODIPY 493/503 (Invitrogen). Stained fat body was examined with Zeiss Axioskop fluorescent microscope.
13C-isotope labeling of Cx. pipiens
Nondiapausing and diapausing female Cx. pipiens after adult eclosion were 13C-isotope labeled by feeding on sponges soaked with three different carbohydrates: 1) 10% sucrose (13C-natural abundance), 2) 10% (m/m, 10 g/100 mL) sucrose and 1% D-[13C6]glucose, 3) 10% D-[13C6]glucose for 7 days after adult eclosion. Isotope-labeled D-[13C6]glucose (uniformly 13C labeled with 99% enrichment) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). Following the 7-days post adult eclosion, mosquitoes were frozen at −80 °C then lyophilized. Lyophilized intact mosquitoes were weighed and packed into a 7-mm zirconia rotor for solid-state NMR analysis. The average dry weight of a mosquito was approximately 1.0 mg.
Solid-state NMR spectrometer
Carbon-13 CPMAS NMR measurements were performed on 7.1-T (proton radio frequency of 300 MHz) Bruker Avance III with a double resonance HX probe. Lyophilized mosquitoes were contained in a 7-mm outer diameter zirconia rotor with Kel-F end-cap spinning at 5 kHz. Proton-carbon matched cross polarization was at 50 kHz with 2-ms contact time30. Spinning sidebands were suppressed using total-suppression of spinning sidebands sequence31 during echo in CPMAS. The proton dipolar decoupling was achieved by applying two-pulse phase modulation (TPPM15)32 on the 1H channel during acquisition. The π pulse length was 5 μs for 13C and the recycle delay was 3 s. The line broadening for spectrum was 20 Hz. 13C-PCMAS spectra were normalized to equal 175-ppm intensity of peptidyl-carbonyl carbons from proteins.
Total lipid and glycogen quantification
Diapausing female Cx. pipiens after adult eclosion were fed two different carbohydrates for 7 days and frozen: 1) 10% (10 g/100 mL) sucrose or 2) 10% glucose. Total lipid and glycogen contents of individual mosquitoes were determined using modified methods described by Van Handel22,23. Briefly, total of 7–8 mosquitoes were measured per each treatment. For lipids, each sample had a single mosquito homogenized in 0.2 mL of chloroform:methanol (1:1) mixture. After evaporating the solvent, 0.2 mL of sulfuric acid was added, and samples were heated at 37 °C for 10 min. After cooling, 2 mL of vanillin reagent (0.12% vanillin in 68% phosphoric acid) was added. Samples were allowed to develop for 5 min, then the absorbance was measured at 525 nm. Sesame oil (Sigma, S3547) was used to generate a standard curve. For glycogen content, a single mosquito was homogenized in 0.2 mL of 2% sodium sulfate, followed by addition of methanol (1 mL) and centrifugation for 1 min. Supernatants were evaporated, and 2 mL of anthrone reagent (0.14% anthrone in 28% sulfuric acid) was added to each sample. Reactions were incubated at 37 °C for 15 min, then the absorbance was measured at 625 nm. Purified glycogen (Sigma, G0885) was used to generate a standard curve. Comparisons of different groups were carried out using the two-tailed unpaired Student’s t test. Differences were considered statistically significant at p < 0.05.
Additional Information
How to cite this article: Chang, J. et al. Solid-state NMR reveals differential carbohydrate utilization in diapausing Culex pipiens. Sci. Rep. 6, 37350; doi: 10.1038/srep37350 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Denlinger, D. L. & Armbruster, P. A. Mosquito diapause. Annu Rev Entomol 59, 73–93 (2014).
Bowen, M. F. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J Med Entomol 29, 843–849 (1992).
Mitchell, C. J. & Briegel, H. Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwinter survival. J Med Entomol 26, 318–326 (1989).
Edman, J. D., Strickman, D., Kittayapong, P. & Scott, T. W. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J. Med. Entomol. 29, 1035–1038 (1992).
Magnarelli, L. A. Bionomics of Psoropora Jerox (Diptera: Culicidae): seasonal occurrence and acquisition of sugars. J. Med. Entomol. 17, 328–332 (1980).
Nayar, J. K. The detection of nectar sugars in field-collected Culex nigripalpus and its application. Ann. Entomol. Soc. Am. 71, 55–59 (1978).
Sim, C. & Denlinger, D. L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA 105, 6777–6781 (2008).
Andersson, I. H. & Jaenson, T. G. Nectar feeding by mosquitoes in Sweden, with special reference to Culex pipiens and Cx torrentium. Med Vet Entomol 1, 59–64 (1987).
Foster, W. A. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 40, 443–474 (1995).
Reisen, W. K., Meyer, R. P. & Milby, M. M. Patterns of fructose feeding by Culex tarsalis (Diptera: Culicidae). J Med Entomol 23, 366–373 (1986).
Foster, W. A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443–474 (1995).
S., P. M. Types of nectar in angiosperms. J. New Phytologist 60, 235–281 (1961).
Eveland, A. L. & Jackson, D. P. Sugars, signalling, and plant development. J. Exp. Bot. 63, 3367–3377 (2012).
Ohto, M. et al. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 127, 252–261 (2001).
Grimstad, P. R. & DeFoliart, G. R. Nectar sources of Wisconsin mosquitoes. J. Med. Entomol. 11, 331–341 (1974).
Magnarelli, L. A. Diurnal nectar feeding of Aedes cantator and A. solicitans (Diptera: Culicidae). Environ. Entomol. 8, 949–955 (1979).
Southwick, E. E., Loper, G. M. & Sadwick, S. E. Nectar production composition, energetics and pollination attractiveness in spring flowers in western New York. Am. J. Bot. 68, 994–1002 (1981).
Schaefer, J. et al. Aromatic cross-links in insect cuticle: detection by solid-state 13C and 15N NMR. Science 235, 1200–1204 (1987).
Fukamizo, T. et al. Analysis of chitin structure by nuclear magnetic resonance spectroscopy and chitinolytic enzyme digestion. Arch Biochem Biophys 249, 15–26 (1986).
Cheetham, N. W. & Tao, L. Solid state NMR studies on the structural an conformational properties of natural maize starches. Carbohydrate Polymers 36, 285–292 (1998).
Li, A. & Denlinger, D. L. Pupal Cuticle Protein Is Abundant During Early Adult Diapause in the Mosquito Culex pipiens. J. Med. Entomol. 46, 1382–1386 (2009).
Van Handel, E. Rapid determination of glycogen and sugar in mosquitoes. J. Am. Mosq. Control Assoc. 1, 299–304 (1985).
Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1, 302–304 (1985).
Marinotti, O. & James, A. A. An α-glucosidase in the salivary glands of the vector mosquito, Aedes aegypti. Insect Biochem. 20, 619–623 (1990).
Moreira-Ferro, C. K., Marinotti, O. & Bijovsky, A. T. Morphological and biochemical analyses of the salivary glands of the malaria vector, Anopheles darlingi. Tissue Cell 31, 264–273 (1999).
Miyamoto, T., Slone, J., Song, X. & Amrein, H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 151, 1113–1125 (2012).
Kreneisz, O., Chen, X., Fridell, Y. W. & Mulkey, D. K. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport 21, 1116–1120 (2010).
Sim, C. & Denlinger, D. L. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol Genomics 39, 202–209 (2009).
Christophers, S. The development of the egg follicle in Anophelines. Paludism, 73–88 (1989).
Stejskal, E. O., Schaefer, J. & McKay, R. A. High-Resolution, Slow-Spinning Magic-Angle Carbon 13 NMR. J. Magn. Reson. 25, 569 (1977).
Dixon, W. T., Schaefer, J., Sefcik, M. D., Stejskal, E. O. & McKay, R. A. Total suppression of sidebands in CPMAS C-13 NMR. J. Magn. Reson. 49, 341–345 (1982).
Bennett, A. E., Reienstra, C. M., Auger, M., Lakshmi, K. V. & Griffin, R. G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 103, 6951–6958 (1995).
Acknowledgements
J.S. was supported by Postdoctoral Fellowship from The Office of Vice Provost for Research at Baylor University. Purchase of the CPMAS NMR probe was supported by NSF EAR IF 1132124. This work was supported in part by the National Institutes of Health under grant number GM116130.
Author information
Authors and Affiliations
Contributions
S.J.K. and C.S. designed the experiments. C.S. and S.K. carried out isotope labeling of diapausing mosquitoes. S.J.K., J.S., B.H. and J.C. carried out solid-state NMR measurements. All authors contributed to the writing of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chang, J., Singh, J., Kim, S. et al. Solid-state NMR reveals differential carbohydrate utilization in diapausing Culex pipiens. Sci Rep 6, 37350 (2016). https://doi.org/10.1038/srep37350
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37350
This article is cited by
-
Cuticular profiling of insecticide resistant Aedes aegypti
Scientific Reports (2023)
-
Mosquito sex and mycobiota contribute to fructose metabolism in the Asian tiger mosquito Aedes albopictus
Microbiome (2022)
-
Semi-field and surveillance data define the natural diapause timeline for Culex pipiens across the United States
Communications Biology (2022)
-
Dsi-RNA knockdown of genes regulated by Foxo reduces glycogen and lipid accumulations in diapausing Culex pipiens
Scientific Reports (2020)
-
Fluorescent cellulose nanocrystals for the detection of lead ions in complete aqueous solution
Cellulose (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.