Abstract
Diabetes mellitus is a chronic disease that is associated with depression. Also, depression is common in adults with type 2 diabetes mellitus (T2DM). Translocator protein (18kDa) (TSPO) and allopregnanolone play an important role in the depression treatment. However, few studies have evaluated TSPO and allopregnanolone in the treatment of depression in T2DM. AC-5216, a ligand for TSPO, produces anxiolytic- and antidepressant-like effects in animal models. The present study aimed to explore antidepressant-like effects of AC-5216 on diabetic rats. Following the development of diabetic model induced by high fat diet (HFD) feeding and streptozotocin (STZ), AC-5216 (0.3 and 1 mg/kg, i.g.) elicited the antidepressant-like effects in behavioral tests while these activities were blocked by TSPO antagonist PK11195 (3 mg/kg, i.p.). The levels of allopregnanolone in the prefrontal cortex and hippocampus were increased by AC-5216 (0.3 and 1 mg/kg, i.g.), which was antagonized by PK11195 (3 mg/kg, i.p.). The increased plasma glucose (PG) and decreased insulin (INS) in HFD-STZ rats were reversed by AC-5216 (0.3 and 1 mg/kg, i.g.). This study indicates that the antidepressant-like effects of AC-5216 on HFD-STZ rats, suggesting that TSPO may represent a novel therapeutic target for depression in T2DM.
Similar content being viewed by others
Introduction
Diabetes mellitus is a metabolic disease that induces decreased quality and expectancy of life, as well as increased costs of care1. World Health Organization (WHO) has forecasted that about 300 million people will suffer from diabetes mellitus by 20252. As compared to type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) is much more prevalent. It comprises around 90% of people with diabetes mellitus that is caused by a combination of resistance to insulin action and an inadequate compensatory insulin secretory response3. The structural and neurophysiological changes in the central nervous system (CNS) are induced by diabetes that are associated with cognitive deficits and the psychiatric disorder, such as depression4,5.
Depression is a common co-morbid condition in T2DM and it has been estimated that people with T2DM are more likely to suffer from depression than general population5. Comorbid depression is also associated with increased risks of diabetic retinopathy, neuropathy, nephropathy, macrovascular complications, and sexual dysfunction6. Patients with depression had a 60% increased risk of developing T2DM7. The possibility is that people with elevated depressive symptoms are less attentive toward a healthy lifestyle, therefore increasing the risks of developing diabetes8. Furthermore, depression in T2DM is related to the diminished quality of life. The impact of depression on quality of life in various chronic diseases (arthritis, angina, asthma and diabetes) shows that quality of life is impaired in patients with diabetes and depression9.
Despite a variety of psychological and pharmacological treatments have been introduced in depression with T2DM, a large proportion of patients had not achieved satisfactory outcomes. One finding refers to the chronic dysregulations of serotonin (5-HT) activity leading to the pathogenesis of depression in T2DM10. Numerous evidences support that selective serotonin reuptake inhibitors (SSRIs) (e.g fluoxetine and sertraline) are effective in the therapy of depression in T2DM10,11. However, SSRIs have disadvantages, including delayed onset of action, limited response with residual symptoms as well as severe side effects, such as insomnia and agitation12. Based on the downsides, investigators need to search the novel pharmacological targets for depression in T2DM treatments.
One major role of neuroactive steroid (i.e., allopregnanolone) is neuroprotection in case of lesion, ischemia or peripheral neuropathies (i.e., diabetes)13. Allopregnanolone, a neuroactive steroid derived from progesterone, is synthesized within the nervous tissue, by means of specific enzymes13. Contrary to progesterone and its metabolite dihydroprogesterone, allopregnanolone is able to interact with γ-aminobutyric acid (GABA) A receptor that is associated with psychiatric disorders, such as schizophrenia, depression, anxiety, and impulsive aggression14,15,16. Lowered levels of allopregnanolone may lead to imbalance in excitatory neurotransmission that causes depressive symptoms17. Conversely, allopregnanolone exerts antidepressant-like effects in preclinical and clinical studies16,18. However, the relationship between allopregnanolone and depression in T2DM has not yet fully understood. Thus, it is reasonable to hypothesis that allopregnanolone may also play a significant role in the treatment of depression in T2DM.
Translocator protein 18kDa (TSPO), previously described as peripheral-type benzodiazepine receptor (PBR), represents the starting point and an important rate-limiting step in neurosteroidogenesis19. It is a five trans-membrane domain protein that is located mainly in the outer mitochondrial membrane in peripheral tissues and central nervous system (CNS). In the CNS, TSPO is basically located in the glial cells and mediates the translocation of cholesterol from outer to inner mitochondrial membrane20. The role of TSPO has received increased attention on the pathophysiology of stress response and stress-related disorders21. The alternation of TSPO expression (or function) is a promising therapeutic target for depression without benzodiazepine-like side effects22. In diabetic study, TSPO activation was effective in ameliorating the severity of diabetic neuropathy by means of increased levels of neuroactive steroid23. Consequently, TSPO ligands may be represented as a promising option on the remedy of neurological disorders. The therapeutic approach could be extremely interesting because it may avoid possible endocrine side effects exerted by systemic treatment of neuroactive steroids.
The TSPO ligand, such as AC-5216 (Emapunil, XBD173), has been shown to exert anxiolytic/antidepressant activity in rodents by neurosteroidogenesis24. In addition, the TSPO ligands reduced the weight gain and improved glucose tolerance to high-fat diet-induced obese rodents23. However, little is known about the importance of TSPO in the treatment of depression in T2DM.
In the present study, the development of diabetic model in rats was induced by high fat diet (HFD) feeding and streptozotocin (STZ). STZ is widely used in T2DM animal models preparation. It is a glucosamine-nitrosourea that acts by alkylating DNA and exposing cells to reactive oxygen species and nitric oxide25. STZ accumulates efficiently in pancreatic β-cells resulting in cell death that routinely deployed to induce experimental diabetes26. Following the development of HFD-STZ rats, we evaluated the pharmacological characteristics of AC-5216. The antidepressant-like effects were assessed by animal behavioral tests, including sucrose preference test (SPT), novelty-suppressed feeding test (NSFT), forced swimming test (FST) and open-field test (OFT). To further evaluate the role of TSPO in the treatment of depression in T2DM, we determined whether pharmacological effects of AC-5216 were antagonized by PK11195 (TSPO antagonist) in HFD-STZ rats. The animals were decapitated after the end of behavioral tests. The levels of allopregnanolone, plasma glucose (PG), insulin (INS), total cholesterol (TC), and triglyceride (TG) were assessed as well.
Materials and Methods
Drugs and administration
AC-5216 was purchased from Medchem Express (USA). PK11195, STZ, metformin (Met) and fluoxetine (Flu) were purchased from Sigma-Aldrich (USA). AC-5216 was prepared as a suspension in 0.5% tragacanth gum solution and administered to rats by gavage (i.g)27. The dose range of AC-5216 (0.1, 0.3, and 1 mg/kg, i.g.) was based on its antidepressant- and anxiolytic- like activities as described previously with minor adjustments24. PK11195, injected intraperitoneally (i.p.), was suspended in saline containing 2% DMSO and 0.8% Tween 8024. The dosage of PK11195 (1 and 3 mg/kg, i.p.) was based on published studies showing the inhibitory effect of PK11195 against AC-5216 and other TSPO ligands24,28,29. STZ dissolved freshly in citrate buffer (pH 4.5)30. With the control effects of blood glucose, Met is the mainstay treatment in the prevention of T2DM and associated comorbidities31. The lowered blood glucose induced by Met in HFD-STZ model mimics the situation of type 2 diabetes relevant to human condition. Consequently, Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and the combination (Met+Flu, MF) were administered as positive control drugs in all behavioral tests respectively based on their antidepressant-like effects on the T2DM rodent model30.
Animals and housing
Sprague-Dawley rats (male, 180 ± 20 g) were purchased from Beijing Vital Laboratory Animal Technology Company (Beijing, China). The animals were maintained in the standard conditions of controlled temperature (23 ± 1 °C), humidity (45%), and lighting (from 06:00 to 18:00). The rats were housed in a 12-h light/dark cycle starting at least 5 days before the experiments with access to food and water freely available. The total number of animals was 100 (ten in each group). All methods were carried out in accordance with National Institute of Health Guide for the care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). The experimental procedures were approved by the institutional committee of Academy of Military Medical Sciences on animal care and use. All efforts were made to minimize animal suffering and reduce the number of animals used in the experiments.
Development of the HFD-STZ rats
The development of the HFD-STZ rats mimics the situation T2DM in human30. Development of HFD-STZ rats was performed by basing the design on previous studies with minor adjustments10,30. After the accommodation to new environment, rats were divided randomly into two groups: the control group (HFD-STZ (−), n = 10) was fed a normal chow diet, whereas the diabetic group (HFD-STZ (+), n = 90) was fed a HFD (59% fat, 26% protein and 20% carbohydrate, as a percentage of total kcal) for two weeks. After the duration of dietary manipulation, the diabetic groups were fasted for 16 h followed by a dose of STZ (40 mg/kg, i.p) for two consecutive days. While the control group was given vehicle citrate buffer (pH 4.5) in a volume of 2 mL/kg, i.p, respectively. PG was determined by a single touch glucometer (OneTouch Ultra 2; LifeScan, High Wycombe, UK). The rat with the non-fasting PG ≥ 300 mg/dl was considered diabetic and selected for further evaluation of the feasible preparation of HFD-STZ rats30. After that, oral glucose tolerance test (OGTT) was performed based on the previous study10. Animals were fasted for 16 h and given glucose at a dose of 2 g/kg, i.p. PG was determined before and after 15, 30, 60, 90 and 120 min of the glucose application. In addition, during the development of HFD-STZ rats, the body weight (BW) was recorded for all of the rats. We found that within the period of HFD-STZ rat spreparation, no significant difference of BW between control group and diabetic group (data not shown). However, OGTT showed severely impaired clearance of acute increase of PG during 120 min monitoring process: the levels of PG in HFD-STZ rats were significantly higher than that of control rats at all of the time points (data not shown).
Behavioral assessments
Two days after the development of HFD-STZ rats, behavioral experiments were started. The sucrose preference test (SPT) (from day 2 to 5), novelty-suppressed feeding test (NSFT) (from day 7 to 8), forced swimming test (FST) (from day 10 to 11), and open-field test (OFT) (day 13) were conducted. To evaluate the effects of repeated treatments on behavior, Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p), MF, AC-5216 (0.1, 0.3, and 1 mg/kg, i.g.) and PK11195 (1 and 3 mg/kg, i.p.) were given once per day from day 1 to 13. Met, Flu, MF and AC-5216 were given 1 h and PK11195 was given 30 min before the behavioral tests, respectively. The control rats (HFD-STZ (-) were given vehicle 0.5% tragacanth gum solution. The outline of treatment schedule design and behavioral tests is shown in Fig. 1.
Sucrose preference test (SPT)
SPT is widely employed to measure major depression in human32. The test was performed as described previously33. Rats were placed in individual cages to train to consume 1% (w/v) sucrose solution for 48 h without food and water supply. Following 16 h water deprivation, one bottle of 1% sucrose solution was replaced by water. The SPT was performed for 1 h. During the test, rats could select between two preweighed bottles, one with 1% sucrose solution and the other with tap water. The sucrose preference was calculated as sucrose intake/(sucrose intake + water intake) × 100%.
Novelty-suppressed feeding test (NSFT)
The NSFT provides a sensitive and reliable measure of depression and motivation level in animals which mimics the situation in human34. The test was performed according to the literature with minor modification35. Briefly, after fasting for 24 h, rats were placed in the corner of the plastic box (76 × 76 × 46 cm) with several pellets placed in the center. The latency to begin eating within 5 min was recorded (defined as rat chewing or biting the pellet, instead of merely sniffing or toying with it). Moreover, home-cage food consumption in 5 min was immediately evaluated to assess effects of drugs on feeding drive.
Forced swimming test (FST)
FST is a well-established measurement for evaluation the effects of antidepressants. The antidepressant-like effects are indicated by decreased duration of immobility. The test is highly reliable to predict the validity of antidepressants29. The procedure comprised two sessions (the pretest and the test) with identical apparatus and conditions (height 40 cm, diameter 20 cm, containing 25 cm of water maintained at 25 °C). During the pretest session, rats were forced to swim for 15 min. After 24 h, rats were placed in the same apparatus for 5 min and the session was designated as a test session. The duration of immobility during 5 min was measured.
Open-field test (OFT)
In order to rule out whether non-specific locomotor effects of AC-5216 on antidepressant-like activity, the locomotor activity was assessed. The test was performed as described previously35. Rats were placed in the corner of plastic box (76 × 76 × 46 cm) for 5-min acclimation, then the number of crossings (squares crossed with all paws) and rears (raising the fore paws) and fecal pellets was recorded in the next 5 min session. The square arena was cleaned with a water-alcohol (10%) solution and dried after each test in order to hide animal cues and prevent the odor influence from the previous animals.
Blood collection and levels of PG, INS, TC, and TG measurement
To evaluate effects of AC-5216 on levels of PG, INS, TC, and TG, which are important parameters in diabetic animal models30,36, the blood was collected and the levels of parameters above were determined followed by the final behavioral test as the previous study with minor adjustments36. The single touch glucometer (OneTouch Ultra 2; LifeScan, High Wycombe, UK) was used to determine the levels of PG collected from the tail vein of rats. The blood sample was centrifuged (2000 g, 25 min) at 4 °C, and supernatants were collected. All samples were maintained at −80 °C until further use. The samples were assayed and quantified by Enzyme Immunoassay kits, respectively (INS (Millipore, USA), TC (Cell Biolabs, USA), and TG (Abcam, USA)). Six samples in each group were used to determine optical density (OD) values, at 450 nm in enzyme-linked immunosorbent assay (ELISA) plate reader and used for statistical analyzes.
Allopregnanolone determination
Altered levels of allopregnanolone have been implicated as one of the possible contributors to the development of depression17. The levels of allopregnanolone in the antidepressant-like activity of AC-5216 were also evaluated by ELISA. The brain and blood preparation for ELISA was based on a literature37. Animals were sacrificed by decapitation at the end of OFT in 24 h after the last drugs treatment. The brains were removed, rinsed of blood, and carefully dissected to remove the prefrontal cortex and hippocampus. Both brain regions, involved in emotional processing, fear conditioning and explicit memory, play a significant role in depression38,39.
The brain regions were extracted by 1 mL extraction buffer per 100 mg tissue and then homogenized in the ice-cold lysis buffer containing 137 mM NaCl, 1% NP40, 10% glycerol, 20 mM Tris-HCl (pH 8.0), 1 μg/mL leupeptin, 1 mM PMSF 10 μg/mL aprotinin, and 0.5 mM sodium vanadate. The tissue homogenate solutions were centrifuged at 13,000 g for 30 min at 4 °C, and then the supernatants were collected. For the measurement of serum allopregnanolone, the blood samples were centrifuged (2000 g, 25 min) at 4 °C, and the supernatants were collected. All samples were maintained at −80 °C until further use. Allopregnanolone was quantified by Enzyme Immunoassay kit (Raybiotech, USA) that was specific for the measurement of allopregnanolone40. Six samples in each group were used to determine OD values at 450 nm in ELISA plate reader and used for statistical analyzes.
Statistical analysis
Unless otherwise specified, statistical analysis was conducted by GraphPad Prism 5.0 (version 2.0; GraphPad Software Inc., San Diego, CA). All data are presented as mean ± standard error of measurement (S.E.M). The statistical significance of experimental observations was determined by one-way analysis of variance (ANOVA) or two-way ANOVA followed by Bonferroni’s multiple comparison tests, as indicated in the results section. For all tests, level of statistical significance was set at p < 0.05.
Results
The effects of AC-5216 on HFD-STZ rats in SPT
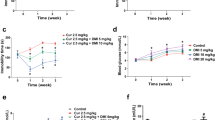
The antidepressant-like effects of AC-5216 on HFD-STZ rats in SPT were shown in Fig. 2. Sucrose preference was significantly decreased in HFD-STZ rats. Similar to Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, AC-5216 (0.3 and 1 mg/kg, i.g) produced antidepressant-like effects, as evidenced by the increase of sucrose preference (one-way ANOVA, F (7, 72) = 9.245, p < 0.05; Fig. 2A). However, this activity (AC-5216, 1 mg/kg, i.g.) was antagonized by PK11195 (3 mg/kg, i.p.) (two-way ANOVA, F (9, 90) = 8.798, p < 0.05; Fig. 2B), indicating that the antidepressant-like effects of AC-5216 in SPT were mediated by TSPO.
The antidepressant-like effects of AC-5216 on HFD-STZ rats in SPT.
The sucrose preference was increased by AC-5216 (A) and this effect was reversed by PK11195 (B) #p < 0.05 vs. vehicle-treated HFD-STZ (−) group; *p < 0.05,**p < 0.01 vs. vehicle-treated HFD-STZ (+) group; $p < 0.05 vs. AC-5216 (1 mg/kg, i.p.) group (n = 10).
The effects of AC-5216 on HFD-STZ rats in NSFT
As shown in Fig. 3, the latency to feed was increased significantly in HFD-STZ rats. Consistent with Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, AC-5216 (1 mg/kg, i.g) produced the antidepressant-like effects, as evidenced by the decrease of latency to feed (one-way ANOVA, F (7, 72) = 4.072, p < 0.05; Fig. 3A). However, the effects (AC-5216, 1 mg/kg, i.g.) were blocked by PK11195 (3 mg/kg, i.p.) (two-way ANOVA, F (9, 90) = 4.914, p < 0.05; Fig. 3B). Moreover, no differences of in home-cage food consumption were observed among the groups (data not shown). These results indicated that the antidepressant-like effects of AC-5216 in NSFT were mediated by TSPO.
The antidepressant-like effects of AC-5216 on HFD-STZ rats in NSFT.
The latency to feed was decreased by AC-5216 (A) and these effects were reversed by PK11195 (B) #p < 0.05 vs. vehicle-treated HFD-STZ (−) group; *p < 0.05 vs.vehicle-treated HFD-STZ (+) group; $p < 0.05 vs. AC-5216 (1 mg/kg, i.p.) group (n = 10).
The effects of AC-5216 on HFD-STZ rats in FST
The antidepressant-like effects of AC-5216 on HFD-STZ rats in FST were shown in Fig. 4. The immobility time was increased significantly in HFD-STZ rats. Similar to Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, AC-5216 (0.3 and 1 mg/kg, i.g.) exerted the antidepressant-like effects, as evidenced by the decreased immobility time (one-way ANOVA, F (7, 72) = 2.328, p < 0.05; Fig. 4A). However, the activities of AC-5216 (1 mg/kg, i.g.) was antagonized by PK11195 (3 mg/kg, i.p.) (two-way ANOVA, F (9, 90) = 3.621, p < 0.05; Fig. 4B), indicating that the antidepressant-like effects of AC-5216 in FST were mediated by TSPO.
The antidepressant-like effects of AC-5216 on HFD-STZ rats in FST.
The immobility time was decreased by AC-5216 (A). The antidepressant-like effects of AC-5216 were antagonized by PK11195 in the immobility time (B). #p < 0.05 vs. vehicle-treated HFD-STZ (−); *p < 0.05 vs. vehicle-treated HFD-STZ (+) group; $p < 0.05 vs. AC-5216 (1 mg/kg, i.p.) group (n = 10).
The effects of AC-5216 on HFD-STZ rats in OFT
The OFT is evaluated whether locomotor activity is affected by various treatments in rats. The effects of treatments on locomotor activity were shown in Fig. 5. One-way ANOVA analysis revealed that similar to the model group, the number of crossings (F (7, 72) = 1.213, p > 0.05; Fig. 5A), rears (F (7, 72) = 0.5026, p > 0.05; Fig. 5B) and fecal pallets (F (7, 72) = 0.1707, p > 0.05; Fig. 5C) was not affected by Met, Flu, MF and AC-5216. Also, two-way ANOVA analysis revealed that PK11195 had no effects on the crossings (F (9, 90) = 0.5635, p > 0.05; Fig. 5D), rears (F (9, 90) = 0.6367, p > 0.05; Fig. 5E) and fecal pallets (F (9, 90) = 0.2325, p > 0.05; Fig. 5F). These results indicated that the antidepressant-like effects of AC-5216 were not affected by locomotor activity in HFD-STZ rats.
The effects of AC-5216 on levels of PG, TC, TG, and INS in HFD-STZ rats
The effects of AC-5216 on the levels of PG, TC, TG, and INS in HFD-STZ rats were shown in Fig. 6. One-way ANOVA analysis revealed that the levels of PG (F (7, 40) = 3.320, p < 0.05; Fig. 6A), TC (F (7, 40) = 3.426, p < 0.05; Fig. 6B), and TG (F (7, 40) = 2.258, p < 0.05; Fig. 6C) were increased significantly while the INS (F (7, 40) = 4.065, p < 0.05; Fig. 6D) was markedly decreased in HFD-STZ rats. Similar to Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, the increase of PG and the decrease of INS were reversed by AC-5216 (0.3 and 1 mg/kg, i.g for PG and 1 mg/kg, i.g for INS, respectively). Both TC and TG were not significantly affected by AC-5216. Moreover, two-way ANOVA analysis revealed that the levels of PG (F (9, 50) = 2.655, p < 0.05; Fig. 6E), TC (F (9, 50) = 2.081, p < 0.05; Fig. 6F), TG (F (9, 50) = 2.889, p < 0.05; Fig. 6G), and INS (F (9, 50) = 2.874, p < 0.05; Fig. 6H) in HFD-STZ rats were not affected by PK11195. These results indicated that AC-5216 induced the increase of PG and the decrease of INS.
The effects of AC-5216 on PG, TC, TG, and INS in HFD-STZ rats.
The increase of PG (A) and the decrease of INS (D) were reversed by AC-5216, which did not affect the levels of TC (B) and TG (C) Moreover, PG (E) TC (F) TG (G) and INS (H) in HFD-STZ rats were not affected by PK11195. #p < 0.05, ##p < 0.01 vs. vehicle-treated HFD-STZ (−) group; *p < 0.05, **p < 0.01 vs. vehicle-treated HFD-STZ (+) group (n = 6).
The effects of AC-5216 on allopregnanolone in HFD-STZ rats
The effects of AC-5216 on allopregnanolone in HFD-STZ rats were illustrated in Fig. 7. One-way ANOVA analysis revealed that the levels of allopregnanolone were decreased significantly in the prefrontal cortex (F (7, 40) = 2.654, p < 0.05; Fig. 7A), hippocampus (F (7, 40) = 1.922, p < 0.05; Fig. 7B), and serum (F (7, 40) = 1.059, p < 0.05; Fig. 7C) in HFD-STZ rats. Similar to Flu (10.8 mg/kg, i.p) and MF, the effects were reversed by AC-5216 in the prefrontal cortex (0.3 and 1 mg/kg, i.g. for prefrontal cortex, Fig. 7A) and hippocampus (1 mg/kg, i.g. for hippocampus, Fig. 7B), but not in serum (Fig. 7C). However, two-way ANOVA analysis revealed that the effects of AC-5216 were totally blocked by PK11195 (3 mg/kg, i.p.) in the prefrontal cortex (F (9, 50) = 2.418, Fig. 7D) and hippocampus (F (9, 50) = 2.509, Fig. 7E) without in serum (F (9, 50) = 1.445, Fig. 7F). The results indicated that the antidepressant-like effects of AC-5216 in HFD-STZ rats were associated with allopregnanolone biosynthesis in the prefrontal cortex and hippocampus, which was mediated by TSPO.
The effects of treatment on allopregnanolone in HFD-STZ rats.
The decreased levels allopregnanolone were reversed by AC-5216 in the prefrontal cortex (A) and hippocampus (B) but not in serum (C). However, the effects of AC-5216 were totally blocked by PK11195 in the prefrontal cortex (D) and hippocampus (E), but not in serum (F). #p < 0.05, ##p < 0.01 vs. vehicle-treated HFD-STZ (−) group; *p < 0.05, **p < 0.01 vs. vehicle-treated HFD-STZ (+) group; $p < 0.05, $$p < 0.01 vs. AC-5216 (1 mg/kg, i.p.) group (n = 6).
Discussion
This study was initiated with the objective of evaluating the role of TSPO and allopregnanolone biosynthesis in the treatment of depression in T2DM. Antidepressant-like pharmacological profile of AC-5216 was determined by animal behavioral tests. Based on the data above, the antidepressant-like activities were produced by AC-5216 in HFD-STZ rats and these effects were antagonized by TSPO antagonist PK11195 without affecting the locomotor activity. Also, the decreased allopregnanolone synthesis in the prefrontal cortex and hippocampus was reversed by AC-5216 while these effects were also antagonized by PK11195. It is indicated that the antidepressant-like effects of AC-5216 in HFD-STZ rats were mediated by TSPO and allopregnanolone biosynthesis.
The diabetic animal model (HFD-STZ rats) is typical, including either irreversible or reversible (insulin resistance) destruction of β-pancreatic cells, which is widely utilized in the T2DM study25. The model simulates the human syndrome of depression in DM and is identified as suitable for testing anti-diabetic agents for the treatment of T2DM26. The alternations of PG, INS, TC, and TG are important parameters to determine T2DM30,36. Comparing to the control group, PG (Fig. 6A), TC (Fig. 6B), and TG (Fig. 6C) were increased while INS (Fig. 6D) was decreased that the animals induced by HFD-STZ administration. These similar alternations in diabetic rodents were consistent with the previous studies demonstrating that the changes resembled the situation of patients with T2DM26,30,41.
Depression is prevalent in the patients with diabetes. The risks of elevated incident depression are increased in individuals with T2DM compared with those in healthy subjects5. Consequently, following the exposure to HFD-STZ treatment, depressive-like behavior was evaluated in rats. Our data indicated that depressive-like behavior was elicited by diabetic procedure, as evidenced by sucrose preference was significantly decreased in SPT (Fig. 2), the latency to feed and the immobility time was increased markedly in NSFT (Fig. 3) and FST (Fig. 4), respectively. All of these parameters are represented as depressive-like symptoms in rodents33. Similarly, clinical reports have indicated that depression is linked to hyperglycemia42. Depressed adults with T2DM have poor control of glycemia as compared to those without mood disorder43. An explanation for relationship between depression and T2DM stems from the study results indicating that blood glucose is itself a potent regulator for mood states44. In particular, hypoglycemia or severe hyperglycemia is able to induce negative emotional states in patients with diabetes43. Other studies has reported that the depressive mood is positively related to the presence of diabetic complications. It has been reported that prevalence of depression is higher among T2DM subjects with retinopathy, neuropathy, nephropathy and peripheral vascular disease (PVD)45,46. An explanation for the association could be the increase in the burden of disease due to complications which can lead to depressive-like symptoms2.
Numerous studies have reported that TSPO plays a significant role in the therapy of depression and symptoms of depression could be reversed by TSPO ligands (e.g AC-5216, YL-IPA08)24,27. However, the importance of TSPO in the remedy of depression in T2DM is still unknown. To evaluate this, several behavioral tests as measures of depression were performed after development of HFD-STZ rats. Interestingly, similar to traditional antidepressant (Flu)47, Met (1.8 mg/kg, i.p) also had antidepressant-like effects on HFD-STZ rodents. These findings were supported by the clinical research that Met produced antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus48. The results of the present and previous studies raise the possibility that supplementary administration of antidiabetic medications may enhance the recovery of depression, comorbid with T2DM, through improvements in cognitive performance. Consistent with Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, AC-5216 produced the antidepressant-like effects in SPT (Fig. 2) (reversed the decreased sucrose preference (Fig. 2A)), in NSFT (Fig. 3) (reversed the increased latency to feed (Fig. 3A)), in FST (Fig. 4) (reversed the decreased immobility time (Fig. 4A)). The similarly effective doses (0.3 and 1 mg/kg, i.g) were consistent among the behavioral tests and in line with anxiolytic-like effects of AC-5216 in the Vogel conflict test and in the social interaction test24,49. Also, our previous studies showed that the behavioral deficits in an animal model of post-traumatic stress disorder (PTSD) were attenuated by AC-521637,50. All the anxiolytic- and anti-PTSD- like effects were at similar doses, which supported our present study. The data above (Figs 2A, 3A, 4A) had implicated that antidepressant-like activity of AC-5216 in HFD-STZ rats was mediated by TSPO on the basis that AC-5216 had high binding affinity on TSPO.
To further evaluate the significance of TSPO on depression in T2DM, PK11195 was applied in the present study. PK11195 is an isoquinoline carboxamide which binds selectively to TSPO and widely utilized in the TSPO antagonism study24,29. The data showed that the antidepressant-like activities of AC-5216 in HFD-STZ rats was antagonized by PK11195 at the dose of 3 mg/kg i.p (Figs 2B, 3B, 4B). All these results were in agreement with previous studies showing the antidepressant-, anxiolytic-, and anti-PTSD- like effects of AC-5216 were blocked by PK1119524,29,37. Taking together, these demonstrate that TSPO plays a significant role in the treatment of depression in T2DM.
We also found that the antidepressant-like effects of AC-5216 were not significantly influenced by locomotor activity (Fig. 5), including crossings (Fig. 5A), rears (Fig. 5B) and fecal pellets (Fig. 5C) in HFD-STZ rats. The findings were consistent with the studies that anxiolytic-, anti-PTSD-, and anti-panic- like effects of AC-5216 were also not mediated by affecting the locomotor activity22,49,50. Our results were also consistent with other reports that TSPO selective ligands with subnanomolar affinity for TSPO such as indolylglyoxylamides, stimulated the steroid biosynthesis and exerted the anxiolytic activities without affecting the locomotor activity in rats51,52. Also, the locomotor activity in HFD-STZ rats was not affected by PK11195 (Fig. 5D–F). The data indicated that TSPO is a potential treatment target for depression in T2DM without affecting locomotor activity. In addition, our data were supported by a previous study showing that TSPO is a promising target for treating neurological disorders without benzodiazepine-like side effects, such as emotional and somatic withdrawal symptoms53.
Based on the data of behavioral tests indicating that the antidepressant-like effects of AC-5216 were mediated by TSPO in HFD-STZ rats, more investigation involved the significance of neurosteroid biosynthesis and pharmacological mechanism of AC-5216 was needed. Neurosteroids serve as neuromodulators at neurotransmitter receptors, (e.g acetylcholine and glutamate receptors), and also affect emotion, memory, learning as well as stress responses54,55. The development and maintenance of stress-associated depression might be ascribed to the altered steroid production20,56. Further, neurosteroids have been reported to exert the protective effects in animal models that mimic a variety of pathogenic aspects of brain dysfunction, including Alzheimer’s disease, traumatic brain injury, and stroke56,57. In diabetic study, levels of neuroactive steroid were decreased in CNS of STZ-treated rats23. Consistent with this finding, our findings showed that levels of allopregnanolone were decreased significantly in the prefrontal cortex (Fig. 7A), hippocampus (Fig. 7B), and serum (Fig. 7C) in HFD-STZ rats. More studies had shown that depression was closely associated with the neurosteroids biosynthesis (e.g allopregnanolone). For instance, decreased allopregnanolone in peripheral blood or cerebrospinal fluid (CSF) was found to relevant to major depression, anxiety disorders, premenstrual dysphoric disorders, negative symptoms in schizophrenia, or impulsive aggression. These might be related to the effects of levels of allopregnanolone on the modulation of GABAA receptor function13,58,59. The evidences from the preclinical and clinical studies suggested that GABA played a role in the pathophysiology of the diabetic-related depression60. The study has shown that the GABAA agonist modulator interacted on the brain by changing the expression of α2 GABAA receptor subunit to elicit the neuroprotective effects on depressive-like behavior with diabetes mellitus61. The protective effects of allopregnanolone have been also reported in animal models of peripheral diabetic neuropathy (i.e., rats rendered diabetic by STZ injection). The neuroactive steroid improves sciatic nerve conduction velocity, mRNA levels of a myelin protein, such as the peripheral myelin protein 22, thermal threshold, skin innervation density57. It is also worth noting that allopregnanolone produces the antidepressant-like effects in T2DM.
On this point of view, it has demonstrated that the decreased levels of allopregnanolone were reversed by Flu (10.8 mg/kg, i.p) and MF. This finding was supported by that SSRIs were able to reverse the decreased allopregnanolone16. Also, clinical studies demonstrated that treatment with SSRIs normalized the allopregnanolone content of CSF in patients with depression62. The observation obtained in the present study indicating that similar to Flu (10.8 mg/kg, i.p) and MF, AC-5216 stimulated the lowered levels of allopregnanolone biosynthesis in the prefrontal cortex (Fig. 7A) and hippocampus (Fig. 7B). AC-5216 may represent an interesting therapeutic perspective based on the explanation that these ligands are able to increase the biosynthesis of neuroactive steroids56. This is consistent with a previous report that anxiolytic-like effects of AC-5216 were associated with the increased levels of allopregnanolone in the brain49. It is interesting to note that other TSPO ligands, such as Ro5-4864 and etifoxine, exerted the neuroprotective effects in various animal models of neurodegeneration by allopregnanolone biosynthesis23,63. However, the increased levels of allopregnanolone by AC-5216 (Fig. 7D,E) were blocked by PK11195, suggesting that the antidepressant-like effects of AC-5216 in T2DM animal model were associated with allopregnanolone biosynthesis, which was mediated via TSPO in the brain. These findings were also consistent with our previous studies showing that the anti-PTSD-like effects of AC-5216 were mediated by TSPO and allopregnanolone biosynthesis37,40. More researches have shown that TSPO activation was effective in ameliorating the severity of diabetic neuropathy through a local increase of neuroactive steroid levels23. We also found that the lower allopregnanolone biosynthesis in serum (Fig. 7C) was not reversed by AC-5216 indicating that action of AC-5216 on depression in T2DM was associated with the stimulation of allopregnanolone biosynthesis in the CNS.
Interesting in our present study, we found that the effects of Flu (10.8 mg/kg, i.p) and MF were similar to Met (1.8 mg/kg, i.p) on levels of PG (Fig. 6A), TC (Fig. 6B). TG (Fig. 6C) and INS (Fig. 6D). Our findings were supported by the previous study that Flu induced lowered blood glucose and in patients with diabetes64. Also, Flu enhanced the insulin sensitivity in obese patients and ameliorated the function of pancreatic β-cell secretory in drug-naive major depressive disorder (MDD) patients65,66. In addition, when it came to TC and TG, data showed that both of the parameters were decreased by chronic Flu treatment in patients with MDD67. Partially in line with Met (1.8 mg/kg, i.p), Flu (10.8 mg/kg, i.p) and MF, HFD-STZ-induced effects on PG (Fig. 6A) and INS (Fig. 6D) were reversed by AC-5216 without affecting TC (Fig. 6B) and TG (Fig. 6C). The results indicated that the antidepressant-like effects of AC-5216 were partially associated with the alternation of PG and INS and in agreement with that administration of TSPO ligands to obese mice reduced weight gain and lowered glucose level68. The possible mechanism is involved in activation of TSPO that improves glucose uptake. The previous study showed that the presence of TSPO by itself might provide the protection for the normal cellular activities of adipocytes68. Knockdown of TSPO in newly differentiated healthy adipocytes led to negative metabolic consequences, including the reduction of insulin stimulated glucose uptake and irregularities in adipokine release. Although many details remain to be elucidated, the data presented together have shown that activation of TSPO by ligands, with the ingestion of excess nutrition, may promote lipid consumption and improve adipocyte function, insulin sensitivity and glucose homeostasis, thus serving as therapeutic strategies to cure obesity and T2DM68. Further studies should be conducted to investigate the putative mechanisms of the interaction on depression in T2DM (e.g allopregnanolone biosynthesis, GABA system), as well as the role of TSPO in the modulation of T2DM aiming at new strategies to treat depression in patients with T2DM.
Conclusion
The present study demonstrated that the role of TSPO and allopregnanolone in the treatment of depression in T2DM. These effects were associated with the allopregnanolone biosynthesis that was mediated by TSPO. The findings advance our knowledge that TSPO selective ligands may be a promising new pharmacological class of drugs for the future treatment of depression in T2DM.
Additional Information
How to cite this article: Qiu, Z.-K. et al. The antidepressant-like activity of AC-5216, a ligand for 18KDa translocator protein (TSPO), in an animal model of diabetes mellitus. Sci. Rep. 6, 37345; doi: 10.1038/srep37345 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abrahamian, H., Hofmann, P., Kinzl, J. & Toplak, H. Diabetes mellitus and comorbid depression: improvement of both diseases with milnacipran. A replication study (results of the Austrian Major Depression Diabetes Mellitus study group). Neuropsychiatr Dis Treat. 8, 355–360 (2012).
Siddiqui, S. Depression in type 2 diabetes mellitus-a brief review. Diabetes Metab Syndr. 8, 62–65 (2014).
Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 6, 456–480 (2015).
McCall, A. L. Diabetes mellitus and the central nervous system. Int Rev Neurobiol. 51, 415–453 (2002).
Myers, A. K., Grannemann, B. D., Lingvay, I. & Trivedi, M. H. Brief report: depression and history of suicide attempts in adults with new-onset Type 2 Diabetes. Psychoneuroendocrinology. 38, 2810–2814 (2013).
Bassett, J., Adelman, A., Gabbay, R. & Aňel-Tiangco, R. M. Relationship between Depression and Treatment Satisfaction among Patients with Type 2 Diabetes. J Diabetes Metab. 3pii,1000210 (2012).
Mezuk, B., Eaton, W., Albrecht, S. & Golden, S. H. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 31, 2383–2390 (2008).
Springer, S. C. et al. Management of type 2 diabetes mellitus in children and adolescents. Pediatrics. 131, e648–e664 (2013).
Penckofer, S., Ferrans, C., Velsor-Friedrich, B. & Savoy, S. The psychological impact of living with diabetes: women’s day to day experiences. Diabetes Educator. 33, 680–690 (2007).
Sestile, C. C., Maraschin, J. C., Rangel, M. P., Cuman, R. K. & Audi, E. A. Antidepressant-like Effect of Insulin in Streptozotocin-induced Type 2 Diabetes Mellitus Rats. Basic Clin Pharmacol Toxicol. 119, 243–248 (2016).
Goodnick, P. J. Use of antidepressants in treatment of comorbid diabetes mellitus and depression as well as in diabetic neuropathy. Ann Clin Psychiatry. 13, 31–41 (2001).
Popovic, D., Vieta, E., Fornaro, M. & Perugi, G. Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRI antidepressants. J Affect Disord. 173, 211–215 (2015).
Melcangi, R. C. & Panzica, G. C. Allopregnanolone: state of the art. Prog Neurobiol. 113, 1–5 (2014).
Bäckström, T. et al. Allopregnanolone and mood disorders. Prog Neurobiol. 113, 88–94 (2014).
Frye, C. A. & Walf, A. A. Hippocampal 3alpha, 5alpha-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 78, 531–540 (2004).
Pinna, G. & Rasmusson, A. M. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 4, 102–116 (2012).
Hellgren, C., Åkerud, H., Skalkidou, A., Bäckström, T. & Sundström-Poromaa, I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 69, 147–153 (2014).
Pallarès, M., Llidó, A., Mòdol, L., Vallée, M. & Darbra, S. Finasteride administration potentiates the disruption of prepulse inhibition induced by forced swim stress. Behav Brain Res. 289, 55–60 (2015).
Papadopoulos,, V. et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 27, 402–409 (2006).
Costa, B., Da Pozzo, E. & Martini, C. Translocator protein as a promising target for novel anxiolytics. Curr Top Med Chem. 12,270–285 (2012).
Rupprecht, R. et al. Translocator protein (18kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 9, 971–988 (2010).
Rupprecht, R. et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 325, 490–493 (2009).
Mitro, N. et al. LXR and TSPO as new therapeutic targets to increase the levels of neuroactive steroids in the central nervous system of diabetic animals. Neurochem Int. 60, 616–621 (2012).
Kita, A. et al. Antianxiety and antidepressant-like effects of AC-5216, a novel mitochondrial benzodiazepine receptor ligand. Br J Pharmacol. 142, 1059–1072 (2004).
Vinerean, H. V., Gazda, L. S., Hall, R. D. & Smith, B. H. Streptozotocin is responsible for the induction and progression of renal tumorigenesis in diabetic Wistar-Furth rats treated with insulin or transplanted with agarose encapsulated porcine islets. Islets. 3, 196–203 (2011).
Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 5, 349–358 (2014).
Zhang, L. M. et al. Antidepressant-like and anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18kDa). Neuropharmacology. 81, 116–125 (2014a).
Miao, Y. L. et al. Midazolam ameliorates the behavior deficits of a rat posttraumatic stress disorder model through dual 18kDa translocator protein and central benzodiazepine receptor and neurosteroidogenesis. PLoS One. 9, e101450 (2014).
Qiu, Z. K. et al. Translocator protein mediates the anxiolytic and antidepressant effects of midazolam. Pharmacol Biochem Behav. 139, 77–83 (2015).
Wang, Y. H., Yin, L. T., Yang, H., Li, X. L. & Wu, K. G. Hypoglycemic and anti-depressant effects of Zuogui Jiangtang Jieyu formulation in a model of unpredictable chronic mild stress in rats with diabetes mellitus. Exp Ther Med. 8, 281–285 (2014).
Salpeter, S. R., Buckley, N. S., Kahn, J. A. & Salpeter, E. E. : Meta-analysis: Metformin treatment in persons at risk for diabetes mellitus. Am J Med. 121, 149–157 (2008).
Bekris, S., Antoniou, K., Daskas, S. & Papadopoulou-Daifoti, Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 161, 45–59 (2005).
Chen, H. X. et al. Antidepressant-like activity of YL-0919: a novel combined selective serotonin reuptake inhibitor and 5-HT1Areceptor agonist. PLoS One. 8, e83271 (2013).
Hattiangady, B., Mishra, V., Kodali, M., Shuai, B., Rao, X. & Shetty, A. K. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci. 8, 78 (2014).
Xue, R. et al. Antidepressant-like effects of 071031B, a novel serotonin and norepinephrine reuptake inhibitor. Eur Neuropsychopharmacol. 23, 728–741 (2013).
Yang, P. et al. Compromised Wound Healing in Ischemic Type 2 Diabetic Rats. PLoS One. 11, e0152068 (2016).
Zhang, L. M. et al. Involvement of allopregnanolone in the anti-PTSD-like effects of AC-5216. J Psychopharmacol. 30, 474–481 (2016).
Liu, L. et al. The identification of metabolic disturbances in the prefrontal cortex of the chronic restraint stress rat model of depression. Behav Brain Res. 305, 148–156 (2016).
Wang, Y., Xu, Y., Sheng, H., Ni, X. & Lu, J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammsome activation in hippocampus. Behav Brain Res. 307, 18–24 (2016).
Zhang, L. M. et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol. 17, 1659–1669 (2014b).
Al-Malki, A. L. Inhibition of α-Glucosidase by Thiosulfinate as a Target for Glucose Modulation in Diabetic Rats. Evid Based Complement Alternat Med. 2016, 7687915. doi: 10.1155/2016/7687915 (2016).
Penckofer, S. et al. Does glycemic variability impact mood and quality of life? Diabetes Technology & Therapeutics. 14, 303–310 (2012).
Hermanns, N. et al. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia. 50, 930–933 (2007).
Verma, S. K. et al. Impact of depression on health related quality of life in patients with diabetes. Annals of the Academy of Medicine Singapore. 39, 913–917 (2010).
de Groot, M., Anderson, R. M. & Freedland, K. E. Association of depression and diabetes complications: a meta-analysis. Psychosomatic Medicine. 63, 619–630 (2001).
Talbot, F. & Nouwen, A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 23, 1556–1562 (2000).
Li, N. et al. TCM Formula Xiaoyaosan Decoction Improves Depressive-Like Behaviors in Rats with Type 2 Diabetes. Evid Based Complement Alternat Med. 2015, 415243 (2015).
Guo, M. et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 41, 650–656 (2014).
Kita, A. & Furukawa, K. Involvement of neurosteroids in the anxiolytic-like effects of AC-5216 in mice. Pharmacol Biochem Behav. 89, 171–178 (2008).
Qiu, Z. K. et al. Repeated administration of AC-5216, a ligand for the 18kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 45, 40–46 (2013).
Costa, B. et al. Anxiolytic properties of a 2-phenylindolglyoxylamide TSPO ligand: stimulation of in vitro neurosteroid production affecting GABAA receptor activity. Psychoneuroendocrinology. 36, 463–472 (2011).
Da, Settimo F. et al. Anxiolytic-like effects of N, N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J Med Chem 2008. 51, 5798–5806 (2008).
Nothdurfter, C. Translocator protein (18kDa) as a target for novel anxiolytics with a favourable side-effect profile. J Neuroendocrinol. 4, 82–92 (2011).
Pibiri, F., Nelson, M., Guidotti, A., Costa, E. & Pinna, G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 5, 5567–5572 (2008).
Pinna, G., Costa, E. & Guidotti, A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 9, 24–30 (2009).
Nothdurfter, C., Rupprecht, R. & Rammes, G. Recent developments in potential anxiolytic agents targeting GABAA/BzR complex or the translocator protein (18kDa) (TSPO). Curr Top Med Chem. 12, 360–370 (2012).
Leonelli, E. et al. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience. 144, 1293–1304 (2007).
Girdler, S. S. & Klatzkin, R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 116, 125–139 (2007).
Purdy, R. H., Morrow, A. L., Moore, Jr. P. H. & Paul, S. M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 88, 4553–4557 (1991).
Yasin Wayhs, C. A., Tannhauser Barros, H. M. & Vargas, C. R. GABAergic Modulation in Diabetic Encephalopathy-Related Depression. Curr Pharm Des. 21, 4980–4988 (2015).
Caletti, G. Antidepressant dose of taurine increases mRNA expression of GABAA receptor α2 subunit and BDNF in the hippocampus of diabetic rats. Behav Brain Res. 283, 11–15 (2015).
Uzunova, V. Proc Natl Acad Sci USA Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. 95, 3239–3244 (1998).
Barron, A. M. et al. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J Neurosci. 33, 8891–8897 (2013).
Biagetti, B. & Corcoy, R. Hypoglycemia associated with fluoxetine treatment in a patient with type 1 diabetes. World J Clin Cases. 1, 169–171 (2013).
Chang, H. H. et al. The change of insulin levels after six weeks antidepressant use in drug-naïve major depressive patients. J Affect Disord. 150, 295–299 (2013).
Maheux, P., Ducros, F., Bourque, J., Garon, J. & Chiasson, J. L. Fluoxetine improves insulin sensitivity in obese patients with non-insulin-dependent diabetes mellitus independently of weight loss. Int J Obes Relat Metab Disord. 21, 97–102 (1997).
Shahsavand Ananloo, E., Ghaeli, P., Kamkar, M. Z. & Sadeghi, M. Comparing the effects of fluoxetine and imipramine on total cholesterol, triglyceride, and weight in patients with major depression. Daru. 21, 4 (2013).
Li, J. & Papadopoulos, V. Translocator protein (18kDa) as a pharmacological target in adipocytes to regulate glucose homeostasis. Biochem Pharmacol. 97, 99–110 (2015).
Acknowledgements
This study was supported by grants from Guangdong Natural Science Foundation of China (No. 2014A030310103) and National Natural Science Foundation of China (No. 81373993).
Author information
Authors and Affiliations
Contributions
All authors participated in the preparation of the manuscript, and read and approved the final manuscript. Zhi-Kun Qiu and Jia-Li He conceived the idea, directed the work and designed the experiments; Zhi-Kun Qiu and Xu Liu performed the experiments and paper writing; The data analyses were performed by Jia Zeng and Yong-Gang Shen; Guan-Hua Zhang, Hong Nie and Ji-sheng Chen provided comments and technical support.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Qiu, ZK., He, JL., Liu, X. et al. The antidepressant-like activity of AC-5216, a ligand for 18KDa translocator protein (TSPO), in an animal model of diabetes mellitus. Sci Rep 6, 37345 (2016). https://doi.org/10.1038/srep37345
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37345
This article is cited by
-
Translocator protein (18kDa) TSPO: a new diagnostic or therapeutic target for stress-related disorders?
Molecular Psychiatry (2022)
-
Regulation of Anxiety and Depression by Mitochondrial Translocator Protein-Mediated Steroidogenesis: the Role of Neurons
Molecular Neurobiology (2021)
-
Visualization of translocator protein (18 kDa) (TSPO) in the retina of diabetic retinopathy rats using fluorine-18-DPA-714
Annals of Nuclear Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.