Abstract
This case–control study identified the association between the arsenic methylation capacity and developmental delays and explored the association of this capacity with the health status of children. We recruited 120 children with developmental delays and 120 age- and sex-matched children without developmental delays. The health status of the children was assessed using the Pediatric Quality of Life Inventory (PedsQL) and Pediatric Outcomes Data Collection Instrument (PODCI). The arsenic methylation capacity was determined by the percentages of inorganic arsenic (InAs%), monomethylarsonic acid (MMAV%), and dimethylarsinic acid (DMAV%) through liquid chromatography and hydride generation atomic absorption spectrometry. Developmental delays were significantly positively associated with the total urinary arsenic concentration, InAs%, and MMAV%, and was significantly negatively associated with DMAV% in a dose-dependent manner. MMAV% was negatively associated with the health-related quality of life (HRQOL; −1.19 to −1.46, P < 0.01) and functional performance (−0.82 to −1.14, P < 0.01), whereas DMAV% was positively associated with HRQOL (0.33–0.35, P < 0.05) and functional performance (0.21–0.39, P < 0.01–0.05) in all children and in those with developmental delays. The arsenic methylation capacity is dose-dependently associated with developmental delays and with the health status of children, particularly those with developmental delays.
Similar content being viewed by others
Introduction
Exposure to environmental toxicants can affect health, particularly that of foetuses, infants, and children, during the sensitive and vulnerable early developmental and growth stages. Among heavy metals, arsenic is considered the most hazardous and toxic contaminant originating from environmental, medicinal, and occupational sources1,2, such as contaminated drinking water, silicon-based computer chips, pesticides, and feed additives for swine and poultry3. Arsenic toxicity depends on the total arsenic concentration and its oxidation state and chemical form4. InAs is methylated to MMAV and DMAV, which are subsequently converted in arsenic metabolism to monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII), respectively, and excreted in the urine in addition to unmetabolised InAs, namely arsenate (AsV) and arsenite (AsIII)2. In most populations, 85% of InAs is methylated to at least MMA and mostly to DMA5. The arsenic methylation process is considered a detoxification process because MMAV and DMAV are less toxic than is InAs6. Arsenic exposure is associated with lung, liver, kidney, bladder, prostate, and skin cancers7. Morphological alterations subsequent to arsenic exposure during rapid brain growth periods evidenced arsenic-induced developmental toxicity8. Moreover, in utero exposure to arsenic can induce epigenetic changes and increase the risk of diseases in later stages of life7. The epidemiological burden caused by arsenic-induced environmental toxicity exerted negative effects on the growth and development of children and is a serious concern worldwide9.
Neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4, which act on p75NTR, TrkA, TrKB, and TrkC receptors, regulate various neurodevelopmental functions10. Neurotrophin-mediated endocytic trafficking regulates various developmental events, such as synaptogenesis, axonal growth, and even degenerative changes11. A recent molecular study reported that precursor and mature BDNF inhibit Schwann cell migration12. The nervous system maintains the most appropriate connections through competition among neurons for limiting the amount of neurotrophins produced by target cells13. Defective neurotrophin functions in the brain caused by a combination of environmental and genetic factors may result in many neurological diseases14. Inhibitory effects of recombinant human neurotrophin on arsenic trioxide-induced neurotoxicity were reported15.
Children with developmental delays have a lower health status, including HRQOL and functional performance, than do those with typical development16,17. Although with a relatively small sample size, we previously reported that higher urinary total arsenic levels and a lower arsenic methylation capacity with a higher MMAV% and lower DMAV% are associated with developmental delays in preschool children in a dose–response manner16. However, no study has established evidence regarding the arsenic methylation capacity and health status of children. Therefore, the present study evaluated the association between the arsenic methylation capacity and developmental delays in a larger sample and explored the association between this capacity and the health status of children.
Results
The sociodemographic characteristics of the children and their mothers are shown in Table 1. The diagnosed developmental delays comprised cognitive dysfunctions (n = 5), speech–language delays (n = 14), gross and fine motor delays (n = 13), social and emotional delays (n = 12), global delays (n = 65), and unclassified delays (n = 11). Furthermore, we observed unclassified developmental disorders (n = 35), attention deficit hyperactivity disorder (n = 37), mental retardation (n = 14), autistic spectrum disorder (n = 10), articulation disorder or stuttering (n = 9), cerebral palsy (n = 7), and central nervous system-related disorders (n = 8). The mothers of the children with developmental delays had a significantly lower educational level than did those of the children without delays.
Table 2 presents the parent-reported health status and family functioning of the children. The children with developmental delays showed a significantly lower HRQOL and poorer functional performance than did those without developmental delays. Compared with the parents of the children without developmental delays, those of the children with developmental delays reported a significantly lower HRQOL, higher family influence, and increased psychological distress. We performed stratified analyses of urinary arsenic indices according to the age and sex of the children and educational level of their mothers. The results revealed that these indices were not associated with the age and sex of the children. Moreover, the children of mothers with a higher educational level exhibited a significantly higher DMAV% (91.75 ± 0.72 vs. 88.89 ± 0.90, P < 0.05), whereas those of mothers with a lower educational level had a significantly higher MMAV% (4.47 ± 0.48 vs. 2.91 ± 0.44, P < 0.05; data not shown).
Table 3 shows the dose–response association between the urinary arsenic indices and developmental delays. After adjustment for multiple variables, namely age, sex, educational level of the mothers, and urine creatinine, trend analyses of exposure strata in tertiles revealed the following dose–response associations. The association of developmental delays with the total urinary arsenic concentration, InAs%, and MMAV% was significantly positive, whereas that with DMAV% was significantly negative. These findings are consistent with our previous study regarding the association between the arsenic methylation capacity and developmental delays16, although the sample size was larger in this study.
Table 4 presents the association of the arsenic methylation capacity with the health status, namely the HRQOL and functional performance, of the participants determined using multiple linear regression analyses after adjustment for age, sex, and educational level of the mothers, according to the developmental status of the children. MMAV% showed a significantly negative association with the HRQOL (−1.19 to −1.46, P < 0.01) and functional performance (−0.82 to −1.14, P < 0.01), whereas DMAV% showed a significantly positive association with the HRQOL (0.33–0.35, P < 0.05) and functional performance (0.21–0.39, P < 0.01–0.05) in all children; the children with developmental delays demonstrated a similar tendency. However, in children without developmental delays, the association among MMAV%, psychosocial health, and global functioning was significantly negative and that among DMAV%, HRQOL, and functional performance was absent. In both groups, the total urinary arsenic concentration and InAs% were not associated with the health status, namely the HRQOL and functional performance (data not shown).
Discussion
The present study provides further evidence regarding the dose–response association between poor arsenic methylation capacity and developmental delays. The health status, namely the HRQOL and functional performance, was negatively associated with MMAV% and positively associated with DMAV% in all children and in those with developmental delays. According to our review of relevant literature, this study is the first to reveal that poor arsenic methylation capacity (higher MMAV% and lower DMAV%) is negatively associated with the health status of children, particularly those with developmental delays.
In Taiwan, humans may be exposed to arsenic through drinking water, edible oil, cereals, rice, and seafood16. Approximately 70% of organic and inorganic arsenic is excreted through the kidney via urine7, and a small portion is excreted through the skin, hair, nails, and faeces18. Therefore, urinary arsenic speciation profiles are crucial for investigating the metabolism, toxicity, and transformation of ingested arsenic19,20, and they can be used as effective biomarkers for internal dosing3. By contrast, total arsenic in blood is an imprecise biomarker of exposure because of the relative toxicity and methylation efficiency of the different arsenic forms and the short-term effect of the exposure measure compared with total lead in blood21. Therefore, we used urinary arsenic species rather than blood arsenic levels to identify the association of the arsenic methylation capacity with developmental delays and the health status of the children in this study.
The primary metabolic pathway of InAs facilitates arsenic elimination through its methylation to MMA and DMA22. Physiological S-adenosylmethionine (SAM) is necessary for the methylation of InAs to MMA and subsequently to DMA23, and both methylation steps are catalysed by arsenic (+3 oxidation state) methyltransferase (AS3MT)22. SAM is biosynthesised through folate- and cobalamin-dependent one-carbon metabolism22. The arsenic methylation capacity is regulated by various enzymes and arsenic metabolism-related genes, such as AS3MT, purine nucleoside phosphorylase, and glutathione S-transferase omega 224. Gene polymorphism in the one-carbon metabolism pathway through plasma folate and homocysteine metabolism may further lead to the risk of cancer25. Future research must identify the association between arsenic metabolism-related genes and developmental delays in children.
Various factors affect arsenic methylation in arsenic-exposed humans, including age, gender, race, lifestyle, inherited genetic characteristics, socioeconomic status, smoking, drinking, exposure route, arsenic species, and dietary factors such as fish, jaggery, tea, fruit, vitamins, N-acetylcysteine, glutathione, and zinc6,9,26,27. The arsenic methylation capacity was higher in children having a higher consumption of folate, meat, eggs, red–orange vegetables, and green leafy vegetables and higher body mass index9. For urinary arsenic species measurement, the participants were suggested to not consume seafood for at least 3 days before urine collection because of increases in DMAV metabolism28. However, recent studies have reported that individual arsenic methylation patterns remained fairly stable for 5 days following arsenic ingestion29,30. We evaluated the association of the arsenic methylation capacity with development delays and the health status rather than the factors associated with the effects of the arsenic metabolism capacity on urinary arsenic excretion in children. Therefore, in this study, we neither assessed the factors related to arsenic metabolism, including neurotrophins and dietary state, nor restricted the diet and nutrition of the children during the intervention period. We could not determine the exact major exposure sources of arsenic in children; however, the urinary arsenic indices measured here can account for exposure to all sources16,31. The association between arsenic metabolism genes and poor arsenic methylation capacity and their associations with developmental delays warrant further investigation.
Studies have reported that arsenic can cross the placental barrier32,33,34. We could not rule out the possibility that developmental delays are caused by the arsenic exposure of the children rather than that of the mothers during pregnancy. Therefore, we further analysed the urinary arsenic methylation profiles of the mothers. These profiles showed no statistically significant differences in total urinary arsenic (15.02 ± 1.85 μg/L vs. 16.67 ± 2.28 μg/L, P = 0.58), InAs% (7.46 ± 0. 85 vs. 5.92 ± 0.99, P = 0.25), MMAV% (5.11 ± 0.94 vs. 4.36 ± 1.32, P = 0.63), and DMAV% (87.44 ± 1.42 vs. 89.72 ± 1.66, P = 0.30) among the mothers of the children with and without developmental delays. The results favour the speculation that developmental delays are caused by the arsenic exposure of the children rather than that of their mothers during pregnancy. However, because of the cross-sectional study design, additional longitudinal studies are required to confirm the speculation.
Although both MMA and DMA are methylated via similar pathways, MMA is considered more toxic than the other metabolites35. Decreased arsenic methylation capacity with high MMA% in urine is related to an increased cancer risk, noncancer diseases, and developmental delays16,35. We demonstrated positive correlations between DMAV % and the health status, namely the HRQOL and functional performance, and negative correlations between MMAV % and the health status. A possible explanation for these results is that a lower arsenic methylation capacity with lower MMAV% and higher DMAV% is either genetically related or influenced by environmental factors, such as long-term dietary and nutritional patterns29, which may be associated with the developmental status of the children; it further affects their health status, including the HRQOL. Because of the cross-sectional study design, future follow-up studies for children enrolled for a certain amount of time are indicated to determine whether the developmental delays can be alleviated and whether the HRQOL can improve with therapeutic interventions, such as changing their dietary habits and increasing the vitamin dosage.
The association of the arsenic methylation capacity with developmental disabilities evidenced by a deteriorated health status of the children was not as significant as that of the arsenic methylation capacity with developmental delays. Poor arsenic methylation capacity may contribute to developmental delays in children through various mechanisms. Furthermore, because of the case–control design with an exposure–outcome timeline constraint, we can claim only the presence of an association of arsenic exposure with developmental disabilities and could not differentiate this association from that of arsenic exposure with developmental delays in the present study. In addition, developmental delays themselves, rather than poor arsenic methylation capacity, may account for developmental disabilities in children. Therefore, the association between the arsenic methylation capacity and developmental disabilities in children may be caused by developmental delays. Additional longitudinal studies must explore the association of arsenic exposure with developmental delays and disabilities in children.
Arsenic exposure can cause developmental neurotoxicity in rat brains36. The oral administration of arsenic resulted in neurochemical and behavioural alterations in an animal model37. The exposure of pregnant rats to low InAs concentrations through drinking water during gestation and lactation can result in the delayed development of sensory–motor reflexes in rat pups and decreased development of locomotor activity in adult rats38. Arsenic exposure affects learning and concentration because arsenic can invade the blood–brain barrier39. A prospective cohort study conducted in Bangladesh reported that environmental arsenic exposure in early life hinders growth by the age of 5 years40. In a US pregnancy cohort study, low-level in utero arsenic exposure during gestation impaired the foetal immune regulation system, potentially causing disease in the future41. In rural Bangladesh, high arsenic exposure reduced the cell-mediated immunity in preschool children42. Concurrent arsenic exposure-induced immunosuppression may contribute to the risk of chronic diseases or infections42. Furthermore, in paediatric populations, an inverse association existed between InAs concentrations and judgment, attention, executive function, language, memory, visuospatial function, and processing speed43. We previously demonstrated a dose–response association between arsenic exposure and developmental delays16. However, the overall evidence did not reveal a specific causal dose–response relationship between arsenic exposure and cognitive function21. The differences in lifetime arsenic exposure and genetic polymorphisms among various populations may contribute to the varying results.
This study has several limitations. First, as mentioned, we could determine only associations between poor arsenic methylation capacity and developmental delays and between the arsenic methylation capacity and the health status of the children; this crucial point may affect the validity of our data interpretation. Second, because urinary arsenic concentrations were measured at a single time point, the exact sources of arsenic exposure for children remain unknown. However, the accuracy of urinary arsenic index evaluation by using spot urine samples might be reliable because all participants maintained their lifestyle and appeared to retain their homeostatic metabolism during the intervention period16. Third, the diagnoses of the children with developmental delays varied; therefore, we cannot extrapolate these results to a specific developmental delay in children, such as attention deficit hyperactivity disorder. However, the inclusion of participants with diverse diagnoses of developmental delays was advantageous because the sample represented various developmental delays among children assessed and treated in clinical settings. Fourth, the controls did not receive the same developmental tests as those received by children with developmental delays; therefore, we could not provide descriptive evaluation data, as provided for the children with developmental delays, to prove that the controls were true controls. Fifth, although all children with developmental delays were diagnosed using standardised developmental tests, we did not use molecular biomarkers to define the children’s diseases or characterise their symptom severity. The measurement of levels of neurotrophins, at least NGF and BDNF, in urine is necessary for enhancing the validity and generalisability of the current findings in the future. Sixth, the children could not completely define their health status because they were very young, had underdeveloped communication skills, or lacked concentration to assess their own views. Although we cannot rule out some probable differences between the children’s and parents’ perspectives about health, we used highly valid and reliable parent-reported health measurements for children. Seventh, HRQOL data were missing for dietary interventions. In addition to genetic polymorphisms, a folate-rich diet might affect the arsenic methylation capacity. Hence, data on the amount of folate or other vitamins and drugs that were consumed during the study might have affected the arsenic methylation capacity. Further information related to diet, nutrition, and drugs is required in future studies. Finally, the sample size was fairly small; a community-based longitudinal study with a larger sample size is required in the future for generalising the results.
Conclusions
The present study confirmed that poor arsenic methylation capacity is associated with developmental delays in a dose-dependent manner in a sample larger than that of previous study. According to our review of relevant literature, this study is the first to demonstrate that poor arsenic methylation capacity (high MMAV and low DMAV) is negatively associated with the health status, namely the HRQOL and functional performance, of children, particularly those with developmental delays.
Methods
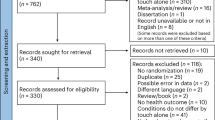
This prospective study was conducted at Shin Kong Wu Ho-Su Memorial Teaching Hospital in Taipei, Taiwan. Children aged 2–10 years with a diagnosis of developmental delays in fine motor, gross motor, cognition, social and emotional, and speech and language domains were eligible for this study. A developmental delay was defined as according to a performance 2 or more standard deviations lower than the mean on age-appropriate, standardised, norm-referenced tests. The assessment tools used included the Bayley III Scale of Infant and Toddler Development, Preschool Language Evaluation Tool, Child Expression Evaluation Tool, Chinese Wechsler Intelligence Scale for Children (3rd edition), Gross Motor Function Measure, and Peabody Developmental Motor Scales. The family structure, the social support system, and hearing and visual acuity were routinely evaluated. Additional electroencephalography, cranial sonography, magnetic resonance imaging, and a genetic chromosomal check-up were conducted according to the children’s conditions. The detailed procedures are presented in our previous studies16,17. In total, 120 children with a diagnosis of developmental delays at the early developmental intervention clinic of the hospital were included, along with their mothers. In addition, 120 age- and sex-matched children who visited the paediatric clinics of the hospital for regular developmental evaluation and did not receive a diagnosis of developmental delays during the same period were recruited as the controls, along with their mothers.
The study was approved by the Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital, in accordance with the World Medical Association Declaration of Helsinki (ClinicalTrials.gov: NCT02523989, date of registration: Aug 13, 2015). Informed written consent for the study was obtained from all of the parents of the children, and they granted permission for their and the children’s recruitment. A well-trained examiner administered structured questionnaires to the parents for assessing the health status of the children and its effect on their family function.
Health status: HRQOL and functional performance of children
The HRQOL was measured using the parent-reported format of the PedsQL Generic Core Scales44. Higher scores represented a higher HRQOL. The Chinese version of the aforementioned scales yields satisfactory, feasible, reliable, and valid results45,46. The PODCI was used to assess the functional performance of the children by using a parent-reported form47, with scores ranging from 0 to 100–100 indicates the highest level of functional performance. The reliability of the Chinese version of the PODCI is satisfactory46,48.
Family functioning: HRQOL, family influence, and psychological distress of parents
The Chinese version of the World Health Organization Quality of Life: Brief Version questionnaire was used to assess the parental HRQOL49. Scores range from 0 to 100, with a higher score indicating a higher HRQOL. This questionnaire has satisfactory reliability46,49. The PedsQL Family Impact Module was used to assess the effect of the children’s chronic conditions on the functioning of their parents and family44. Higher scores indicate a lower effect on the family. Moreover, the Chinese version of this module has satisfactory reliability46. The Hospital Anxiety and Depression Scale was used to measure parental psychological distress50; its Chinese version has satisfactory reliability46,48.
Urine sample collection and analysis
Spot urine samples were collected from each participant, immediately frozen, and stored at −20 °C. In all urine samples, the levels of 4 forms of arsenic, AsIII, AsV, MMAV, and DMAV, were measured through high-performance liquid chromatography (Waters 501; Waters Associates, Milford, MA, USA) combined with hydride generation atomic absorption spectrometry (Perkin–Elmer Flow Injection Analysis System 400-AA 100; Perkin–Elmer, Waltham, MA, USA). The detailed protocol was presented in our previous study31. The recovery rates of AsIII, AsV, MMAV, and DMAV ranged from 93.8% to 102.2%, with detection limits of 0.02, 0.07, 0.05, and 0.08 μg/L, respectively. For assessing the validity of the measurements, we purchased freeze-dried SRM 2670 urine, containing 480 ± 100 μg/L arsenic, from the National Institute of Standards and Technology (Gaithersburg, MD, USA). We detected arsenic at 507 ± 17 μg/L in the SRM 2670 standard (n = 4). For stabilising the urinary arsenic profiles, analyses were performed within 6 months of sample collection51.
Statistical analysis
The results are expressed as the mean ± standard deviation. Chi-squared and t tests were used for analysing the demographic data of both study groups. All urinary arsenic profiles were normalised using urinary creatinine (μg/g or mg/g creatinine). The total urinary arsenic concentration (μg/g creatinine) was defined as the sum of AsIII, AsV, MMAV, and DMAV concentrations. The relative proportion of each arsenic species (InAs%, MMAV%, and DMAV%) was calculated by dividing the species concentrations by the total arsenic concentration. Multivariate logistic regression models were used to estimate odds ratios (ORs) and 95% CIs for determining the association between the urinary arsenic profile and developmental delay risk. Furthermore, multiple linear regression analyses were performed to identify the association between the arsenic methylation capacity and the health status of the children. Cut-off points for the values of various arsenic indices were the respective tertiles of the controls for dose–response analyses. Significance tests for the linear trend among ORs across exposure strata were performed by categorising exposure variables and considering scored variables as continuous. The potential confounding factors, namely age, sex, birth weight of the children, gestational weeks of their mothers, and urine creatinine, were adjusted in the multivariate models. All data were analysed using the Statistical Analysis Software package (version 9.1.2; SAS, Cary, NC, USA). The statistical significance level was set at a 2-sided P value of <05. The sample size was calculated assuming a standardised effect (mean difference and standard deviation) of 0.5. To achieve 95% power at a significance level of.05, the number of participants in each group had to be at least 11016.
Additional Information
How to cite this article: Hsueh, Y.-M. et al. Association of Arsenic Methylation Capacity with Developmental Delays and Health Status in Children: A Prospective Case–Control Trial. Sci. Rep. 6, 37287; doi: 10.1038/srep37287 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Rodríguez-Barranco, M. et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454–455, 562–577 (2013).
Falck, A. J. et al. Developmental Exposure to Environmental Toxicants. Pediatr Clin North Am 62, 1173–1197 (2015).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Lead. Atlanta, GA: US. Department of Health and Human Services, Public Health Service (2007).
Garcia-Vargas, G. G. & Cebrian, M. E. Health effects of arsenic. In: Wang L. W., ed. Toxicology of metals. Boca Raton : CRC Press 423–438 (1996).
Basu, A. et al. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environ Health Perspect 119, 1308–1313 (2011).
Yu, H., Liu, S., Li, M. & Wu, B. Influence of diet, vitamin, tea, trace elements and exogenous antioxidants on arsenic metabolism and toxicity. Environ Geochem Health 38, 339–351 (2016).
Abdul, K. S., Jayasinghe, S. S., Chandana, E. P., Jayasumana, C. & De Silva, P. M. Arsenic and human health effects: A review. Environ Toxicol Pharmacol 40, 828–846 (2015).
Dhar, P., Mohari, N. & Mehra, R. D. Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology 234(1–2), 10–20 (2007).
Kordas, K. et al. Low-level arsenic exposure: Nutritional and dietary predictors in first-grade Uruguayan children. Environ Res 147, 16–23 (2016).
Bothwell, M. NGF, BDNF, NT3, and NT4. Handb Exp Pharmacol 220, 3–15 (2014).
Ascano, M., Bodmer, D. & Kuruvilla, R. Endocytic trafficking of neurotrophins in neural development. Trends in Cell Biology 22, 266–273 (2012).
Ding, Y. Q. et al. ProBDNF inhibits collective migration and chemotaxis of rat Schwann cells. Tissue Cell 48, 503–510 (2016).
Katrin, Deinhardt & Moses, V. Chao. I. Intercellular Signaling in Development and Disease: Cell Signaling Collection how to reference books (ed. Katrin, D. ) 303–307 (Elsevier’s, 2011).
Park, H. & Poo, M. M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14, 7–23 (2013).
Lou, Y. et al. [Inhibitory effects of recombinant human neurotrophin-4/5 protein on neurotoxicity caused by arsenic trioxide]. Zhonghua Yu Fang Yi Xue Za Zhi. 33, 295–297 (1999).
Hsieh, R. L. et al. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int J Hyg Environ Health 217, 678–686 (2014).
Hsieh, R. L. et al. Quality of life and impact of children with unclassified developmental delays. J Paediatr Child Health. 49, E116–E121 (2013).
Ghosh, A., Majumder, S., Awal, M. A. & Rao, D. R. Arsenic exposure to dairy cows in Bangladesh. Arch Environ Contam Toxicol 64, 151–159 (2013).
Cui, J., Shi, J., Jiang, G. & Jing, C. Arsenic levels and speciation from ingestion exposures to biomarkers in Shanxi, China: implications for human health. Environ Sci Technol 47, 5419–5424 (2013).
Mandal, B. K., Ogra, Y., Anzai, K. & Suzuki, K. T. Speciation of arsenic in biological samples. Toxicol Appl Pharmacol 198, 307–318 (2004).
Tsuji, J. S., Garry, M. R., Perez, V. & Chang, E. T. Low-level arsenic exposure and developmental neurotoxicity in children: A systematic review and risk assessment. Toxicology 337, 91–107 (2015).
Howe, C. G. et al. Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr. 144, 690–697 (2014).
Paul, S. & Giri, A. K. Epimutagenesis: A prospective mechanism to remediate arsenic-induced toxicity. Environ Int 81, 8–17 (2015).
Huang, Y. K. et al. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem Toxicol 46, 929–938 (2008).
Chung, C. J. et al. Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer Causes Control 21, 1605–1613 (2010).
Shen,. H. et al. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 13, 205 (2016).
Jain, R. B. Association of arsenic exposure with smoking, alcohol, and caffeine consumption: data from NHANES 2005-2010. Environ Toxicol Pharmacol. 39, 651–658 (2015).
Ma, M. & Le, X. C. Effect of arsenosugar ingestion on urinary arsenic speciation. Clin Chem. 44, 539–550 (1998).
Steinmaus, C., Yuan, Y., Kalman, D., Atallah, R. & Smith, A. H. Intraindividual variability in arsenic methylation in a U.S. population. Cancer Epidemiol Biomarkers Prev 14, 919–924 (2005).
Concha, G., Vogler, G., Nermell, B. & Vahter, M. Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Occup Environ Health 75, 576–580 (2002).
Hsueh, Y. M. et al. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A 54, 431–444 (1998).
Kaya-Akyüzlü, D., Kayaaltı, Z., Doğan, D. & Söylemezoğlu, T. Does maternal MDR1 C1236T polymorphism have an effect on placental arsenic levels? Environ Toxicol Pharmacol. 41, 142–146 (2016).
Punshon, T. et al. Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort. J Expo Sci Environ Epidemiol. 25, 599–603 (2015).
Vahter, M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 29, 381–399, doi: 10.1146/annurev-nutr-080508-141102 (2009).
Chen, Y. et al. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol. 178, 372–381 (2013).
Dhar, P., Mohari, N. & Mehra, R. D. Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology 234, 10–20 (2007).
García-Medina, N. E. et al. Conditioned flavor aversion and brain Fos expression following exposure to arsenic. Toxicology 235, 73–82 (2007).
Gumilar, F., Lencinas, I., Bras, C., Giannuzzi, L. & Minetti, A. Locomotor activity and sensory-motor developmental alterations in rat offspring exposed to arsenic prenatally and via lactation. Neurotoxicol Teratol 49, 1–9 (2015).
Mundey et al. Antioxidant Potential of Ocimum sanctum in Arsenic Induced Nervous Tissue Damage. Braz J. Vet. Pathol 6, 95–101 (2013).
Gardner, R. M., Kippler, M., Tofail, F., Bottai, M., Hamadani, J., Grandér, M. et al. Environmental exposure to metals and children’s growth to age 5 years: a prospective cohort study. Am J Epidemiol 177, 1356–1367 (2013).
Nadeau, K. C. et al. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin Immunol 155, 188–197 (2014).
Ahmed, S. et al. Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh. Toxicol Sci 141, 166–175 (2014).
Edwards, M., Hall, J., Gong, G. & O’Bryant, S. E. Arsenic exposure, AS3MT polymorphism, and neuropsychological functioning among rural dwelling adults and elders: a cross-sectional study. Environmental Health 13, 15 (2014).
Varni, J. W., Seid, M. & Rode, C. A. The PedsQL: measurement model for the pediatric quality of life inventory. Med. Care 37, 126–139 (1999).
Chan, L. F., Chow, S. M. & Lo, S. K. Preliminary validation of the Chinese version of the Pediatric Quality of Life Inventory. Int. J. Rehabil. Res. 28, 219–227 (2005).
Hsieh, R. L., Huang, H. Y. & Lee, W. C. The Correlation of Pediatric Outcome Data Collection Instrument with Health Related Quality of Life, Emotion in Children and Their Parents with Developmental Delays. Taipei: Taiwan Academy of Physical Medicine and Rehabilitation (2009).
Haynes, R. J. & Sullivan, E. The Pediatric Orthopaedic Society of North America pediatric orthopaedic functional health questionnaire: an analysis of normals. J Pediatr Orthop 21, 619–621 (2001).
Hsieh, R. L., Lin, M. I., Huang, H. Y. & Lee, W. C. The relationship between pediatric outcomes data collection instruments and functional impairment in developmentally delayed Chinese children and their parents’ health: implications for child and family-centered medicine. Int J Pers Cent Med 1, 1–8 (2011).
Hwang, H. F., Liang, W. M., Chiu, Y. N. & Lin, M. R. Suitability of the WHOQOL-BREF for community-dwelling older people in Taiwan. Age. Ageing 32, 593–600 (2003).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr Scand 67, 361–370 (1983).
Chen, Y. C., Amarasiriwardena, C. J., Hsueh, Y. M. & Christiani, D. C. Stability of arsenic species and insoluble arsenic in human urine. Cancer Epidemiol Biomarkers Prev 11, 1427–1433 (2002).
Acknowledgements
This study was supported by research grants from Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-103-DR-36, SKH-8302-104-DR-29, SKH-8302-105-DR-24, SKH-TMU-102-10, and SKH-TMU-100-06) and Taiwan’s Ministry of Science and Technology (MOST 105-2314-B-341-002 and MOST 105-2314-B-002-049-MY3).
Author information
Authors and Affiliations
Contributions
R.L.H. coordinated and designed the study. C.Y.L., S.N.C., and S.R.H. contributed to case collection and sample analysis. H.S.S., M.I.L., and S.C.M. assisted in case collection. Y.M.H. and W.J.C analysed the data. Y.M.H. and R.L.H. drafted the manuscript, and other authors critically revised it. All authors have provided approval for publication.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hsueh, YM., Chen, WJ., Lee, CY. et al. Association of Arsenic Methylation Capacity with Developmental Delays and Health Status in Children: A Prospective Case–Control Trial. Sci Rep 6, 37287 (2016). https://doi.org/10.1038/srep37287
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37287
This article is cited by
-
Polymorphism of nucleotide binding domain-like receptor protein 3 (NLRP3) increases susceptibility of total urinary arsenic to renal cell carcinoma
Scientific Reports (2020)
-
Changes in urinary arsenic species and methylation capacity in original arsenic exposure cohort after water quality improvement
Environmental Geochemistry and Health (2020)
-
Developmental toxicity of arsenic: a drift from the classical dose–response relationship
Archives of Toxicology (2020)
-
Exposure to arsenic in utero is associated with various types of DNA damage and micronuclei in newborns: a birth cohort study
Environmental Health (2019)
-
Serum folate levels and urinary arsenic methylation profiles in the US population: NHANES, 2003–2012
Journal of Exposure Science & Environmental Epidemiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.