Abstract
Ice-free cryopreservation, referred to as vitrification, is receiving increased attention in the human and animal assisted reproduction. However, it introduces the detrimental osmotic stress by adding and removing high contents of cryoprotectants. In this study, we evaluated the effects of normalizing cell volume regulation by adding glycine, an organic osmolyte, during vitrification of mouse germinal vesicle stage oocyte and/or subsequent maturation on its development. The data showed that glycine supplementation in either vitrification/thawing or maturation medium significantly improved the cytoplasmic maturation of MII oocytes manifested by spindle assembly, chromosomal alignment, mitochondrial distribution, euploidy rate, and blastocyst development following fertilization in vitro, compared to the control without glycine treatment. Furthermore, glycine addition during both vitrification/thawing and maturation further enhanced the oocyte quality demonstrated by various markers, including ATP contents and embryo development. Lastly, the effect of anti-apoptosis was also observed when glycine was added during vitrification. Our result suggests that reducing osmotic stress induced by vitrification could improve the development of vitrified mouse oocyte.
Similar content being viewed by others
Introduction
Cryopreservation of oocytes, is standard practice in Assisted Reproductive Technologies1,2,3 (ART). Indeed, the experimental connotation, once associated with oocyte cryopreservation has been removed4,5. Pregnancy rates with cryopreserved oocytes equal those using fresh or cryopreserved embryos and the incidence of birth anomalies does not differ between them6,7,8. The use of oocytes avoids ethical concerns and legal restrictions associated with embryo preservation. It reduces the risk and costs of repeated ovarian stimulation, since excess oocytes can be stored. Freezing oocytes is attractive to women without a partner or soon to lose ovarian function due to surgery, chemotherapy or radiation. In addition, women are increasingly choosing to delay motherhood because of demanding careers and/or financial concerns. In support, some companies (ie. Apple and Facebook) have offered to sponsor women employees who choose to freeze their eggs to delay pregnancy9.
Historically, oocytes were frozen in the second metaphase (MII) of meiosis (mature oocytes). Unfortunately, MII oocytes are very sensitive to the destructive effects of freezing because of their high membrane permeability and complicated constructions. Freezing induced damages include loss of cell membrane, meiotic spindle disorganization, chromatid disjunction and aneuploidy10,11,12, abnormal mitochondrial distribution and activity (ie. ATP production), premature cortical granule release and zona pellucida hardening3,13,14,15.
To circumvent the problems associated with MII oocytes, research efforts have recently focused on the cryopreservation of germinal vesicle (GV) stage, or immature oocytes14. Because GV oocytes have not yet developed a spindle apparatus, they are thought to be more resistant to freezing-induced damage. Nevertheless, GV oocytes are susceptible to freezing inflicted injury and their rate of development into blastocysts remains low compared to MII oocytes16. A major difficulty in the use of frozen GV oocytes is that after thawing, they must undergo in vitro maturation in order to be fertilized and develop into embryos17,18. The optimal incubation media that will ensure successful in vitro maturation of GV oocytes (developmental competence), has yet to be established.
Oocyte freezing is often accomplished through vitrification, an ice-free cryopreservation process achieved by ultra-rapid cooling rates in a small volume of media19. Unfortunately, the concentrations of cryoprotectants required for vitrification are much greater than those used in slow cooling, resulting in a hypertonic environment and abnormal osmotic pressures that are detrimental to cell viability20,21.
Physiologically, embryos appear to maintain cell volume as they pass through the oviducts and uterus by first importing inorganic ions and later small organic molecules called osmolytes22. Some putative organic osmolytes, are amino acids or amino acid derivatives such as glutamine, glycine, betaine, proline and beta-alanine. Of these, glycine appears to convey the greatest level of protection against the hypertonicity created by the cryoprotectants23,24. Moreover, a single powerful transporter (GLYT1) for glycine is present and active in embryos until compaction25. Using MII oocytes, or 1 and 2-cell stage mouse embryos, it was shown that the accumulation of glycine varied proportionately with the osmolarity of the incubation media26,27,28. Moreover, glycine supplementation, allowed 1-cell stage mouse embryos to develop through the 2-cell stage block in media that was 70 mOsM more hypertonic when compared to media without glycine24,27. Inhibition of GLYT1 completely abolishes the development of 1-cell mouse embryos in hypertonic media as well as osmosensitive glycine accumulation27. Glycine supplementation of mouse MII oocytes during vitrification maintained mitochondrial distribution, mitochondrial membrane potential and development to the blastocyst stage, when compared to those without glycine29. Collectively, these findings suggest that embryonic cells take up glycine, in part, to balance external osmolarity.
While GLYT1 activity is undetectable in GV oocytes in situ (which also contain very little endogenous glycine), transporter activity is induced upon ovulation or soon after removal from the ovarian follicle28. It would not be unreasonable to propose that glycine supplementation during in vitro maturation, might increase the developmental competence of the gametes. Therefore our objectives were to determine the effects of glycine supplementation during vitrification and in vitro maturation on the developmental competance of GV oocytes, as determined by (1). Oocyte survivability and progression to the blastocyst stage, (2). Assembly of meiotic spindle and chromosomal allignment, (3). Rate of oocyte aneuploidy, (4). Mitochondrial distribution and activity (ATP production), and (5). Rate of apoptosis.
Results
Glycine supplementation improved the preimplantation development following COCs vitrification
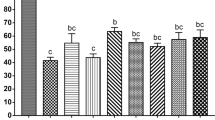
To test whether glycine supplementation in the vitrification and thawing medium increases the survival rate of oocytes at GV stage, we freezed and thawed the COCs with or without physiological levels of glycine at 1 mM, which was found to be effective in maintaining normal cell volume in previous study, respectively. The results indicated that there was no significant difference in the survival rate (P > 0.05, N = 5) of oocytes, manifested by whether they had evenly granulated cytoplasm with apparent perivitelline space (viable) or degenerated cytoplasm without perivitelline space (nonviable), between glycine (90.3 ± 1.0%, n = 141) and control (88.5 ± 1.3%, n = 141) groups (Fig. 1a). To further examined whether glycine supplementation improves the oocyte maturation following COCs vitrification, we compared the nuclear maturation rate among groups of adding 1 mM glycine into either vitrification medium or maturation medium, or both vitrification and maturation medium. The data showed that there were no significant difference among the examined groups (Control: 93.4 ± 1.6%, n = 124; Vitrified + Gly: 91.6 ± 1.2%, n = 125; IVM + Gly: 93.6 ± 1.6%, n = 135; and Vitrified + IVM + Gly: 97.6 ± 1.0%, n = 133) (P > 0.05, N = 5) (Fig. 1b). However, adding 1 mM glycine into either vitrification medium or maturation medium significantly increased the percentages of 2-cell embryo (51.4 ± 1.4%, n = 117; 45.7 ± 2.9%, n = 124, respectively) and blastocyst (22.7 ± 1.8%, n = 60; 19.0 ± 1.8%, n = 56, respectively), compared to those in the control group without glycine supplementation (2-cell: 32.8 ± 1.5%, n = 116; blastocyst: 8.7 ± 3.6%, n = 38) (P < 0.05, N = 5), but no significant difference was found between glycine supplementation in vitrification medium and that in maturation medium alone (P > 0.05, N = 5). Interestingly, adding glycine into both vitrification and maturation medium further enhanced the percentages of 2-cell embryo (65.7 ± 2.2%, n = 130) and blastocyst (32.7 ± 1.7%, n = 85), compared to those of glycine supplementation in either vitrification medium or maturation medium (P < 0.05, N = 5). (Fig. 1c,d). It is worth noting that there was no glycine supplementation in the embryo culture medium during all the experiments.
Effects of Glycine supplementation during vitrification and/or in vitro maturation on oocytes’ survival, maturation and subsequent preimplantation development.
(a) The survival rate of vitrified GV oocyte with or without glycine addition during vitrification/thawing. (b) The percentage of MII oocytes (number of MII oocytes/number of survival GV oocytes) from various glycine treatment groups. (c) The rate of 2-cell embryo (number of 2-cell embryos/number of MII oocytes) after in vitro fertilization. d. The percentage of blastocyst (number of blastocysts/number of 2-cell embryos) from different glycine treatment groups. Control: without glycine treatment; IVM + Gly: glycine treatment during in vitro maturation only; Vitrified + Gly: glycine treatment during vitrification/thawing only; Vitrified + IVM + Gly: glycine treatment during both vitrification/thawing and maturation in vitro. Different low case letters above columns indicate statistical differences at P < 0.05.
Loading oocytes with glycine during vitrification and/or maturation improved MII-spindle assembly
Correct bipolar spindle assemble is essential for the alignment of all chromosomes at the spindle equator and accurate chromosome segregation in mammalian oocyte, which is served as an important marker of oocyte quality. We speculated that addition of glycine during vitrification and/or maturation increased the formation of blastocyst following COCs freezing, maturation and fertilization in vitro as it improved the MII-spindle assembly and chromosome alignment. As shown in Fig. 2. The oocyte spindles were classified into three groups according to morphology: (1) normal spindles with dense, bipolar (barrel-shaped or elliptical) microtubules; (2) abnormal spindles, partial or total disorganization, clumped or dispersed distribution, and multiple spindle-like structures; and (3) absent or no meiotic spindle around the chromosomes. Chromosome alignment was judged into two groups: (1) normal, chromosomes were compact in one area along the equator of the spindle; and (2) abnormal, chromosomes were distributed scatteredly and far away from the equator of spindle (Fig. 2). In parallel with the data that glycine supplementation improved the preimplantation development following COCs vitrification, adding 1 mM glycine into either vitrification medium or maturation medium significantly increased the rates of normal spindle assembly (55.8 ± 4.5%, n = 68; 51.1 ± 3.6%, n = 59, respectively) and chromosome alignment (57.1 ± 3.5%, n = 68; 53.0 ± 4.5%, n = 59, respectively), compared to those in the control group without glycine supplementation (Spindle: 37.2 ± 1.7%, n = 70; Chromosome: 37.2 ± 1.7%, n = 70) (P < 0.05, n = 4). No significantly difference was found between glycine supplementation in vitrification medium and that in maturation medium alone. Interestingly, adding glycine into both vitrification and maturation medium further enhanced the percentages of normal spindle assembly (75.0 ± 3.4%, n = 66) and chromosome alignment (77.7 ± 3.3%, n = 66), compared to those of glycine supplementation in either vitrification medium or maturation medium (P < 0.05, N = 4) (Fig. 2d,e).
Effects of Glycine addition during vitrification and/or in vitro maturation of GV oocytes on the meiotic spindle assembly and chromosomal alignment.
(a, b and c) Representative fluorescence image of oocyte with either normal meiotic spindle organization and chromosomal alignment (a) or abnormal spindle assembly and chromosome alignment (b) or without spindle assembly (c). (d) Comparison of spindle morphology in MII oocyte from different glycine treatment groups. (e) Comparison of chromosome alignment in MII oocyte from various glycine treatment groups. Green: spindle, Blue: DNA. Control: without glycine treatment; IVM + Gly: glycine treatment during in vitro maturation only; Vitrified + Gly: glycine treatment during vitrification/thawing only; Vitrified + IVM + Gly: glycine treatment during both vitrification/thawing and maturation in vitro. Different low case letters above columns indicate statistical differences at P < 0.05. Scale bar (a–c) 10 μm.
Glycine addition either in the vitrification/thawing medium or in the maturation medium decreased aneuploidy in the MII oocytes following COCs vitrification
Since addition of glycine enhanced the percentage of normal spindle assembly and chromosome alignment, we further examined whether glycine supplementation could decrease the rate of aneuploidy of MII oocyte following COCs vitrification at GV stage. As expected, the aneuploidy rate was significantly decreased when adding glycine into the vitrification medium and/or IVM medium, compared to the control without glycine addition (P < 0.05, N = 5) (Fig. 3 and Table 1). However, no significantly difference was found among addition of glycine into either vitrification medium or maturation medium, or both vitrification and maturation medium (P > 0.05, N = 5).
Glycine supplementation during vitrification of COCs and their subsequent maturation decreased the mitochondrial damage caused by freezing
Vitrification usually disrupts the localization and function of mitochondrial in oocytes, which is often blamed for abnormal spindle assembly. We next tested the distribution and function of alive mitochondrial in the MII oocytes following COCs vitrification and their maturation with or without glycine treatment. Roughly, mitochondrial distribution was classified into two categories: (1) mitochondrial diffusing evenly throughout the oocyte, namely evenly distributed mitochondrial, which is found in the majority of non-vitrified MII oocytes30 (normal distribution); the (2) mitochondrial mainly surrounding the cortical area, termed peripherally concentrated mitochondrial (abnormal distribution). In consistent with embryo development and spindle assembly data, the oocytes that were treated with glycine either during vitrification (56.1 ± 3.9%, n = 80) or during maturation (45.6 ± 1.9%, n = 79) had a higher chance to be with normal mitochondrial distribution compared to the counterparts that were never treated with glycine (33.4 ± 2.7%, n = 82) (P < 0.05, N = 4) (Fig. 4). While little change was seen in the oocytes that were treated with glycine during maturation in improving mitochondrial localization compared to these treated with glycine during vitrification (P > 0.05, N = 4). On the other hand, glycine-treatment during both vitrification and maturation could further increase the proportion of MII oocytes with normal mitochondrial distribution (69.6 ± 1.3%, n = 92), when compared with glycine-treatment during either vitrification or maturation alone (P < 0.05, N = 4).
Effects of loading GV oocytes with glycine during vitrification and/or in vitro maturation on mitochondrial distribution in the subsequent MII oocytes.
(a) Example of normal mitochondrial distribution in MII oocyte: mitochondria were distributed throughout the whole cytoplasm including central region. (b) Example of abnormal mitochondrial distribution in MII oocyte: mitochondria were mainly localized around cortical area in the oocyte with no or little mitochondrial presented in the center of oocyte. Blue: DNA, red: mitochondria. (c) Comparison of mitochondrial distribution in MII oocyte from vitrified COCs with different glycine treatments. Control: without glycine treatment; IVM + Gly: glycine treatment during in vitro maturation only; Vitrified + Gly: glycine treatment during vitrification/thawing only; Vitrified + IVM + Gly: glycine treatment during both vitrification/thawing and maturation in vitro. Different low case letters above columns indicate statistical differences at P < 0.05. Scale bar (a,b) 10 μm.
Glycine addition during both vitrification of COCs and their subsequent maturation increased ATP levels in MII oocyte
The most prominent roles of mitochondrial are to produce the ATP and to regulate cellular metabolism. To further validate whether glycine supplementation during vitrification or maturation lessen the mitochondrial impairment induced by vitrification at GV stage, we evaluated the ATP contents in the resultant MII oocytes. As shown in Fig. 5, glycine addition in either vitrification/warming (1.62 ± 0.1 pmol/oocyte) or maturation medium (1.45 ± 0.1 pmol/oocyte) did not help to enhance the ATP levels in the resultant MII oocytes compared to the control without glycine addition (1.26 ± 0.1 pmol/oocyte). However, the ATP contents in MII oocytes were significantly increased when glycine was added through vitrification/warming and IVM (2.2 ± 0.2 pmol/oocyte) compared to that of the counterparts that were never treated with glycine, or co-cultured with glycine either during vitrification/warming or during maturation alone (P < 0.05, N = 5).
Effects of loading GV oocytes with glycine during vitrification and/or in vitro maturation on the ATP levels of the resultant MII oocytes.
Control: without glycine treatment; IVM + Gly: glycine treatment during in vitro maturation only; Vitrified + Gly: glycine treatment during vitrification/thawing only; Vitrified + IVM + Gly: glycine treatment during both vitrification/thawing and maturation in vitro. Data are indicated as mean ± SEM. Different letters above columns indicate significant difference at a level of P < 0.05.
Glycine treatment during vitrification and thawing, but not during maturation, inhibited apoptosis in the vitrified GV oocytes and following MII oocytes
To assess the effect of glycine-treatment during vitrification and/or maturation following COCs freezing on oocyte apoptosis, we stained the resultant oocytes with Annexin-V (early apoptosis) and TUNEL (late apoptosis) staining (Fig. 6). The results exhibited that glycine supplementation in the vitrification and thawing medium (16.6 ± 1.9%, n = 113) decreased the occurrence of early apoptosis in vitrified GV oocytes, compared to the control without glycine supplementation (40.8 ± 2.1%, n = 109) (P < 0.05, N = 4) (Fig. 6c,g). While the apoptotic percentage of MII oocytes from COCs treated with glycine during vitrification/thawing (Vitrified + Gly: 28.1 ± 2.4%, n = 104; Vitrified + IVM + Gly: 20.8 ± 3.7%, n = 106) was dramatically lower than that from COCs without glycine-treatment during vitrification/thawing (Control: 46.6 ± 2.2%, n = 101; IVM + Gly: 38.8 ± 2.1%) (P < 0.05, N = 4) (Fig. 6d,h). Glycine treatment during maturation was less efficient than glycine treatment during vitrification/thawing in preventing the early apoptosis of MII oocytes since no significant difference was found in either groups between COCs with or without glycine treatment during maturation or groups between COCs treated with glycine during vitrification/thawing and these during both vitrification/thawing and maturation (P > 0.05, N = 4). In addition, no late apoptosis staining was observed in all examined samples.
Glycine treatment during GV oocyte vitrification and/or in vitro maturation protected against apoptosis in oocyte at both GV and MII stages.
(a–f) Representative images of early and late apoptosis labeled with Annexin-V (c: GV oocyte; d: MII oocyte) and TUNEL (e: GV oocyte; f: MII oocyte) staining, respectively, and their negative control (a: GV oocyte; b: MII oocyte). Blue: DNA; green: Annexin-V or TUNEL staining. Scale bar (a–f), 10 μm. (g) The rate of early apoptosis in GV oocyte following vitrirication/thawing with or without glycine treatment. (h) The percentage of early apoptosis in MII oocytes following vitrirication/thawing and maturation of COCs with different glycine treatments. Control: without glycine treatment; IVM + Gly: glycine treatment during in vitro maturation only; Vitrified + Gly: glycine treatment during vitrification/thawing only; Vitrified + IVM + Gly: glycine treatment during both vitrification/thawing and maturation in vitro. Different low case letters above columns indicate statistical differences at P < 0.05.
Discussion
Our current results demonstrate for the first time that glycine supplementation during either vitrification/warming or maturation improves cytoplasmic maturation of MII oocytes following vitrified mouse oocytes at GV stage and their subsequent embryo developmental competency. Moreover, addition of glycine during both vitrification/warming and maturation further maximize the beneficial effects of glycine in enhancing the maturation and the resultant embryo development following COCs vitrification. Thus we think that the osmolyte glycine play a protective role during both cryopreservation and subsequent maturation and could be applied as a new nontoxic cryoprotectant for mouse GV oocytes.
There is mounting evidence to suggest that vitrification gives better overall outcomes than slow cooling in mammalian oocytes31,32,33. Compared to controlled rate cooling cryopreservation, vitrification eliminates mechanical injury from ice, transcends the need to find an optimal cooling and warming rate, avoids the need for specialized equipment to control cooling rate, and enables cooling to be rapid enough to outrun chilling injury, but it complicates the osmotic effects of adding and removing high concentrations of cryoprotective agents (CPAs)34,35. An oocyte initially shrinks rapidly in response to the high extracellular osmolarity, which promotes exchange of intracellular water with permeable CPAs during the vitrification process. As a result, there is an extreme and very rapid increase in inorganic ions in oocytes. Then during the thawing process, exposure to impermeable CPAs, which are critical for success of thawing to prevent cell volume increase above the desired level to death, is reserved until the final step to maintain as large a cell volume as possible. Early studies of osmotic stress focused primarily on minimum or maximum cell volume, particularly because over the minimum or maximum cell volume would lead to cell death. Currently, the issues of cryosurvival have largely been overcome and people are focusing efforts on reducing sublethal damage induced by osmotic stress to increase developmental competence in oocytes or embryos since cell volume homeostasis is a key factor in successful early embryo development. On the other hand, glycine serving as a novel organic osmolyte, but not any of the four known somatic cell organic osmolyte, to overcome the developmental block induced by hypertonic environment was well documented36,37. Furthermore, the initiation of glycine transport via the GLYT1 transporter that normally occurs when ovulation is triggered in vivo or when oocytes are removed from the follicle and placed into in vitro culture also initiates the control of cell volume using glycine in fully grown GV oocytes28. Thus, as our speculation, glycine supplementation during vitrification and maturation exerts its advantage roles in reducing the osmotic stress when adding and removing high concentrations of cryoprotective agents during vitrification and thawing.
The osmotic stress during dehydration and rehydration of vitrification process is a well-documented detrimental factor in oocytes at the GV stage, which has been reported in mice14, porcine38 and human oocytes39, resulting in the irreversible damage to the organelle and ultrastructure, including abnormal spindle assembly and chromosome segregation, mis-distributed mitochondrial during oocyte meiosis, and continued development40,41. In consistent with our study, addition of 1 mM glycine, a class of diverse, small, neutral organic compounds accumulated by cells to provide intracellular osmotic support to replace ions that can disrupt cellular physiology at higher concentrations42,43, to the vitrification solutions improved the ability of the mouse MII oocytes to maintain their mitochondrial physiology and increase the blastocyst development29. It has been suggested that meiotic spindle assembly, which is critical for correct sister chromatids segregation and subsequent embryo development10,44, have been associated with mitochondrial distribution and activity in both human and mouse oocytes45. In our study, as expected, glycine supplementation during vitrification and maturation following mouse COCs vitrification enhanced the meiotic spindle assembly, chromosome alignment, ATP contents, and reduced the percentage of aneuploidy in the resultant MII oocytes.
Moreover, studies have reported that various cell types, including cardiac fibroblasts46, medullary epithelial cells47 and rat alveolar type II cells48 were apoptotic when cultured in the hyperosmotic medium. Although no direct link has been established between osmotic stress and oocyte apoptosis, cell death through apoptosis were also happened after oocyte vitrification49,50,51. Glycine prevents the cells against apoptosis has been reported in rat endothelial cells52, mice neuronal cells53 and chick embryos54. For mouse oocytes, we demonstrated for the first time that the glycine supplementation during vitrification/thawing could inhibit early apoptosis (detected by Annexin V binding) in vitrified GV oocytes. However, whether glycine addition during embryos culture could further enhance the outcome of mouse COCs vitrification has yet to be established.
Oocyte cryopreservation is considered relatively inefficient since, at least in part, the oocyte is a large single cell with relatively low surface-to-volume ratio. However, it has numerous advantages over embryo cryopreservation. Our current study suggests that optimization of cell volume regulation by adding organic osmolyte of glycine during freezing-thawing and/or maturation in vitro could improve other mammalian and human oocyte vitrification at GV stage.
Materials and Methods
Unless otherwise specified, all chemicals used in the study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Mouse
All animal experiments were conducted exactly in accordance with the guide to the Care and Use of Experimental Animal issued by the Animal Ethics Committee of Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences (Permit Number: 2014–0035). In addition, these experimental procedures had been approved by the committee before we did our experiments. Kunming white mouse, a native breed widely used in biological research in China, was housed in a temperature-controlled room with 14 h light/10 h dark cycle, fed with commercial diet and water ad libitum. Mice of 5–6 weeks old were injected intraperitoneally each with 5 IU equine chorionic gonadotropin (eCG), and sacrificed 45–47 h later by cervical dislocation for oocyte collection.
Collection of oocytes
Fully-grown GV-stage oocytes surrounded by cumulus cells (CCs), named COCs, were isolated by puncturing large antral follicles with a pair of 26-gauge needles. Only COCs with more than three layers of CCs and homogenous cytoplasm oocytes ~80 μm in diameter were selected for the following experiments.
Vitrification and thawing of oocytes
COCs were vitrified by cryoloop (Hampton Research company, USA) method as previously described55. Briefly, the base medium (BM) of vitrification and warming solutions was MEM-alpha supplemented with 10% FBS. For vitrification, COCs were firstly transferred into equilibrium solution (BM containing 7.5% ethylene glycol (EG), 7.5% dimethylsulfoxide (DMSO), and 0.25 M trehalose) for 3 min. Then COCs were moved into a vitrification solution (BM containing 15% EG and 15% DMSO). Three to five COCs were placed with as little solution as possible on the thin film, which was formed by surface tension following the cryoloop was dipped into the vitrification solution, and directly plunged into liquid nitrogen. The best vitrification results could be got if the whole process was less than 1 min from cells were transferred into vitrification medium to they were vitrified. To warm the vitrified COCs, the cryoloop containing vitrified COCs was immediately dipped into warming solution I (BM supplemented with 1 M trehalose) for 3 min. Then COCs were transferred into warming solution II (BM supplemented with 0.5 M trehalose) and BM, respectively, for 3 min each. The whole thawing procedures were done at 37 °C. The viability of COCs was determined morphologically by whether they had evenly granulated cytoplasm with apparent perivitelline space (viable) or degenerated cytoplasm without perivitelline space (nonviable). Only viable COCs were used for the following experiments.
Oocytes maturation in vitro
The maturation medium was TCM-199 medium (Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal calf serum (FCS) (Gibco, Grand Island, NY), 1 mg/ml 17 β-estradiol, 24.2 mg/l sodium pyruvate, 0.05 IU/ml FSH, 0.05 IU/ml LH, 10 ng/ml EGF. A group of 20–30 COCs were cultured in a 100 μl drops of maturation medium at 37 °C in an atmospehere of 5% CO2 and saturated humidity for 16 h.
Glycine supplementation
Glycine was dissolved in the medium at a final concentration of 1 mM and sterilized by filtration.
Fertilization and embryo culture
Sperms were collected from the cauda epididymis of fertile male mice (Kunming white mice) and were placed at the bottom of a test tube containing T6 medium supplemented with 10 mg/ml bovine serum albumin (BSA). After 3–5 min, these highly motile spermatozoa in the supernatant medium were transferred and capacitated in the same medium for 1.5 h. After being washed with fertilization medium (T6 containing 20 mg/ml BSA), groups of 20–30 COCs were transferred to 100 μl droplets of pre-warmed fertilization medium and fertilized by 1 × 106/ml capacitated sperms for 6 h. After fertilization, these supposed fertilized eggs were firstly cultured in the CZB medium until 4-cell embryo stage and then moved to CZB medium supplemented with 5.5 mM glucose for further development to blastocyst stage. All culture conditions were at 37 °C under 5% CO2 in humidified atmosphere.
Meiotic spindle assembly and chromosomal alignment
Before immunofluorescence staining of spindle, oocytes were treated with 300 μg/ml hyaluronidase for 5 min at 37 °C and denuded of cumulus cells by repeated pipetting in an M2 medium. Denuded oocytes were fixed in a solution containing 2% paraformaldehyde and microtubule stabilizing buffer56 for 60 min at room temperature. Then, oocytes were transferred into blocking buffer (PBS supplemented with 1% BSA and 0.01% Triton X-100) for 1 h at room temperature and incubated with mouse anti-alpha-tubulin-FITC monoclonal antibody (Sigma) diluted 1:200 in the blocking buffer for 60 min at room temperature. After washing thoroughly, the oocytes were transferred to a small drop of Prolong Antifade mounting medium containing 4, 6-diamidino-2-phenylindole (DAPI) and mounted on microscope slides.
Mitochondrial distribution
COCs were removed cumulus cell and then were cultured in M2 medium containing 200 μM Mitotracker Red (Mitotracker Red FM; Molecular Probes, Eugene, OR, USA) for 30 min at 37 °C. After washing, oocytes were fixed in 4% paraformaldehyde in PBS for 20 min. After permeated by 0.5% Triton X-100 for 20 min, oocytes were transferred into a drop of Prolong Antifade mounting medium containing DAPI on a microscope slide and covered with a coverslip.
Oocyte chromosome spreads
Chromosome spreads were carried out according to Hodges and Hunt57 with minor modifications. In brief, the zona pellucida of oocytes was removed by treatment with 0.5 units/μl pronase at 37 °C for 20 sec. Zona-free oocytes were rinsed in M2 medium to detach polar bodies. The oocytes were individually plated onto Plus-charged histology slides that were covered by the fixative solution (PBS containing 4% paraformaldehyde (w/v), 0.15% triton X-100 (v/v) and 3 mM dithiothreitol, pH 9.2). After these slides were air-dried, they were incubated with 10 μg/ml PI and DAPI for 10 min. Finally, these slides were mounted in the Prolong Antifade mounting medium following washing thoroughly with PBS. Chromosomes were observed under a confocal microscope.
Measurement of ATP Contents in MII oocytes
After 16 h of IVM, oocytes were treated with 300 μg/ml hyaluronidase for 5 min at 37 °C and denuded of cumulus cells by repeated pipetting in an M2 medium. Only matured oocytes with first polar body were selected for ATP assay. 30 oocytes in each group were snap-frozen in a microfuge tube containing 200 μl of ultrapure water and stored at −80 °C. ATP contents in oocytes were determined by using the assay kit (Bioluminescent Somatic Cell Assay Kit, FL-ASC, St Louis, MO) based on the luciferin–luciferase reaction as previously described56. The bioluminescence of each sample was measured by high-sensitivity luminometer (Berthold LB 9508, Germany) including a standard curve with different ATP concentrations in each assay.
Annexin V staining
Early-stage apoptosis of oocytes was detected using Annexin V FLUOS Staining Kit (Roche, Germany). Briefly, denuded oocytes were transferred into liquid mixture containing Annexin-V buffer, Annexin-V fluos, and PI for 15 min at 37 °C in the dark. After three washes in PBS containing 0.1% Tween 20 and 0.01% Triton X-100, oocytes were mounted with the Prolong Antifade mounting medium containing DAPI on a microscope slide and covered by a coverslip. PI used in this experiment was to distinguish live cells from dead cells. PI can only pass through the cell when cytoplasmic membrane has lost its integrity. The oocytes were divided into three groups according to Anguita et al.58 (1) viable oocytes: non Annexin-V staining; (2) Early apoptotic oocytes: oocytes membrane were stained by Annexin-V; and (3) necrotic oocytes: PI positive red nuclei.
TUNEL staining
DNA fragmentation was determined by TUNEL staining (Roche, Germany). Denuded Oocytes were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature. Then oocytes were permeabilized in PBS containing 0.1% Triton X-100 and 0.1% sodium citrate for 30 min at room temperature, followed incubation for 1 h at 37 °C in the dark with TUNEL reaction mixture (containing fluorescein isothiocyanate conjugated dUTP and terminal deoxynucleotidyl transferase). Finally, these oocytes were mounted in the Prolong Antifade mounting medium following washing thoroughly with PBS containing 0.1% Tween 20 and 0.01% Triton X-100. During each experiment, a group of oocytes treated with RQ1 RNase-free DNase (50 U/ml) at 37 °C for 1 h was used as a positive control; while another group of oocytes incubated with TUNEL reaction mixture without the terminal deoxynucleotidyl transferase was used as a negative control.
Laser confocal microscope
All immunofluorescence stained oocytes were observed under a Nikon laser scanning confocal microscope (Nikon C2 plus Si). When DAPI fluorescence was monitored, the excitation light wavelength was 405 nm and emission light wavelength was 450–480 nm. When FITC, Annexin V and TUNEL fluorescence was monitored, the excitation light wavelength was 488 nm and emission light wavelength was 515–535 nm. Both PI and Mitotracker Red fluorescence was scanned, the excitation light wavelength was 543 nm and emission light wavelength was 590–630 nm. Images were merged by Nikon Confocal Software.
Data analysis
Each experiment was repeated at least three times. Statistical analyses were performed using Statistics Package for Social Science (SPSS 17.0; SPSS Inc., Chicago, IL). All data were expressed as mean ± SEM. N indicates the number of independent experiments, while n indicates the total number of individual oocytes or embryos. The percentage data were arc-sine transformed before statistical analysis. Data were analyzed by either student’s t-test (two groups) or one-way ANOVA followed by the Duncan multiple comparison test (four groups). All results were considered to be statistically significant at a level of P < 0.05.
Additional Information
How to cite this article: Cao, X.-Y. et al. Glycine increases preimplantation development of mouse oocytes following vitrification at the germinal vesicle stage. Sci. Rep. 6, 37262; doi: 10.1038/srep37262 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Cobo, A. et al. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril 105, 755–764, doi: 10.1016/j.fertnstert.2015.11.027 (2016).
Doyle, J. O. et al. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril 105, 459–466, doi: 10.1016/j.fertnstert.2015.10.026 (2016).
Konc, J., Kanyo, K., Kriston, R., Somoskoi, B. & Cseh, S. Cryopreservation of Embryos and Oocytes in Human Assisted Reproduction. Biomed Res Int, doi: Artn 30726810.1155/2014/307268 (2014).
Ethics Committee of American Society for Reproductive, M. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril 100, 1224–1231, doi: 10.1016/j.fertnstert.2013.08.041 (2013).
Loren, A. W. et al. Fertility Preservation for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 31, 2500–2510, doi: 10.1200/Jco.2013.49.2678 (2013).
Noyes, N., Porcu, E. & Borini, A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online 18, 769–776 (2009).
Noyes, N. et al. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril 94, 2078–2082, doi: 10.1016/j.fertnstert.2010.01.019 (2010).
Ubaldi, F. et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod 25, 1199–1205, doi: 10.1093/humrep/deq046 (2010).
D, F. Perk up: Facebook and Apple now pay for women to freeze eggs. Nbc News (2014).
Chen, S. U. et al. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing-a review article. Mol Cell Endocrinol 202, 101–107, doi: 10.1016/S0303-7207(03)00070-4 (2003).
Rho, G. J. et al. Microtubulin configuration and mitochondrial distribution after ultra-rapid cooling of bovine oocytes. Mol Reprod Dev 63, 464–470, doi: 10.1002/mrd.10196 (2002).
Saunders, K. M. & Parks, J. E. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro-matured bovine oocytes. Biol Reprod 61, 178–187, doi: 10.1095/biolreprod61.1.178 (1999).
Mandelbaum, J. et al. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur J Obstet Gyn R B 113, S17–S23, doi: 10.1016/j.ejogrb.2003.11.005 (2004).
Moawad, A. R., Xu, B. Z., Tan, S. L. & Taketo, T. L-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum Reprod 29, 2256–2268, doi: 10.1093/humrep/deu201 (2014).
Ghetler, Y. et al. Human oocyte cryopreservation and the fate of cortical granules. Fertil Steril 86, 210–216, doi: 10.1016/j.fertnstert.2005.12.061 (2006).
Aono, N. et al. Successful production of blastocysts following ultrarapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J Reprod Dev 49, 501–506 (2003).
Moawad, A. R., Tan, S. L., Xu, B. Z., Chen, H. Y. & Taketo, T. L-Carnitine Supplementation During Vitrification of Mouse Oocytes at the Germinal Vesicle Stage Improves Preimplantation Development Following Maturation and Fertilization In Vitro. Biol Reprod 88, doi: ARTN 10410.1095/biolreprod.112.107433 (2013).
Ruppert-Lingham, C. J., Paynter, S. J., Godfrey, J., Fuller, B. J. & Shaw, R. W. Developmental potential of murine germinal vesicle stage cumulus-oocyte complexes following exposure to dimethylsulphoxide or cryopreservation: loss of membrane integrity of cumulus cells after thawing. Hum Reprod 18, 392–398, doi: 10.1093/humrep/deg071 (2003).
Jin, B., Kleinhans, F. W. & Mazur, P. Survivals of mouse oocytes approach 100% after vitrification in 3-fold diluted media and ultra-rapid warming by an IR laser pulse. Cryobiology 68, 419–430, doi: 10.1016/j.cryobiol.2014.03.005 (2014).
Kuleshova, L. L. & Lopata, A. Vitrification can be more favorable than slow cooling. Fertil Steril 78, 449–454, doi: Pii S0015-0282(02)03305-8Doi 10.1016/S0015-0282(02)03305-8 (2002).
Mullen, S. F. et al. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod 19, 1148–1154, doi: 10.1093/humrep/deh201 (2004).
Baltz, J. M. & Zhou, C. X. Cell volume regulation in mammalian oocytes and preimplantation embryos. Mol Reprod Dev 79, 821–831, doi: 10.1002/mrd.22117 (2012).
Van Winkle, L. J., Haghighat, N. & Campione, A. L. Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid. J Exp Zool 253, 215–219, doi: 10.1002/jez.1402530211 (1990).
Hadi, T., Hammer, M. A., Algire, C., Richards, T. & Baltz, J. M. Similar effects of osmolarity, glucose, and phosphate on cleavage past the 2-cell stage in mouse embryos from outbred and F1 hybrid females. Biol Reprod 72, 179–187, doi: 10.1095/biolreprod.104.033324 (2005).
Van Winkle, L. J., Haghighat, N., Campione, A. L. & Gorman, J. M. Glycine transport in mouse eggs and preimplantation conceptuses. Biochim Biophys Acta 941, 241–256 (1988).
Dawson, K. M. & Baltz, J. M. Organic osmolytes and embryos: substrates of the Gly and beta transport systems protect mouse zygotes against the effects of raised osmolarity. Biol Reprod 56, 1550–1558 (1997).
Steeves, C. L. et al. The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos. P Natl Acad Sci USA 100, 13982–13987, doi: 10.1073/pnas.2334537100 (2003).
Tartia, A. P. et al. Cell volume regulation is initiated in mouse oocytes after ovulation. Development 136, 2247–2254, doi: 10.1242/dev.036756 (2009).
Zander-Fox, D., Cashman, K. S. & Lane, M. The presence of 1 mM glycine in vitrification solutions protects oocyte mitochondrial homeostasis and improves blastocyst development. J Assist Reprod Gen 30, 107–116, doi: 10.1007/s10815-012-9898-4 (2013).
Yu, Y., Dumollard, R., Rossbach, A., Lai, F. A. & Swann, K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol 224, 672–680, doi: 10.1002/jcp.22171 (2010).
Cao, Y. X. et al. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril 92, 1306–1311 (2009).
Fadini, R. et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online 19, 171–180 (2009).
Edgar, D. H. & Gook, D. A. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update 18, 536–554, doi: 10.1093/humupd/dms016 (2012).
Fahy, G. M. & Wowk, B. Principles of cryopreservation by vitrification. Methods Mol Biol 1257, 21–82 (2015).
Keros, V. & Fuller, B. J. Cryopreservation of mammalian oocytes. Methods Mol Biol 1257, 289–304 (2015).
Richards, T., Wang, F., Liu, L. & Baltz, J. M. Rescue of postcompaction-stage mouse embryo development from hypertonicity by amino acid transporter substrates that may function as organic osmolytes. Biol Reprod 82, 769–777 (2010).
Baltz, J. M. & Tartia, A. P. Cell volume regulation in oocytes and early embryos: connecting physiology to successful culture media. Human reproduction update 00, 1–11 (2009).
Wu, C. et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev 73, 1454–1462, doi: 10.1002/mrd.20579 (2006).
Boiso, I. et al. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 17, 1885–1891 (2002).
Hunter, J. E., Fuller, B. J., Bernard, A., Jackson, A. & Shaw, R. W. Vitrification of human oocytes following minimal exposure to cryoprotectants; initial studies on fertilization and embryonic development. Hum Reprod 10, 1184–1188 (1995).
Agca, Y., Liu, J., Critser, E. S. & Critser, J. K. Fundamental cryobiology of rat immature and mature oocytes: Hydraulic conductivity in the presence of Me2SO, Me2SO permeability, and their activation energies. Journal of Experimental Zoology 286, 523–533 (2000).
Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. Living with water stress: evolution of osmolyte systems. Science 217, 1214–1222 (1982).
Zhou, C. & Baltz, J. M. JAK2 mediates the acute response to decreased cell volume in mouse preimplantation embryos by activating NHE1. J Cell Physiol 228, 428–438 (2013).
Keefe, D., Liu, L., Wang, W. & Silva, C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online 7, 24–29 (2003).
Jones, A., Van Blerkom, J., Davis, P. & Toledo, A. A. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod 19, 1861–1866 (2004).
Mockridge, J. W. et al. IGF-1 regulates cardiac fibroblast apoptosis induced by osmotic stress. Biochem Biophys Res Commun 273, 322–327, doi: 10.1006/bbrc.2000.2934 (2000).
Dmitrieva, N., Kultz, D., Michea, L., Ferraris, J. & Burg, M. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J Biol Chem 275, 18243–18247, doi: 10.1074/jbc.M000522200 (2000).
Edwards, Y. S., Sutherland, L. M., Power, J. H., Nicholas, T. E. & Murray, A. W. Osmotic stress induces both secretion and apoptosis in rat alveolar type II cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 275, L670–L678 (1998).
Men, H., Monson, R. L., Parrish, J. J. & Rutledge, J. J. Degeneration of cryopreserved bovine oocytes via apoptosis during subsequent culture. Cryobiology 47, 73–81 (2003).
Vallorani, C. et al. Pig oocyte vitrification by Cryotop method and the activation of the apoptotic cascade. Anim Reprod Sci 135, 68–74, doi: 10.1016/j.anireprosci.2012.08.020 (2012).
Dai, J. et al. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 71, 291–298 (2015).
Zhang, Y. et al. Glycine prevents apoptosis of rat sinusoidal endothelial cells caused by deprivation of vascular endothelial growth factor. Hepatology 32, 542–546, doi: 10.1053/jhep.2000.16605 (2000).
Lu, Y. et al. Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem Int 61, 649–658, doi: 10.1016/j.neuint.2012.07.005 (2012).
Miller, R. R. Jr. et al. Exogenous glycine partially attenuates homocysteine-induced apoptosis and membrane peroxidation in chick embryos. Comp Biochem Physiol C Toxicol Pharmacol 144, 25–33, doi: 10.1016/j.cbpc.2006.05.005 (2006).
Lane, M., Bavister, B. D., Lyons, E. A. & Forest, K. T. Containerless vitrification of mammalian oocytes and embryos-Adapting a proven method for flash-cooling protein crystals to the cryopreservation of live cells. Nat Biotechnol 17, 1234–1236, doi: 10.1038/70795 (1999).
Xu, B., Noohi, S., Shin, J. S., Tan, S. L. & Taketo, T. Bi-directional communication with the cumulus cells is involved in the deficiency of XY oocytes in the components essential for proper second meiotic spindle assembly. Developmental biology 385, 242–252 (2014).
Hodges, C. A. & Hunt, P. A. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma 111, 165–169, doi: 10.1007/s00412-002-0195-3 (2002).
Anguita, B. et al. Effect of the apoptosis rate observed in oocytes and cumulus cells on embryo development in prepubertal goats. Anim Reprod Sci 116, 95–106, doi: 10.1016/j.anireprosci.2009.01.007 (2009).
Acknowledgements
This study was supported by Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences; Central Public-interest Scientific Institution Basal Research Fund (2015ZL012); National Natural science foundation of China (31501958); Jilin Scientific and technological development program (20140520168JH); The natural science fund project in Anhui province (1508085QC59).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: B.X. and X.Y.C. Performed the experiments: X.Y.C., S.Y.W., Y.L., M.Z., M.J.X., TC. Analyzed the data: X.Y.C. and B. X. Wrote the paper: B. X., X.Y.C. and J.R.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cao, XY., Rose, J., Wang, SY. et al. Glycine increases preimplantation development of mouse oocytes following vitrification at the germinal vesicle stage. Sci Rep 6, 37262 (2016). https://doi.org/10.1038/srep37262
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37262
This article is cited by
-
Cryopreservation of NK and T Cells Without DMSO for Adoptive Cell-Based Immunotherapy
BioDrugs (2021)
-
Supplement of Betaine into Embryo Culture Medium Can Rescue Injury Effect of Ethanol on Mouse Embryo Development
Scientific Reports (2018)
-
Urea influences amino acid turnover in bovine cumulus-oocyte complexes, cumulus cells and denuded oocytes, and affects in vitro fertilization outcome
Scientific Reports (2018)
-
Resveratrol promotes the embryonic development of vitrified mouse oocytes after in vitro fertilization
In Vitro Cellular & Developmental Biology - Animal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.