Abstract
Potential benefits of carbohydrate counting for glycemic control in patients with type 1 diabetes mellitus (T1DM) remain inconclusive. Our aim is to systematically assess the efficacy of carbohydrate counting in patients with T1DM. We searched PubMed, Embase, Web of Science, Cochrane Library and the Chinese Biology Medicine (CBM) up to December 2015. Randomized controlled trials (RCTs) with at least 3 months follow-up that evaluated carbohydrate counting compared with usual or other diabetes dietary education in patients with T1DM were included. Overall meta-analysis identified a significant decrease in HbA1c concentration with carbohydrate counting versus other diabetes diet method or usual diabetes dietary education (SMD: −0.35, 95%CI: −0.65 to −0.05, P = 0.023). Subgroup analysis restricted to trials which compared carbohydrate counting with usual diabetes dietary found a significant decrease in HbA1c in carbohydrate counting group (SMD: −0.68, 95%CI: −0.98 to −0.38, P = 0.000), and a similar result has emerged from six studies in adults (SMD: −0.40, 95%CI: −0.78 to −0.02, P = 0.037). Carbohydrate counting may confer positive impact on glucose control. Larger clinical trials are warranted to validate this positive impact.
Similar content being viewed by others
Introduction
Type 1 diabetes mellitus (T1DM) is one of the most challenging medical disorders, and one of the key therapeutic goals to prevent or delay long-term diabetes complications in T1DM is the achievement and maintenance of near-normal glycemic control1. Only insulin treatment is not enough to rely on, dietary adjustments also play an important role in the regulation of blood glucose. Carbohydrates are a major determinant of postprandial blood glucose. Carbohydrate counting is a meal planning approach used with clients who have diabetes that focuses on carbohydrate as the primary nutrient affecting postprandial glycemic response. Advanced carbohydrate counting allows adjustment of the prandial insulin dose for actual carbohydrate intake in T1DM patients on intensive insulin therapy. Therefore, by calculating the carbohydrate amounts in each meal, insulin doses required to preserve postprandial blood glucose within normal limits can be predicted2,3. The current guidelines recommend that the algorithms for prandial insulin calculation take into account the carbohydrate amount of the meal4.
However, the efficacy of carbohydrate counting is not fully understood. At present, study to inquiry the effect of carbohydrate counting on T1DM patients is relatively little. Evidence from randomized controlled trials (RCTs) reported inconsistent results5,6,7. Previous systematic review8 or meta-analysis9 did not cite a clear conclusion. Furthermore, three recent trials10,11,12 with adequate power have been published and involve new evidence. Therefore, we performed this meta-analysis to evaluate the efficiency of carbohydrate counting on glycemic control in people with T1DM.
Methods
Literature search
This study was conducted following the Cochrane Collaboration and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) statement13,14. We identified relevant studies by searching the electronic databases in Pubmed, EMBASE, Cochrane Library, Web of Science and China Biology Medicine (CBM) for RCTs of carbohydrate counting from inception until December 2015. The search strategy included following terms: “carbohydrate counting”, “Type 1 diabetes mellitus”, “Glycated hemoglobin” and “HbAlc” (an example of specific strategy is shown in Supplementary material: Table S1). We read titles and abstracts of retrieved records to eliminate studies those were clearly irrelevant, and read full text of all remaining articles to decide eligible studies. Those discrepancies were resolved by discussion and consensus. Reference lists of identified trials and review articles were also hand-screened to identify any other relevant studies.
Eligility criteria
Trials satisfying the following criteria were included: (1) design: randomised and quasi-randomised controlled clinical trials with at least 3 months’ follow-up; (2) population: T1DM who had injected insulin a minimum of three months; (3) intervention: carbohydrate counting versus other diabetes diet method or usual diabetes dietary education; (4) data: adequate information provided to calculate the standardized mean difference (SMD) and the corresponding 95% confidence interval (CI). We did not use any language limitations. Pregnant women with T1DM also be included in this study.
Data extraction
Data was extracted and placed into a spreadsheet from each included study. The following information was collected: first author, year of publication, country of origin, number of patients, intervention, control, outcomes data (glycosylated hemoglobin (HbAlc) (%), hypoglycemia events, insulin dose and body mass index (BMI)) and follow up. We also contacted corresponding authors to verify extracted data and to request the missing data. The change in HbA1c concentration was predefined primary outcome, and secondary outcomes were the change in hypoglycemia events, insulin dose and BMI.
Risk of bias assessment
Risk of bias was assessed by using the Cochrane Collaboration’s tool15. Each study was assessed and scored as “high”, “low”, or “unclear” risk of bias to the following criteria: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. Blinding of patients and clinicians was extremely difficult and generally not feasible in these trials, and we judged that the primary outcome was less prone to be influenced by lack of blinding. Therefore, studies with high risk of bias for any one or more key domains except blinding were considered as at high risk of bias; while studies with low risk of bias for all key domains except blinding were considered as at low risk of bias; otherwise they were considered as at unclear risk of bias.
Quality of evidence assessment
The quality of evidence for primary and secondary outcomes was assessed according to GRADE methodology for risk of bias, inconsistency, indirectness, imprecision, and publication bias; classified as high, moderate, low or very low. Summary tables were constructed by the GRADE system16,17,18,19 (GRADE version 3.6).
The literature search, data extraction, risk of bias assessment and evidence grade assessment were done independently by two authors (SF and LL) using a same approach. Disagreements were resolved by discussion among all authors.
Statistical analysis
Since all the observation indexes are continuous, and the measurement time of outcome is inconsistent in different studies, thus we pooled the SMD with corresponding 95% CI by using the random-effects model. Heterogeneity across studies was explored by using the I2 statistic20 (the I2 > 50% indicated significant heterogeneity), and publication bias was assessed by using Begg’s test and Egger’s test (P < 0.05 was considered statistically significant for publication bias). Sensitivity analysis was conducted to investigate the stability and reliability of results.
Result
Trial selection and risk of bias assessment
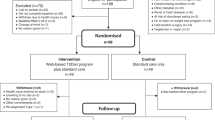
The initial search found 924 articles. After removing duplicates and screening the titles and abstracts, 18 articles were selected for full-text review, and 10 articles5,6,7,10,11,12,21,22,23,24 met the inclusion criteria. While one of them was excluded due to lack of essential data, and we failed to get the raw data from original author25. One more article24 from reference lists of identified trials also met the inclusion criteria and was included in this study. Totally 10 articles were included in the meta-analysis, the literature review process was showed in Fig. 1. According to the Cochrane Collaboration’s tool, two trials9,24 were categorized as at low risk of bias, five as at unclear5,6,10,12,21, and three as at high risk of bias7,11,23. All details of the risk of bias are supplied in Figs 2 and 3.
Characteristics of articles
These ten studies involving 773 participants were published from 2000 to 2014. Four studies5,7,10,12 enrolled children and adolescents, and the remaining six studies included adults6,11,21,22,23,24. Among ten included studies, five compared the carbohydrate counting with other diabetes diet method5,6,7,11,12, and the remaining five compared the carbohydrate counting with usual diabetes dietary education10,21,22,23,24. All studies reported changes in HbA1c concentration, four studies5,7,12,21 reported changes in daily insulin dosage, three studies5,22,24 reported changes in hypoglycemia event frequency and two studies7,21 reported changes in BMI. Detailed characteristics of eligible studies were provided in Table 1.
Primary outcome
The primary outcome is HbA1c concentration. All studies totaling 773 participants provided data on HbA1c concentration. Compared with other diabetes diet method or usual diabetes dietary education, carbohydrate counting significantly reduced HbA1c concentration (SMD: −0.35, 95%CI: −0.65 to −0.05, P = 0.023), with significant heterogeneity (I2 = 71.2%, P < 0.001). The heterogeneity among these studies could be related to different population and control group.
Subgroup analysis and sensitivity analysis
We performed subgroup analysis according to population and control group. Results showed that compared with usual diabetes dietary education, carbohydrate counting significantly reduced HbA1c concentration (SMD: −0.68, 95%CI: −0.98 to −0.38, P < 0.001), with no significant heterogeneity (I2 = 48.5%, P = 0.101). And a similar result has emerged from six studies in adults (SMD: −0.40, 95%CI: −0.78 to −0.02, P = 0.037), with significant heterogeneity (I2 = 70.0%, P = 0.005). All results of subgroup analyses are presented in Figs 4 and 5. And sensitivity analysis showed that present results possess superior reliability (Supplementary material: Figure S1).
Secondary outcomes
Secondary outcomes including the change in hypoglycemia events, insulin doses and BMI. There are four studies5,7,12,21 reported insulin doses, while the data one study reported was suspectable21, and we failed to obtain raw data from authors. Thus it was excluded and three studies were included in the meta-analysis. There are three studies5,22,24 reported hypoglycemia events and three reported BMI data7,21, respectively. Compared with other diabetes diet method or usual diabetes dietary education, carbohydrate counting did not significantly reduce the hypoglycaemia events (SMD: −0.14, 95%CI: −0.39 to 0.10, P = 0.254; I2 = 0.0%, P = 0.758), insulin dosage (SMD: 0.04, 95%CI: −0.24 to 0.31, P = 0.788; I2 = 0.0%, P = 0.945) or BMI (SMD: −0.06, 95%CI: −0.39 to 0.28, P = 0.749; I2 = 0.0%, P = 0.328) (Fig. 6).
Strength of evidence and publication bias
The quality of evidences was evaluated by GRADE system. The level of evidence was at level B and moderate recommendation for HbA1c concentration. All evidence profiles for the primary and secondary outcomes were provided in Table 2. For the meta-analysis of carbohydrate counting on HbA1c concentration, no publication bias was observed by Begg’s test and Egger’s test (Begg’s, P = 0.721; Egger’s, P = 0.688) (Supplementary material: Figure S2).
Discussion
Main findings
This meta-analysis systematically reviewed the current available literature and found that (1) In general, compared with other diabetes diet method or usual diabetes dietary, carbohydrate counting significantly reduced HbA1c concentration, evidence of this benefit was consistent in previous meta-analysis. While subgroup analysis restricted to trials which compared carbohydrate counting with other diabetes diet method found no significant decrease in HbA1c concentration in carbohydrate counting group. Comparing carbohydrate counting with other dietary method is in fact examining the impact of carbohydrate counting plus education in a more general sense, thus the efficiency of carbohydrate counting on glycemic control might be exaggerated. (2) We found that carbohydrate counting significantly reduced HbA1c concentration in adult group, while not in children and teenagers group. It may be because that adults are more likely to learn and apply carbohydrate counting.
Comparison with previous meta-analysis
In our study, the effect of carbohydrate counting reducing HbA1c concentration is consistent with previous meta-analysis11. While differences between our study and previous analysis should be noted. First, previous meta-analysis included seven trials totaling 703 participants. We included six of the seven trials, the other one was excluded due to lack of essential data, and we failed to get the raw data from authors. While we added four new trials, and we also added subgroup analysis according to the control group, got a more stable and reliable conclusion by eliminating interference factors. Our meta-analysis found that heterogeneity among trials mainly is from the design of different control group, rather than population. In addition, we evaluated the quality of evidence and the strength of recommendations. Therefore, our current meta-analysis was the latest and the most comprehensive one.
Guidance for clinical practice
First, our study found that carbohydrate counting has a positive effect on reducing HbA1c concentration. This effect is stable and reliable, and carbohydrate counting should be recommended for the routine treatment of T1DM. Second, up to now, little attention was paid to the study of carbohydrate counting’s effect on hypoglycemia events, insulin doses and BMI. The impact of carbohydrate counting on these aspects is a direction of future research. Finally, considering the dietary education in a more general sense may exaggerate the effect of carbohydrate counting, more clinical trials compared carbohydrate counting with dietary education in a more general sense are warranted to validate the positive impact of carbohydrate counting.
Limitations
Our study also has limitations. Though high quality of studies included in this meta-analysis, the sample sizes of these studies are small, and there is significant heterogeneity among studies, the reliability of results can be affected. More high quality trials with large samples are needed to confirm current results.
Conclusion
Our meta-analysis suggested that carbohydrate counting plays an important role in reducing HbA1c concentration, and this positive impact still needs evaluation by high-quality randomly controlled experiments.
Additional Information
How to cite this article: Fu, S. et al. Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: a systematic review and meta-analysis. Sci. Rep. 6, 37067; doi: 10.1038/srep37067 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med 329, 977–986 (1993).
Kawamura, T. The importance of carbohydrate counting in the treatment of children with diabetes. Pediatr Diabetes 8 (Suppl 6), 57–62 (2007).
Sheard, N. F. et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American diabetes association. Diabetes Care 27, 2266–2271 (2004).
American Diabetes Association (2013) Standards of medical care in diabet. Diabetes Care 36 (Suppl 1), S11–S66 (2013).
Gilbertson, H. R. et al. The effect of flexible low glycemic index dietary advice versus measured carbohydrate exchange diets on glycemic control in children with type 1 diabetes. Diabetes care 1137–1143 (2001).
Kalergis, M. et al. Optimizing insulin delivery: assessment of three strategies in intensive diabetes management. Diabetes, obesity & metabolism 2, 299–305 (2000).
Goksen, D., Altinok, Y. A., Ozen, S., Demir, G. & Darcan, S. Effects of carbohydrate counting method on metabolic control in children with type 1 diabetes mellitus. JCRPE Journal of Clinical Research in Pediatric Endocrinology 74–78 (2014).
Kirstine, J. B., Alan, W. B., Peter, P., Stephen, C. & Jennie, C. B. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2, 133–140 (2014).
Schmidt, S., Schelde, B. & Nørgaard, K. Effects of advanced carbohydrate counting in patients with Type 1 diabetes: a systematic review. Diabet. Med 31, 886–896 (2014).
de Albuquerque, I. Z. et al. Carbohydrate counting, nutritional status and metabolic profile of adolescents with type 1 diabetes mellitus. Scientia Medica 24(4), 21 (2014).
Bell, K. J., Barclay, A. W., Petocz, P., Colagiuri, S. & Brand-Miller, J. C. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2(2), 133–140 (2014).
Enander, R., Gundevall, C., Strömgren, A., Chaplin, J. & Hanas, R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatric diabetes 545–551 (2012).
Higgins, J. P. T. & Green, S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, Available from: http://www.cochrane.org/handbook (2011).
Moher, D., LIberti, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Swiglo, B. A., Murad, M. H., Schunemann, H. J., Kunz, R. & Vigersky, R. A. A case for clarity, consistency, helpfulness: state- of -the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinal Metab 93(3), 666–673 (2008).
Guyatt, G. H. et al. What is “quality of evidence” and why is it important to clinicians? BMJ 336(7651), 995–998 (2008).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650), 924–926 (2008).
Guyatt, G. H. et al. Going from evidence to recommen dations. Bmj 336(7652), 1049–1051 (2008).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003).
Trento, M. et al. Carbohydrate counting improves coping ability and metabolic control in patients with type 1 diabetes managed by group care. J Endocrinol Invest. 34, 101–105 (2011).
Schmidt, S. et al. Use of an automated bolus calculator in MDI-treated type 1 diabetes: The BolusCal Study, a randomized controlled pilot study. Diabetes Care 35, 984–990 (2012).
Scavone, G. et al. Effect of carbohydrate counting and medical nutritional therapy on glycemic control in Type 1 diabetic subjects: a pilot study. Diabet Med 27, 477–479 (2010).
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 325, 746 (2002).
Laurenzi, A. et al. Effects of carbohydrate counting on glucose control and quality of life over 24 weeks in adult patients with type 1 diabetes on continuous subcutaneous insulin infusion: a randomized, prospective clinical trial (GIOCAR). Diabetes Care 34, 823–827 (2011).
Acknowledgements
This study was supported by National Key Clinical Specialties Construction Program of China and The First Affiliated Hospital of Chongqing Medical University Nursing Research Fund (Fund Number: HLJJ2013-17). The funding source of the study was not part of the design of study, collection of information, analysis of data, nor preparation of this manuscript. We thank Dr Jinhui Tian for his statistical guidance, and also thank authors who contributed data of their studies.
Author information
Authors and Affiliations
Contributions
All authors’ responsibilities were as follows: Z.L. designed the subject and revised the article. S.F., L.L. developed inclusion and exclusion criteria, developed and performed the search strategy, conducted the statistical analysis and wrote the article. S.D., L.Z. screened relevant literature, made decisions according to inclusion and exclusion criteria.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fu, S., Li, L., Deng, S. et al. Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: a systematic review and meta-analysis. Sci Rep 6, 37067 (2016). https://doi.org/10.1038/srep37067
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep37067
This article is cited by
-
AdultCarbQuiz for the Portuguese population with type 1 diabetes mellitus: translation, cultural adaptation and validation of its metabolic impact
Acta Diabetologica (2024)
-
Diabetes mellitus-related hospital admissions and prescriptions of antidiabetic agents in England and Wales: an ecological study
BMC Endocrine Disorders (2023)
-
Carbohydrate Counting vs. Fixed Meal Plan in Indian Children with Type 1 Diabetes Mellitus: A Randomized Controlled Trial
Indian Journal of Pediatrics (2023)
-
Automation of insulin bolus dose calculation in type 1 diabetes: a feasibility study
International Journal of Diabetes in Developing Countries (2023)
-
Japanese Clinical Practice Guideline for Diabetes 2019
Diabetology International (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.