Abstract
Insecticide resistance is a growing threat to mosquito control programs around the world, thus creating the need to discover novel target sites and target-specific compounds for insecticide development. Emerging evidence suggests that mosquito inward rectifier potassium (Kir) channels represent viable molecular targets for developing insecticides with new mechanisms of action. Here we describe the discovery and characterization of VU041, a submicromolar-affinity inhibitor of Anopheles (An.) gambiae and Aedes (Ae.) aegypti Kir1 channels that incapacitates adult female mosquitoes from representative insecticide-susceptible and -resistant strains of An. gambiae (G3 and Akron, respectively) and Ae. aegypti (Liverpool and Puerto Rico, respectively) following topical application. VU041 is selective for mosquito Kir channels over several mammalian orthologs, with the exception of Kir2.1, and is not lethal to honey bees. Medicinal chemistry was used to develop an analog, termed VU730, which retains activity toward mosquito Kir1 but is not active against Kir2.1 or other mammalian Kir channels. Thus, VU041 and VU730 are promising chemical scaffolds for developing new classes of insecticides to combat insecticide-resistant mosquitoes and the transmission of mosquito-borne diseases, such as Zika virus, without harmful effects on humans and beneficial insects.
Similar content being viewed by others
Introduction
Mosquitoes are vectors of numerous human pathogens that impose enormous health and socioeconomic burdens on the developing world. The malaria vector An. gambiae and the dengue/yellow fever vector Ae. aegypti are collectively responsible for hundreds of millions of cases of malaria and dengue fever annually, leading to over 500,000 deaths per year1,2,3. Moreover, Ae. aegypti is suspected as the primary vector in the recent outbreak of Zika virus in Latin America and the Caribbean; Zika virus has been causally linked to dramatic increases in the number of cases of microcephaly and Guillan-Barré syndrome4,5. The two major classes of insecticides used in vector control programs are pyrethroids and anticholinergics (i.e., carbamates/organophosphates). These agents work, respectively, by blocking inactivation of voltage-gated sodium channels or inhibiting acetylcholinesterase enzymes expressed in the nervous system6,7,8. Moreover, they act on all developmental stages and sexes, creating intense selective pressure for target site resistance (e.g., knockdown resistance, kdr) and/or metabolic resistance (e.g., elevated expression of cytochrome P450 monoxygenases)9,10,11. The development of resistance and lack of novel, validated target sites that can be exploited for mosquito control complicate efforts to mitigate the spread of emerging mosquito-borne pathogens that have recently circumscribed the globe, such as Zika and chikungunya viruses. Thus, new classes of insecticides acting on novel molecular targets are needed to bolster integrated vector control programs and limit the spread of mosquito-borne diseases.
Recent genetic and pharmacological evidence suggests that inward rectifier potassium (Kir) channels could represent viable targets for new mosquitocides. In Drosophila melanogaster, embryonic depletion of Kir1, Kir2, or Kir3 mRNA levels leads to death or defects in wing development12. Knocking down Kir1 and Kir2 mRNA expression in the heart and Malpighian (renal) tubules of Drosophila, respectively, inhibits the immune response against cardiotropic viruses13 and transepithelial secretion of fluid and K+ 14. In yellow fever mosquitoes (i.e. Ae. aegypti), pharmacological inhibition of Kir1 with structurally-distinct small molecules (i.e. VU573, VU590, or VU625) disrupts the secretion of fluid and K+ in isolated Malpighian tubules, and impairs flight, urine production, and K+ homeostasis in intact females15,16,17,18. A major limitation of the potential use of these inhibitors as adulticides is their inability to penetrate the mosquito cuticle, thereby requiring microinjection to induce toxicity. Thus, one of our goals is to identify Kir1 inhibitors that can kill and/or incapacitate mosquitoes after topical application. Another goal is to identify inhibitors that are specific for mosquito Kir channels over mammalian Kir channels, since the latter play fundamental roles in nerve, muscle, endocrine, and epithelial cell function19.
In the present study, we employed high-throughput screening to identify a Kir1 inhibitor, termed VU041, which exhibits similar toxicity to adult female mosquitoes from representative insecticide-susceptible and -resistant strains of An. gambiae (G3 and Akron, respectively) and Ae. aegypti (Liverpool and Puerto Rico, respectively). Moreover, topical VU041 application to adult female mosquitoes of both species inhibits their fecundity. Importantly, VU041 is selective for mosquito Kir channels over mammalian Kir channel orthologs and non-lethal to adult honey bees (Apis mellifera). Thus, VU041 represents a promising chemical scaffold for the development of a new generation of insecticides to control mosquitoes without harmful effects on humans and beneficial insects.
Results
Discovery of VU041
Approximately 26,000 compounds were screened for pharmacological modulators of AnKir1 channel activity, leading to the discovery of 121 confirmed AnKir1 inhibitors. We focused on 1-(3,4-dihydroquinolin-1(2H)-yl)-2-(3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-indazol-1-yl)ethan-1-one (termed ‘VU041′ hereafter, Fig. 1a), because of its 1) potent inhibition of AnKir1-dependent thallium (Tl+) flux in vitro (e.g., Fig. 1b,c, Table S1) and 2) high partition coefficient (cLogP > 4), making it likely to penetrate the mosquito cuticle20.
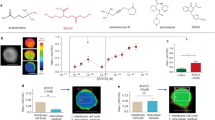
Design and characterization of AnKir1 small-molecule inhibitors.
(a) Modular approach to assess two areas of diversification of VU041 through a medicinal chemistry campaign: heterocyclic portion (red shading) and the dihydroquinoline portion (blue shading) of the molecule. See text for details. (b) Representative fluorescence traces showing dose-dependent inhibition of the AnKir1-mediated Tl+ flux by VU041 with concentrations ranging from 0.12 to 30 μM. The arrows indicate the addition of extracellular Tl+. (c) Concentration-response curves (CRCs) for VU041 and VU937 derived from Tl+ flux assays. The IC50 and Hill-coefficient values for VU041 are 2.5 μM (95% CI: 1.9–3.3 μM) and 1.1, respectively. The IC50 for VU937 was not determined due to less than 50% inhibition at 30 μM. Data are mean ± SEM; n = 5 independent experiments performed in triplicate. (d) Representative current-voltage plots of AnKir1 current in the presence of control saline, 0.5 μM VU041, and 2 mM Ba (positive control). (e) Time-course and reversibility of VU041-dependent inhibition of AnKir1 heterologously expressed in T-Rex-HEK293 cells. The cells were voltage clamped at a holding potential of −75 mV, stepped to −120 mV and then ramped to +120 mV every five seconds before returning to −75 mV. After current stabilization in control (C) saline, the superfusate was switched to one containing 10 μM VU041 until steady inhibition was reached. After this, VU041 was washed out of the bath in exchange for control (C) saline to allow wash-out. Barium (Ba) was added at the end of each experiment to block remaining AnKir1 current. (f) CRCs of VU041, VU730, and VU937 derived from patch clamp experiments (n = 4–6) against heterologously expressed AnKir1 cells.
In whole-cell patch clamp experiments (Fig. 1d–f), VU041 inhibited AnKir1 with an IC50 of 496 nM (95% CI: 396–619 nM; Hill coefficient value of 1.3), making it the 2nd most potent in vitro inhibitor of mosquito Kir1 channels discovered to date; VU625 remains the most potent in vitro inhibitor (IC50 ~ 100 nM), but is not topically toxic to mosquitoes which prevents practical use16. AnKir1 inhibition by VU041 was rapid and partially reversible (Fig. 1e). Two-electrode voltage clamp experiments in Xenopus oocytes revealed that VU041 preferentially inhibited AeKir1 over AeKir2B, both of which are expressed in the Malpighian tubules (Fig. S2).
The selectivity of VU041 for mosquito vs. mammalian Kir channels was evaluated in quantitative Tl+ flux experiments against AnKir1, AeKir1, and a panel of Kir channels that play critical physiological roles in mammals: Kir1.1 (kidney), Kir2.1 (heart, brain), Kir4.1 (kidney, brain), Kir6.2/SUR1 (pancreas, brain), and Kir7.1 (broadly expressed). VU041 inhibited AnKir1 and AeKir1 with IC50 values of 2.5 μM and 1.7 μM, respectively. Importantly, VU041 inhibited mammalian Kir1.1, Kir4.1, Kir6.2/SUR1, and Kir7.1 by less than 10% at a concentration of 30 μM. The only mammalian Kir channel tested that VU041 inhibited appreciably was Kir2.1 (IC50 of 12.7 μM; Table S1).
Lead optimization around the VU041 scaffold to enhance potency and mosquito selectivity
Lead optimization was initiated with the goal of improving the selectivity of VU041 for AnKir1 vs. Kir2.1. The first analog library was designed to keep the right-hand dihydroquinoline constant and evaluate the left-hand heterocyclic portion (Fig. 1a; red highlight). One compound (VU730, 5) retained its activity toward AnKir1 (IC50 = 2.4 μM in Tl+ flux assays [Table S2]; IC50 = 717 nM in patch clamp experiments [Fig. 1f]), but lost activity toward Kir2.1 (IC50 > 30 μM in Tl+ flux assays [Table S2]). Our next library kept the left-hand trifluoromethyl tetrahydropyrazole constant while altering the right-hand amide portion of the molecule (Fig. 1a; blue highlight). Although none of the compounds in this series showed an increase in potency against AnKir1 (Table S3), VU937 (Compound 18) inhibited AnKir1 channel activity in patch clamp experiments by 60-fold less than VU041 (IC50 = 29.7 μM; 95% CI: 17.7–49.9 μM) (Fig. 1f; Table S3). Due to the significant loss of potency, VU937 was used in subsequent experiments as an ‘inactive’ analog to confirm that any toxic or physiological effects of VU041 on mosquitoes were associated with its inhibition of Kir1.
VU041 is equally toxic to insecticide-susceptible and -resistant strains of mosquitoes
To determine if VU041 was topically toxic to mosquitoes, we applied the compound to the cuticles of insecticide-susceptible and insecticide-resistant strains of An. gambiae and Ae. aegypti (adult females) and assessed efficacy 24 h later. The resistant ‘Akron’ strain of An. gambiae is resistant to permethrin (33-fold) and propoxur (101-fold) when compared to the susceptible G3 strain of An. gambiae and is known to confer resistance through target-site (kdr and Modified AcetylCholine Esterase (MACE) and metabolic resistance mechanisms21,22,23,24. The resistant ‘Puerto Rico’ (PR) strain of Ae. aegypti possesses target-site (kdr) resistance (J.J. Becnel and BEI resources, personal communications), which contrasts from another Puerto Rican strain that possesses elevated mRNA levels encoding CYP450 enzymes25. Importantly, the ED50, or effective dose to incapacitate 50% of the mosquitoes, for VU041 was similar between the susceptible and resistant strains for each species (Fig. 2a,b; Table 1). In both species, VU937 was not toxic (Table 1, Fig. S3b), suggesting that the toxicity of VU041 was associated with its inhibition of Kir1 channels.
Toxicological characterization of VU041 in mosquitoes.
(a) Toxicity of the susceptible (G3) and multi-resistant (Akron) strains of An. gambiae mosquitoes (adult females) 24 h after topical exposure to VU041 using n = 3 replicates of 30 mosquitoes per dose tested. (b) Toxicity of the susceptible (LVP) and pyrethroid-resistant (PR) strains of Ae. aegypti mosquitoes (adult females) 24 h after topical exposure to VU041 using n = 4–8 replicates of 10 mosquitoes per dose tested. (c) Still images showing mosquito (adult female An. gambiae, G3) abdomens 24 h post-blood meal; mosquitoes were treated topically with vehicle (negative control), VU937, or VU041 immediately after feeding. An abdomen from a non-blood fed (NBF) mosquito is shown for comparison. (d) diameters of mosquito abdomens during the first 24 h post-blood meal (PBM) after topical exposure to EtOH (n = 20), VU041 (n = 22), or VU937 (n = 18). Values are means ± SEM. Uppercase letters represent statistical significance as determined by a one-way ANOVA with a Tukey’s posttest (P < 0.05). (e) Amount of urine excreted by adult female Ae. aegypti (LVP) mosquitoes 1 h after injection with 900 nL of K+-PBS. Two hours prior to the injection, mosquitoes were treated topically with the vehicle, VU937 (1.7 μg/mg mosquito), or VU041 (1.7 μg/mg mosquito). Values are means ± SEM; n = 14, 8, and 11 trials of 5 mosquitoes each for the vehicle, VU937, and VU041 treatments, respectively. Upper-case letters indicate statistical categorization of the means as determined by a one-way ANOVA with a Newman-Keuls post-test (P < 0.05).
Consistent with VU041 eliciting similar efficacy in both strains of An. gambiae, pre-treatment of the susceptible (G3) strain with piperonyl butoxide (PBO), an inhibitor of cytochrome P450 monoxygenases (CYP450s), only enhanced the efficacy of VU041 by ~3-fold, whereas pre-treatment with S,S,S-tributyl phosphorotrithioate (DEF), an inhibitor of carboxyesterases, did not enhance toxicity (Table 1). Inhibition of CYP450s in the AKRON strain, which possess up to a 12-fold overexpression of some CYP450 genes21, enhanced toxicity 3-fold more than the G3 strain likely due to the increased levels of metabolic enzymes causing altered pharmacokinetics and pharmacodynamics in the resistant strain. Thus, VU041 is only moderately metabolized by cytochrome P450 enzymes and does not appear to be metabolized by esterases. Experiments in the G3 strain of An. gambiae with VU730, which does not inhibit mammalian Kir2.1, revealed a similar ED50 as that for VU041 (Table 1). Thus, VU041 is the first small-molecule inhibitor of mosquito Kir1 channels that exhibits topical toxicity in both insecticide-susceptible and -resistant lines of mosquitoes. Moreover, VU041 can be modified to reduce its inhibition of mammalian Kir2.1 without affecting its efficacy as a mosquitocide (e.g., VU730).
VU041 inhibits renal excretory function in mosquitoes
A signature feature of inhibiting Kir channels in mosquitoes is impairment of fluid secretion/urine production in Malpighian tubules, which reduces the mosquito’s capacity for diuresis15,16,17,18. Diuresis plays an especially important role in adult female mosquitoes after a blood meal by excreting the excess fluid and electrolytes that are absorbed into the hemolymph26,27. We therefore evaluated whether VU041 disrupts fluid-volume regulation associated with blood meal processing in An. gambiae mosquitoes. Immediately after engorgement, mosquitoes were treated with an ED30 dose of VU041, and their abdominal diameters were measured over the following 24 h. In vehicle (control)- and VU937-treated mosquitoes, abdominal diameter increased approximately 2-fold immediately following blood feeding (Fig. 2c,d), and then decreased significantly over 24 h (Fig. 2c,d). In striking contrast, although the abdominal diameter of VU041-treated mosquitoes increased similarly, it did not change over the following 24 h period, consistent with VU041-dependent inhibition of fluid-volume excretion.
To directly determine whether VU041 impairs mosquito excretion, we performed an in vivo diuresis assay on adult female Ae. aegypti, using an approach we recently established for this species15,16, but the inhibitors were applied topically instead of injecting them into the hemolymph. The diuretic capacities of control and VU937-treated mosquitoes were similar to each other, whereas that of VU041-treated mosquitoes was significantly lower by ~51% compared to controls (Fig. 2e). Taken together with the data in Fig. 2c,d, these results suggest that VU041 impairs renal excretory function and fluid-volume regulation during blood meal processing in mosquitoes.
VU041 reduces mosquito fecundity
Given that VU041 disrupts blood meal processing and diuresis in mosquitoes (Fig. 2c–e), and that knock-down of AnKir1 expression via RNA interference reduces fecundity28, we hypothesized that VU041 would also reduce egg laying after blood feeding. Adult female mosquitoes of both species were topically treated with ~1 μg/mg mosquito (An. gambiae) or 3.4 μg/mg mosquito (Ae. aegypti) of VU041 or up to ~10 μg/mg mosquito (solubility limits) of VU937 within 1 h after engorgement, and the total number of eggs laid per mosquito were counted 72 h post blood feeding. For both An. gambiae and Ae. aegypti, the control and VU937-treated mosquitoes laid a similar median number of eggs per mosquito, whereas the VU041-treated mosquitoes laid a significantly lower median number of eggs per mosquito (Fig. 3). Thus, VU041 reduces mosquito fecundity.
Effects of VU041 on mosquito fecundity.
Number of eggs laid per female 72 h PBM for (a) An. gambiae and (b) Ae. aegypti. Mosquitoes were topically treated with the vehicle (control), VU937, or VU041 within 1 h following engorgement with blood. Each data point represents the egg output of an individual mosquito. Red bars indicate the median number of eggs laid for each treatment. The median number of eggs laid by VU041 mosquitoes was significantly lower than those laid for control and VU937-treated mosquitoes (P < 0.05), as determined by a Kruskal-Wallis ANOVA with a Dunn’s post-hoc analysis.
VU041 is not lethal to adult honeybees
Insecticide selectivity against pollinators is important given recent concerns over the role insecticides play in declines in pollinator health29. To determine if VU041 is toxic to honey bees, we treated 3-day old adult honey bees topically on the thoracic notum with a limit dose of VU041 (1 mg/bee; i.e., ~10 μg/mg), and assessed toxicity 48 h later compared to negative (vehicle) and positive (0.1 μg/bee bifenthrin) controls. As shown in Fig. 4, VU041 did not cause significant mortality to honey bees within 48 h compared to the vehicle(Fisher’s Exact Test, P = 0.74, N = 130), whereas application of bifenthrin resulted in 100% mortality at 48 h (P < 0.001, N = 87). Thus, VU041 is non-lethal to adult honey bees when applied topically.
Toxicity of VU041 to the honey bee, A. mellifera.
Percentage of bees alive (gray) or dead (black) 48 h after topical application of (a) solvent (control) or VU041 (1 mg per bee), or (b) solvent (control) or bifenthrin (0.3 μg per bee). P value indicates significant difference between control and treatment as determined by a Fisher’s Exact Test (P < 0.05).
Discussion
Resistance of mosquitoes to conventional insecticides that target the nervous system, such as pyrethroids, carbamates, and organophosphates, is a major hurdle to the integrated management of mosquito-borne diseases11. As discussed below, the results of the present study provide compelling data that a small molecule inhibitor of mosquito Kir channels (VU041) offers a new active compound for the development of mosquitocides that overcome insecticide resistance and may also be safe to humans and pollinators.
VU041 overcomes insecticide-resistance mechanisms
The Akron strain of An. gambiae used in the present study carries multiple resistance mechanisms including 1) mutations in a voltage-gated Na+-channel (kdr) that offers resistance to pyrethroids, 2) mutations in an AChE (MACE, ace-1R) that confers resistance to carbamates30,31,32,33, and 3) metabolic resistance derived from increased biochemical levels of CYP450s and carboxylesterases21. The Puerto Rican (PR) strain of Ae. aegypti used in this study is resistant to pyrethroids only through a point mutation (kdr) in the voltage-gated sodium channel.
A priori, one would not expect a mosquito strain with only target-site resistance in Na+ channels, such as the PR strain of Ae. aegypti used in this study, to exhibit resistance to a small molecule inhibitor of Kir channels. As such, VU041 showed similar efficacy against the LVP and PR strains of Ae. aegypti. However, mosquito strains with both target-site and metabolic resistance, such as the Akron strain of An. gambiae, may have a greater capacity to detoxify a small molecule, regardless of its molecular target. Importantly, VU041 showed similar efficacy against the Akron and G3 strains of An. gambiae, suggesting that VU041 is able to avert the elevated xenobiotic detoxification mechanisms of the Akron mosquitoes. Consistent with this finding, inhibition of CYP450s with PBO or esterases with DEF nominally enhanced the toxicity of VU041 against the G3 strain of An. gambiae (Table 1), suggesting that it is not efficiently metabolized by these important detoxifying enzymes. Inhibition of CYP450s in the Akron strain enhanced toxicity of VU041 at a greater level when compared to the G3 strain, presumably due to the significant overexpression of multiple CYP450 genes in this resistant strain. These data are consistent with VU041 being a modest substrate of this class of detoxification enzymes.
Potential mechanisms/modes of action of VU041 on mosquitoes
Our in vivo experiments of blood meal processing and diuretic capacity suggest that one mechanism of action of VU041 is the disruption of excretory functions mediated by Malpighian tubules. This finding is consistent with previous studies by our group that indicate Kir channels are an important mechanism of transepithelial K+ and fluid secretion in mosquito Malpighian tubules, where they localize to the basolateral membrane of the epithelial cells18,34,35,36. The inhibition of Kir channels in Malpighian tubules is expected to disrupt the processing of blood meals by limiting the excretion of blood-derived electrolytes and water that are absorbed into the hemolymph. In addition, potential effects on the midgut’s digestion of blood and absorption of ions/fluid from the blood cannot be ruled out given that the mosquito midgut is a site of Kir mRNA expression and barium-sensitive K+ transport28,37,38. Our experiments in An. gambiae demonstrate that mosquitoes treated with VU041 retain a large abdominal girth 24 h after blood feeding compared to control mosquitoes, suggesting an impairment of blood meal processing, perhaps by disrupting the post-prandial diuresis and/or blood digestion. Our experiments in Ae. aegypti confirm that VU041 at least inhibits mosquito diuretic capacity, consistent with impairment of Malpighian tubule function as a contributor to the toxicity of this compound. This novel mechanism of action of VU041 on a physiological target outside of the nervous system may also contribute to its efficacy against the Akron and PR strains, which are resistant to neurotoxic carbamates and/or pyrethroids.
The combined effects of VU041 on blood-meal processing and diuresis may also contribute to its inhibitory effects on mosquito fecundity in An. gambiae and Ae. aegypti. That is, if VU041 inhibits blood meal digestion, nutrient absorption, and/or solute/metabolite excretion, then vitellogenesis may not proceed efficiently. Furthermore, a direct effect of VU041 on the ovaries cannot be ruled out. In a previous study on adult female An. gambiae, we demonstrated that Kir1 mRNA levels are enriched in the ovaries and Kir1 mRNA knockdown reduces fecundity28. Thus, the reduced fecundity observed in the current and previous study may be a combined effect of inhibiting Kir1 in the excretory and reproductive systems.
Selectivity of VU041 for mosquito vs. mammalian Kir channels
The selectivity of new mosquitocides is of critical importance, because mosquito control methods often are implemented within or near human dwellings, especially in tropical regions with endemic malaria or dengue/Zika fever (e.g., aerial sprays, insecticide-treated bed nets). The results of our in vitro screening assays for VU041 show that this compound has a relatively clean ancillary pharmacology against a panel of mammalian Kir channels with no activity against Kir1.1, Kir4.1, Kir7.1, and Kir6.2/SUR1. However, VU041 moderately inhibits Kir2.1, which is highly expressed in the human heart; inhibition of this channel may have deleterious consequences on heart function39,40. Thus, we developed analogs of VU041 to determine if any structural changes led to increased selectivity for AnKir1 vs. Kir2.1. Remarkably, compound VU730 retained its inhibitor activity against AnKir1 without measureable inhibition of Kir2.1. Importantly, we confirmed that VU730 also retained its topical mosquitocidal toxicity with a similar potency as VU041. Although additional experiments will be necessary to demonstrate whether VU730 is non-toxic to mammals, our data suggest the exciting possibility that VU041 offers a chemical scaffold for the development of potent inhibitors of mosquito Kir channels with topical mosquitocidal activity and minimal inhibition of mammalian Kir channels.
Selectivity of VU041 for mosquitoes vs. adult honey bees
The selectivity of new mosquitocides is also important to limit effects on beneficial insects, such as honey bees and other pollinators, which contribute to over $24 billion USD to the US economy (https://www.whitehouse.gov/the-press-office/2014/06/20/fact-sheet-economic-challenge-posed-declining-pollinator-populations). Recent studies have cited significant concerns over the effects of broad-spectrum insecticides (e.g., neonicotinoids, pyrethroids) on pollinator health29. Remarkably, a dose of 10 μg VU041 per mg adult honey bee (A. mellifera) was not toxic within 48 h. A similar dose in mosquitoes would exhibit ~100% efficacy within 24 h. The honey bee ortholog of Kir1 shares only ~55% amino acid identity with mosquito Kir1 channels41. Thus, the interaction of VU041 with mosquito Kir1 channels may occur in a domain that is not conserved with the bee Kir1 channel. Alternatively, the chemical composition of the honey bee cuticle may substantially differ from that of mosquitoes in a manner that reduces the penetration of VU041 into the hemolymph and/or the VU041 molecule may be more efficiently detoxified by bees relative to mosquitoes. Additional studies will be required to better understand the lack of activity in adult bees and to confirm that the compound is not toxic to adult and larval bees when added to their diets. Future studies will also be required to determine if VU041 has any chronic sub-lethal effects on the performance of honey bee colonies. However, the data of the present study at least provide the exciting possibility that VU041 could serve as scaffold for developing inhibitors of mosquito Kir channels that are toxic to mosquitoes with nominal effects on beneficial insects.
Potential of VU041 for practical use against mosquitoes
Although VU041 represents a significant advance in the development of new mosquitocides targeting Kir channels (i.e., topical efficacy, potentially safe to humans and bees), it is important to note that this molecule requires additional chemical optimization and formulation development to improve its efficacy for use as a traditional mosquitocide in the field. However, the present study shows that VU041 impairs blood meal processing, renal function, and fecundity at relatively low, sub-lethal doses. Thus, if sub-lethal doses of Kir channel inhibitors can be delivered to adult female mosquitoes in the field, then we may expect to impair the longevitiy/fecundity of adult females instead of causing immediate death. As has been previously suggested, such an approach may dampen the propagation of resitance genes in a population and lead to the sustainable control of mosquito-borne diseases more effectively than traditional neurotoxic insecticides42,43.
To conclude, our study reports the first topically active, mosquito-selective, and resistance-mitigating small molecule inhibitor of a Kir channel. These data provide additional proof-of-concept and validation that Kir channels are viable insecticide target sites, as we have proposed in previous studies15,16,17,18. Future work will be aimed at increasing the in vitro and in vivo potency of these Kir channel inhibitors as well as fully elucidating their mechanisms of toxicity.
Methods
Stable T-REx-HEK293 cell line expressing Kir channels
The open-reading frame of a full-length cDNA encoding AnKir1 cloned from An. gambiae Malpighian tubules (Genbank Accession # KJ596497)17 was sub-cloned into the pcDNA5/TO expression vector (Life Technologies) and used for stable cell line generation, as described previously44,45. Expression constructs for mammalian Kir channels were developed and validated in previous studies by our group46,47,48.
Chemical Synthesis and Lead Optimization
Methods for chemical synthesis are provided in the Supplemental Methods section.
High Throughput Screening
Tl+ flux assays were performed essentially as described previously17,44,47. Briefly, stably transfected T-Rex-HEK-293 cells expressing AnKir1 channels were cultured overnight in 384-well plates (20,000 cells/20 μL/well black-walled, clear-bottomed BD PureCoat amine-coated plates (BD, Bedford, MA) with a plating media containing DMEM, 10% dialyzed FBS and 1 μg/mL tetracycline. Approximately twenty-four hours after cell plating, the cell culture medium was replaced with a dye-loading solution containing assay buffer (Hanks Balanced Salt Solution with 20 mM HEPES, pH 7.3), 0.01% (w/v) Pluronic F-127 (Life Technologies, Carlsbad, CA), and 1.2 μM of the thallium-sensitive dye Thallos-AM (TEFlabs, Austin, TX). Following 1 hr incubation at room temperature, the dye-loading solution was washed from the plates and replaced with 20 μL/well of assay buffer.
Whole-cell patch clamp electrophysiology
Transiently transfected HEK-293T cells expressing AnKir1 cells were voltage clamped in the whole-cell configuration of the patch clamp technique, as described previously17,47,48. The extracellular bath solution contained (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, 10 HEPES free acid, pH 7.4, 290 mOsm/kg H2O. The pipette solution contained (in mM): 135 KCl, 2 MgCl2, 1 EGTA, 10 HEPES free acid, 2 Na2ATP (Roche, Indianapolis, IN), pH 7.3, 275 mOsm. Cells were voltage clamped at −75 mV, stepped to −120 mV for 200 msec, and then ramped to 120 mV at a rate of 2.4 mV/msec. Concentration-response curves (CRCs) were constructed by measuring the effects of increasing doses of inhibitors on AnKir1 currents at −120 mV. All recordings were made at room temperature (20–23 °C).
Two-electrode voltage clamp electrophysiology
Heterologous expression of AeKir1 or AeKir2B was performed in Xenopus laevis oocytes as described previously16,17. Current-voltage (I-V) relationships of oocytes were acquired by clamping the membrane potential (Vm) near the spontaneous, resting Vm and then initiating a voltage-stepping protocol (via the Clampex module of pCLAMP) consisting of 20 mV steps from −140 mV to +40 mV (100 ms each). Additional details are provided in the Supplemental Methods.
Mosquito colonies
An. gambiae mosquitoes, G3 strain (MRA-112), were reared in an environmental chamber at 27 °C and 75% relative humidity at Vanderbilt University, Department of Biological Sciences, Nashville, TN. An. gambiae, Akron strain (MRA-913, isolated in Benin), was reared in a separate environmental chamber at the Emerging Pathogens Institute, University of Florida, Gainesville, FL at 27 °C and 75% relative humidity. The Akron strain of An. gambiae was selected every 5th generation for anticholinergic and pyrethroid resistance by exposing adult mosquitoes to bendiocarb (12.5 μg/bottle) and permethrin (21.5 μg/bottle) using the CDC bottle assay. The survivors of each sex were then mixed and allowed to breed (personal communication, Mr. Paul Howell, Centers for Disease Control and Prevention, Atlanta, GA). Furthermore, to ensure resistance, the mosquitoes from each resistant egg cohort were exposed to an LC99 dose (based on G3 toxicity values) of permethrin and propoxur. The mosquitoes used in this study were derived from the same colony that was used to demonstrate the resistance of the Akron strain to propoxur and permethrin that is attributed to both target site and metabolic resistance through upregulation of CYP450 enzymes21,22,23,49.
An established colony of Ae. aegypti mosquitoes, Liverpool strain (LVP-IB12, MRA-735), was reared and maintained as previously described15 in an environmental chamber at 28 °C and 80% relative humidity at the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University, Wooster, OH. When needed, eggs from a pyrethroid-resistant strain of Ae. aegypti, Puerto Rico strain (PR, NR-48830), were obtained from BEI Resources, NIAID, NIH and reared to adults. The third-instar larvae of the resistant strain of Ae. aegypti were exposed to permethrin (0.1 mg/ml) every third generation to maintain the resistance trait (personal communication, Mr. Paul Howell, Centers for Disease Control and Prevention, Atlanta, GA). Adult mosquitoes of all strains were fed a 10% sucrose solution ad libitum and held under a 12 h/12 h light cycle. All experiments were carried out on adult females at 3–5 days post-eclosion.
Toxicology experiments in An. gambiae
Topical toxicity bioassays were performed based on the method of Pridgeon et al.50. Briefly, non-blood fed adult female mosquitoes were chilled on ice for 1–3 minutes, during which 200 nL of chemical (dissolved in 95% ethanol) was applied onto the abdomen of the insect using a handheld Hamilton® micro-applicator. For synergism studies, 500 ng of the synergist (i.e., piperonyl butoxide [PBO] or S,S,S-tributyl phosphorotrithioate [DEF]) per milligram of mosquito (ng/mg) was applied to the abdomen 4 h prior to application of the insecticide. For each compound, 6–8 doses that resulted in toxicity ranging between 0% and 100% were applied to a minimum of 30 mosquitoes each, and repeated 3 times on mosquito broods. The three ED50 values obtained were then averaged and presented as a mean. An ethanol-only treatment was included in each experiment as a negative control. Treated mosquitoes were transferred to small cages with access to 10% sucrose and held under rearing conditions for 24 h. Mortality was recorded at 24 h. Mortality data were pooled and analyzed by log-probit using Poloplus® to determine 24 h ED50 values, after correcting for control mortality using Abbot’s formula51.
Blood meal processing studies were performed with similar methods as described above, with the exception that An. gambiae were blood fed on anesthetized mice. All methods were carried out in accordance with Vanderbilt Institutional Animal Care and Use Committee approval (protocol M/13/164 to Dr. Julian Hillyer, Department of Biology, Vanderbilt University). Upon completion of blood feeding, female mosquitoes with a fully distended abdomen were selected and 200 nL of chemical at a non-lethal concentration (1 μg/mg) was applied directly to the abdomen. Any mosquito that died during the 24 h observation period was excluded from the analysis. Images of the abdomens were acquired at 0, 2, 5, 8, and 24 h through the dorsal cuticle and were measured at the widest point of the abdomen. These images were captured using bright-field illumination on a Nikon 90i light microscope (Nikon Corp., Tokyo, Japan) connected to a Photometrics CoolSNAP HQ2 high-sensitivity monochrome CCD camera (Roper Scientific, Ottobrunn, Germany). Digital images were acquired using Nikon Advanced Research NIS-Elements software. Mean abdominal diameters were compared using a one-way ANOVA with a Tukey’s post-hoc analysis (Prism 6, Graphpad Software, La Jolla, CA).
Toxicity experiments in Ae. aegypti
Topical toxicity bioassays in adult female Ae. aegypti (LVP and PR strains) were performed as described previously52. Briefly, for a given dose, 10 non-blood fed mosquitoes were immobilized on ice and 500 nL of VU041 was applied to the thorax of each using a handheld Hamilton® microapplicator. A solvent-only treatment was included in each experiment as a negative control. Treated mosquitoes were transferred to small cages with access to 10% sucrose and held under rearing conditions for 24 h. The efficacy of a dose was measured as the percentage of treated mosquitoes in a cage that were flightless or dead at 24 h. Four to eight replicates of 10 mosquitoes were performed per dose. The ED50 values were determined using a non-linear curve fit analysis (log [inhibitor] vs. response variable-slope) in Prism 6 (Graphpad Software).
The efficacy of VU041 was compared to that of its inactive analog (i.e., VU937) only in LVP mosquitoes. In these experiments, two groups of 10 mosquitoes were treated with a dose of VU041 or VU937 near the ED50 of VU041 (3.24 μg/mg mosquito), and their condition was assessed 24 h later, as described above. Six replicate experiments of 10 mosquitoes were performed. The mean efficacies of the solvent, VU041, and VU937 were analyzed using a one-way ANOVA with a Newman-Keuls post hoc analysis (Prism 6, Graphpad Software).
Diuresis experiments in Ae. aegypti
The excretory capacity of adult female Ae. aegypti (LVP strain) was measured as described previously15,16. In brief, groups of 5 mosquitoes were treated with a sub-lethal dose of VU041 (1.7 μg/mg mosquito), VU937 (1.7 μg/mg mosquito), or solvent 2 h before injecting the hemolymph of each mosquito with 900 nL of a potassium-enriched, phosphate-buffered saline (K+-PBS) using a Nanoject II microinjector (Drummond Scientific Company, Broomall, PA). The K+-PBS consisted of the following (in mM): 92.2 NaCl, 47.5 KCl, 10 Na2HPO4 and 2 KH2PO4 (pH 7.5). Each treatment group of 5 mosquitoes was transferred into a separate graduated, packed-cell volume tube (MidSci, St. Louis, MO) and held for 1 h at 28 °C. The volume excreted by the mosquitoes was measured visually via the graduated column at the bottom of the tube. At least 8 replicates (5 mosquitoes per replicate) were performed for each treatment. All mosquitoes were confirmed to be alive at the end of 1 h. The mean volumes excreted by solvent-, VU041-, and VU937-treated mosquitoes were analyzed using a one-way ANOVA with a Newman-Keuls post hoc analysis (Prism 6, Graphpad Software).
Mosquito fecundity experiments
To determine the effects of VU041 on fecundity in An. gambiae, we used an assay similar to Raphemot et al.28 Briefly, adult female mosquitoes were given access to an anesthetized mouse for 60 min. After this time period, engorged mosquitoes were immobilized on ice and 200 nL of VU041 (ED30: 1 μg/mg of mosquito), VU937 (10 μg/mg of mosquito), or solvent was applied directly to their abdomens. After treatment with the respective drugs, individual female mosquitoes were transferred to Drosophila vials (Fisher Scientific, Pittsburg, OA) containing 2 mL of water. The total number of eggs were counted 72-hours after being transferred to the vial. Any mosquitoes that died during this 72-hour period were excluded from the analysis. All assays were performed in an environmental chamber that was maintained at 27 °C and 75% relative humidity and mosquitoes were given access to 10% sucrose solution ad libitum. At least 25 female mosquitoes were used per replicate for each treatment group; each treatment was repeated on three separate broods, giving a total number of individuals studied ranging from 75–113 for each group.
To determine the effects of VU041 on fecundity in Ae. aegypti, adult female mosquitoes were allowed to feed for 1 h on heparinized rabbit blood (Hemostat) presented in a membrane feeder (Hemotek). After the feeding period, the mosquitoes were immobilized on ice, visually inspected for blood engorgement, and topically treated with 500 nL of solvent, VU041 (3.4 μg/mg mosquito), or VU937 (3.4 μg/mg mosquito). The mosquitoes were returned to rearing conditions for 24 h after which they were transferred to individual egg laying tubes consisting of a glass tube 21 × 70 mm (Fisher Scientific, Pittsburg, PA) with a piece of coffee filter (Melitta USA, Clearwater, FL) cut to fit the bottom of the tube. The filter was wetted with 150 μl of dH2O and the open end of the tube was plugged with a cotton ball. The mosquitoes in their individual egg laying tubes were returned to rearing conditions for an additional 48 h, and the number of eggs laid were counted. Any mosquitoes that died during the 72 h period after blood feeding were excluded from the analysis. Thirty female mosquitoes were used per replicate for each treatment group; each treatment was repeated on four separate broods, giving a total number of individuals studied ranging from 87–113 for each treatment group. For both species, the median number of eggs laid per mosquito was compared using a Kruskal-Wallis ANOVA with a Dunn’s post-hoc analysis (Prism 6, Graphpad Software).
Honeybee rearing and toxicity experiments
Frames of late-stage honey bee (A. mellifera) pupae were taken from four colonies at The Ohio State University Honey Bee Lab in Wooster, OH and maintained in a dark humid incubator at 34 °C (Darwin Chambers Co., St. Louis, MO, model H024) until adult bees emerged. New adults were brushed from frames daily and placed in wooden screen cages (21 × 14 × 12 cm) provisioned with 1:1 (w/w) sucrose in water.
Acute toxicity experiments in adult bees were performed as follows. Twenty-four hours after emergence, adult honey bees in cages were anaesthetized with carbon dioxide, divided into groups of approximately 20 bees and placed in plastic-coated paper cups (177 cm3; UNIQ Paper Yogurt Cup, Frozen Dessert Supplies, Gilbert, AZ) covered with cotton cheesecloth. Smaller groups of bees were anaesthetized a second time and dosed on the thoracic notum with 10 μl of VU041 (100 μg/μl) or 10 μl of the vehicle. As a positive control, some bees were treated with 3 μl of bifenthrin (0.1 μg/μl); negative controls consisted of bees treated with 3 μl of solvent. Applications were made using a 20 μl micropipette (Fisherbrand Finnpipette F2, Fisher Scientific, Pittsburgh, PA) with disposable plastic tips. After treatment, bees were returned to the paper cups and provided with sugar syrup in punctured 1.5 ml microcentrifuge tubes. Toxicity to honey bees was recorded at 48 h versus the 24 h used for mosquitoes to ensure VU041 did not cause delayed toxicity due to the larger body size of the bee. Bees showing no movement on inspection at 48 h after treatment were scored as dead. A Fisher’s Exact Test was used to compare the proportion of mortality induced by VU041 and bifenthrin compared to their respective vehicle controls.
Additional Information
How to cite this article: Swale, D. R. et al. An insecticide resistance-breaking mosquitocide targeting inward rectifier potassium channels in vectors of Zika virus and malaria. Sci. Rep. 6, 36954; doi: 10.1038/srep36954 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Hemingway, J. & Ranson, H. Insecticide resistance in insect vectors of human disease. Annual review of entomology 45, 371–391 (2000).
Shepard, D. S., Undurraga, E. A. & Halasa, Y. A. Economic and disease burden of dengue in Southeast Asia. PLoS neglected tropical diseases 7, e2055 (2013).
Rasmussen, S. A., Jamieson, D. J., Honein, M. A. & Petersen, L. R. Zika Virus and Birth Defects–Reviewing the Evidence for Causality. N Engl J Med 374, 1981–1987 (2016).
Cao-Lormeau, V. M. et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387, 1531–1539 (2016).
Fukuto, T. R. Mechanism of action of organophosphorus and carbamate insecticides. Environmental health perspectives 87, 245–254 (1990).
Casida, J. E. Pest toxicology: the primary mechanisms of pesticide action. Chemical research in toxicology 22, 609–619 (2009).
Soderlund, D. M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Archives of toxicology 86, 165–181 (2012).
Wondji, C. S. et al. Impact of pyrethroid resistance on operational malaria control in Malawi. Proc Natl Acad Sci USA 109, 19063–19070 (2012).
Saavedra-Rodriguez, K. et al. Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect Mol Biol 21, 61–77 (2012).
Liu, N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60, 537–559 (2015).
Dahal, G. R. et al. An inwardly rectifying K+ channel is required for patterning. Development 139, 3653–3664 (2012).
Eleftherianos, I. et al. ATP-sensitive potassium channel (KATP)-dependent regulation of cardiotropic viral infections. Proc Natl Acad Sci USA 108, 12024–12029 (2011).
Wu, Y., Baum, M., Huang, C. L. & Rodan, A. R. Two Inwardly Rectifying Potassium Channels, Irk1 and Irk2, Play Redundant Roles in Drosophila Renal Tubule Function. Am J Physiol Regul Integr Comp Physiol, ajpregu 00148, 02015 (2015).
Raphemot, R. et al. Eliciting renal failure in mosquitoes with a small-molecule inhibitor of inward-rectifying potassium channels. PloS one 8, e64905 (2013).
Rouhier, M. F., Raphemot, R., Denton, J. S. & Piermarini, P. M. Pharmacological validation of an inward-rectifier potassium (Kir) channel as an insecticide target in the yellow fever mosquito Aedes aegypti. PloS one 9, e100700 (2014).
Raphemot, R. et al. Discovery and characterization of a potent and selective inhibitor of Aedes aegypti inward rectifier potassium channels. PloS one 9, e110772 (2014).
Beyenbach, K. W., Yu, Y., Piermarini, P. M. & Denton, J. Targeting renal epithelial channels for the control of insect vectors. Tissue Barriers (2015).
Hibino, H. et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90, 291–366 (2010).
Webb, J. E. & Green, R. A. On the penetration of insecticides through the insect cuticle. J Exp Biol 22, 8–20 (1945).
Djouaka, R. F. et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics 9, 538 (2008).
Ranson, H. et al. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27, 91–98 (2011).
Weill, M. et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol 13, 1–7 (2004).
Ahoua Alou, L. P. et al. Distribution of ace-1R and resistance to carbamates and organophosphates in Anopheles gambiae s.s. populations from Cote d’Ivoire. Malar J 9, 167 (2010).
Reid, W. R. et al. Transcriptional analysis of four family 4 P450s in a Puerto Rico strain of Aedes aegypti (Diptera: Culicidae) compared with an Orlando strain and their possible functional roles in permethrin resistance. Journal of medical entomology 51, 605–615 (2014).
Williams, J. C., Hagedorn, H. H. & Beyenbach, K. W. Dynamic changes in flow rate and composition of urine during the post blood meal diuresis in Aedes aegypti. Journal of Comparative Physiology [B] 153, 257–266 (1983).
Coast, G. M. Neuroendocrine control of ionic homeostasis in blood-sucking insects. J Exp Biol 212, 378–386 (2009).
Raphemot, R. et al. Molecular and functional characterization of Anopheles gambiae inward rectifier potassium (Kir1) channels: A novel role in egg production. Insect Biochem Mol Biol 51, 10–19 (2014).
Goulson, D., Nicholls, E., Botias, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015).
Wong, D. M. et al. Select small core structure carbamates exhibit high contact toxicity to “carbamate-resistant” strain malaria mosquitoes, Anopheles gambiae (Akron). PloS one 7, e46712 (2012).
Wong, D. M. et al. Aryl methylcarbamates: potency and selectivity towards wild-type and carbamate-insensitive (G119S) Anopheles gambiae acetylcholinesterase, and toxicity to G3 strain An. gambiae. Chemico-biological interactions 203, 314–318 (2013).
Djogbenou, L. et al. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. Journal of medical entomology 44, 805–810 (2007).
Yadouleton, A. W. et al. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malaria journal 8, 103 (2009).
Piermarini, P. M., Rouhier, M. F., Schepel, M., Kosse, C. & Beyenbach, K. W. Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti. Insect Biochem Mol Biol 43, 75–90 (2013).
Piermarini, P. M. et al. Localization and role of inward rectifier K channels in Malpighian tubules of the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol (2015).
Rouhier, M. F. et al. Excretion of NaCl and KCl loads in mosquitoes. 2. Effects of the small molecule Kir channel modulator VU573 and its inactive analog VU342. Am J Physiol Regul Integr Comp Physiol 307, R850–R861 (2014).
Rouhier, M. F. & Piermarini, P. M. Identification of life-stage and tissue-specific splice variants of an inward rectifying potassium (Kir) channel in the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol 48, 91–99 (2014).
Pacey, E. K. & O’Donnell, M. J. Transport of H(+), Na(+) and K(+) across the posterior midgut of blood-fed mosquitoes (Aedes aegypti). J Insect Physiol 61, 42–50 (2014).
Plaster, N. M. et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 105, 511–519 (2001).
Rodriguez-Menchaca, A. A. et al. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc Natl Acad Sci USA 105, 1364–1368 (2008).
Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443, 931–949 (2006).
Read, A. F., Lynch, P. A. & Thomas, M. B. How to make evolution-proof insecticides for malaria control. PLoS Biol 7, e1000058 (2009).
Foy, B. D., Kobylinski, K. C., da Silva, I. M., Rasgon, J. L. & Sylla, M. Endectocides for malaria control. Trends Parasitol 27, 423–428 (2011).
Lewis, L. M. et al. High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1. Mol Pharmacol 76, 1094–1103 (2009).
Raphemot, R. et al. Development and validation of fluorescence-based and automated patch clamp-based functional assays for the inward rectifier potassium channel kir4.1. Assay Drug Dev Technol 11, 532–543 (2013).
Bhave, G. et al. Development of a selective small-molecule inhibitor of Kir1.1, the renal outer medullary potassium channel. Mol Pharmacol 79, 42–50 (2011).
Raphemot, R. et al. Discovery, characterization, and structure-activity relationships of an inhibitor of inward rectifier potassium (Kir) channels with preference for kir2.3, kir3.x, and kir7.1. Front Pharmacol 2, 75 (2011).
Raphemot, R. et al. Direct Activation of beta-cell KATP Channels with a Novel Xanthine Derivative. Mol Pharmacol (2014).
Mutunga, J. M. et al. Carbamate and pyrethroid resistance in the akron strain of Anopheles gambiae. Pestic Biochem Physiol 121, 116–121 (2015).
Pridgeon, J. W. et al. Susceptibility of Aedes aegypti, Culex quinquefasciatus Say, and Anopheles quadrimaculatus Say to 19 pesticides with different modes of action. J Med Entomol 45, 82–87 (2008).
Abbott, W. S. A method of computing the effectiveness of an insecticide. 1925. Journal of the American Mosquito Control Association 3, 302–303 (1987).
Calkins, T. L. & Piermarini, P. M. Pharmacological and Genetic Evidence for Gap Junctions as Potential New Insecticide Targets in the Yellow Fever Mosquito, Aedes aegypti. PLoS One 10, e0137084 (2015).
Acknowledgements
The authors thank Nuris Acosta (The Ohio State University), and the Vanderbilt High-Throughput Screening Center for technical assistance, and Dr. Julián F. Hillyer, (Vanderbilt University) for providing the Anopheles gambiae (G3 strain) that were used for toxicology experiments. Funded by a grant from the Foundation for the National Institutes of Health through the Vector-Based Transmission of Control: Discovery Research (VCTR) program of the Grand Challenges in Global Health initiative. This work was also supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK082884; JSD).
Author information
Authors and Affiliations
Contributions
D.R.S., A.G., E.A.I., E.D., F.K., L.Y., P.M.R. performed experiments. D.W.E., S.R.B., C.R.H. provided new chemical reagents. D.R.S., D.W.E., S.R.B., A.G., E.A.I., E.D., F.K., R.M.J., L.Y., C.R.H., P.M.R., J.S.D. performed data analysis. D.R.S., R.M.J., J.R.B., C.R.H., P.M.P., J.S.D. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Swale, D., Engers, D., Bollinger, S. et al. An insecticide resistance-breaking mosquitocide targeting inward rectifier potassium channels in vectors of Zika virus and malaria. Sci Rep 6, 36954 (2016). https://doi.org/10.1038/srep36954
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36954
This article is cited by
-
Potassium ion channels as a molecular target to reduce virus infection and mortality of honey bee colonies
Virology Journal (2023)
-
Inwardly Rectifying Potassium (Kir) Channels Represent a Critical Ion Conductance Pathway in the Nervous Systems of Insects
Scientific Reports (2018)
-
ATP-sensitive inwardly rectifying potassium channel regulation of viral infections in honey bees
Scientific Reports (2017)
-
Bti-based insecticide enhances the predatory abilities of the backswimmer Buenoa tarsalis (Hemiptera: Notonectidae)
Ecotoxicology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.