Abstract
Though not uncommon in other animals, heterospecific mating is rarely reported in arachnids. We investigated sexual interactions among four closely related and syntopical African golden orbweb spiders, Nephila inaurata, N. fenestrata, N. komaci, and N. senegalensis. In two South African localities, female webs were often inhabited by heterospecific males that sometimes outnumbered conspecifics. Species association of males with females was random in nature. In subsequent laboratory choice experiments, N. inaurata males chose heterospecific females in 30% of trials. We also observed natural mating interactions between N. inaurata males and N. komaci females, and between N. komaci males and N. inaurata females in laboratory experiments. While heterospecific mating in the laboratory never produced offspring, conspecific mating did. We discuss potential ecological and evolutionary consequences of heterospecific mating interactions in Nephila that may be particularly costly to the rarer species.
Similar content being viewed by others
Introduction
Closely related species with overlapping ranges that compete for resources are often subjected to ecological research, e.g. in studies of species distribution1 and competition for resources2,3. Less attention has been given to species interacting in a mating context, beyond the obvious cases of hybridization. Sexual encounters between species may be costly and non-adaptive, but are not uncommon, with 167 species spanning the animal kingdom reported to engage in sexual interactions with heterospecifics4. When heterospecific sexual interactions affect the fitness of individuals of either species, theory predicts the occurrence of reproductive interference5. Reproductive interference typically results from incomplete species recognition, reinforced by strong male-male competition, resulting in indiscriminate male mating attempts and relaxed female choice5. While reproductive interference may be common6, the effects on population dynamics and community structure remain unexplored.
In arachnids, heterospecific sexual interactions are confined to spider mites7,8, and two anecdotal accounts exist of laboratory-staged mating trials in cursorial spiders, Ctenidae9 and Lycosidae10, but have so far not been reported in orbweb spiders. Heterospecific sexual interactions are expected to be rare in species where sexual cannibalism is prevalent, since, in theory, cannibalism in the context of female choice would allow females to avert such mistakes. One may thus expect that spiders, particularly sexually dimorphic taxa known for sexual cannibalism11, would not engage in heterospecific matings.
However, our field discovery and subsequent laboratory studies, surprisingly, suggest otherwise. We investigated field sex ratios in, and species interactions between, four golden orbweb spiders (genus Nephila) co-occurring in an area in north-eastern KwaZulu-Natal, South Africa: Nephila inaurata (Walckenaer, 1841), N. fenestrata (Thorell, 1859), N. komaci Kuntner & Coddington, 2009, and N. senegalensis (Walckenaer, 1841). Their relative phylogenetic proximity12, their co-occurrence, and similar life histories suggest interspecific competition for niche and mating opportunities. In particular, N. inaurata and N. komaci are ecologically indistinguishable where they co-habit, but N. inaurata is widespread and locally abundant13 whereas N. komaci is severely limited in range and locally rare14. We report on the occurrence of heterospecific interactions among the four Nephila species in the field, and of mating between N. komaci and N. inaurata. These interactions are surprising given that sexual cannibalism is common in Nephila12, and could play an important role in determining the community structure of Nephila, possibly through reproductive interference.
Results
We observed a total of 127 haphazardly encountered females of the four Nephila species in the two sites in KwaZulu-Natal, South Africa. Most webs of subadult and adult females were occupied by at least one male. Of these, heterospecific males were present on 50%, 20–60%, and 66.67% of N. fenestrata, N. inaurata, and N. komaci webs, respectively (Fig. 1; Table 1).
Interspecific interactions in Nephila.
(a) feeding female N. fenestrata with a conspecific male mating, and a N. inaurata male (left, large) within her web in iSimangaliso NP. (b) female N. komaci (lower, right), with a conspecific (left, small) and a heterospecific male (N. inaurata, top, large) within her web in Ndumo Game Reserve.
In iSimangaliso Wetland Park, we observed 29 N. fenestrata and 32 N. inaurata females. On the webs of both species we observed conspecific as well as heterospecific males. Sixty-two percent of N. fenestrata females (n = 18) had at least one male cohabiting in the web (2.5 ± 1.67, n = 44 males). Of the recovered males (n = 35), 31% (n = 11) were heterospecific. We observed a total of 24 males in 10 of the observed N. inaurata webs, including juvenile webs (2.56 ± 1.71); of the collected males (n = 17), 10 were heterospecific (59%).
In Ndumo Game Reserve, 83% (10 of 12 webs) of N. inaurata webs were inhabited by at least one male (1.40 ± 0.66; n = 14 males), and two of the collected males (n = 9) were heterospecific (22%). We observed 37 webs of female N. komaci, of which 65% (n = 24) were inhabited by at least one male (1.50 ± 0.82, n = 36 males), these being mostly heterospecific (65%, n = 17 of 26 collected males). We observed 17 N. senegalensis webs. Only nine males were found on N. senegalensis webs (1.13 ± 0.33), of which four were conspecifics; the other five males were lost and could not be identified. We observed two copulations between N. inaurata males and N. komaci females.
Male association with webs was random between N. inaurata and N. fenestrata in iSimangaliso (Fisher’s exact test, n = 52, p = 0.544), and not random among the three species in Ndumo (Fisher’s exact test, n = 39, p < 0.001); there, N. komaci webs were occupied predominantly by heterospecific males (65.38%, n = 17 heterospecific males; 34.62%, n = 9 conspecific males).
Male sizes varied among (ANOVA: F134,3 = 12.482, p < 0.001) and within species (Fig. 2). Post hoc comparisons using Tukey HSD revealed that N. inaurata males were larger than N. fenestrata males (p-adjust = 0.02, n = 100), while N. komaci males were smaller than both N. inaurata (p-adjust < 0.001, n = 92) and N. senegalensis males (p-adjust < 0.001, n = 38).
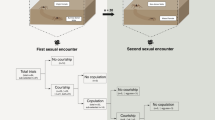
In mate choice experiments (Fig. 3), N. inaurata males chose conspecific versus heterospecific females in 11/16 (68.75%), versus in 5/16 trials (31.25%), respectively. Conspecific and heterospecific mate choice occurred randomly (binomial test: p = 0.21, n = 16). In these trials, one male copulated with a conspecific female. We observed courtship, but no copulation, in additional four conspecific couples and two heterospecific couples. We observed female aggression in four cases, three of which occurred in conspecific couples.
In mating trials between N. komaci males and N. inaurata females, all males (n = 9) courted females, and of these, eight males (88.89%) mated using both palps. We monitored all heterospecifically mated females, of which only two produced egg-sacs, but these were unviable.
Discussion
We report on the first heterospecific sexual interactions among orbweb spiders. Our field and laboratory results suggest that heterospecific coexistence in webs, and even mating interactions, are common in Nephila. We predominantly detected random species association of males with females in nature, and found no preference for conspecific females by N. inaurata males in the laboratory trials. Our results suggest that heterospecific interactions of Nephila males may involve several mistakes in locating, courting to, and mating with a “correct” (conspecific) female. As explained below, the phenomenon might be a result of incomplete species recognition reinforced by male – male competition and male biased sex ratios.
The high proportions of Nephila webs occupied by heterospecific males in nature (20–60%) may be a consequence of incomplete species recognition, where males randomly occupy heterospecific and conspecific webs of congeners. Random mate choice was also confirmed in laboratory experiments, however, N. inaurata males chose a conspecific female in 70% of trials. On the other hand, in the absence of a choice between a con- and a heterospecific female, N. komaci males always engaged in mating interactions with heterospecific females (N. inaurata). These results imply that Nephila males are in fact able, to some degree, to identify conspecifics on webs, but when on heterospecific webs, they indiscriminately engage in mating interactions.
Nephila spiders are extremely sexually size dimorphic, and occur in male biased sex ratios15,16. Females commonly co-habit with multiple males, which compete for mating opportunities. In direct male – male competition larger males usually outcompete smaller rivals17,18,19,20. On average among the species studied here, N. komaci males are the smallest and are thus expected to be outcompeted, in heterospecific interactions, by larger N. inaurata or N. senegalensis. Indeed, our field observations found that most males in N. komaci webs (~65%) were heterospecific. Additionally, local variation in species composition and phenology, relative abundances, and species-specific sex ratios likely affect the proportion of heterospecific males. For example, the proportion of heterospecific males in N. inaurata webs greatly differ between the two locations in our study. In iSimangaliso Wetland Park, where N. inaurata co-occurs with N. fenestrata, 60% of N. inaurata webs harbored heterospecific males. On the other hand, in Ndumo Game Reserve, where N. inaurata co-occurs with N. komaci and N. senegalensis, only 20% of N. inaurata webs harbored heterospecific males.
Heterospecific sexual interactions could interfere with reproduction outputs of sympatric species. If so, occupying a heterospecific female web could affect male, perhaps even female fitness. Several risks exist for males. First, strong male-male competition in Nephila usually results in larger males outcompeting smaller ones17,18,19,20, and thus the syntopic species with smaller males is likely at a disadvantage. For example, N. komaci males are on average smaller than the other two co-occurring species, which implies that interspecies male competition could lower their reproductive success simply due to an increased number of competing, larger males. This may explain the rarity of N. komaci in nature. In addition to male competition, males also risk being cannibalized by females, as is common in Nephila11,21. Finally, if at least some sperm gets transferred at copulations with heterospecific females, males risk becoming sperm depleted because adult Nephila males are incapable of recharging their palps with sperm22,23.
For females, heterospecific males are a threat to their own reproductive success. Failure in species recognition poses a cost, as females need to balance between being choosy (by means of sexual cannibalism) on the one hand, and accepting enough males in order to successfully reproduce on the other. Because of female gigantism, Nephila females are expected to be sperm depleted, and at least in laboratory conditions, they need to mate more than twice before they produce a viable eggsac24,25. Moreover, none of the females that in our trials mated with both a conspecific and a heterospecific were able to produce viable egg sacs, while those that only mated with conspecifics did. While this evidence is preliminary, it suggests that heterospecific mating negatively affects female fitness. A similar phenomenon has been observed between two closely related Panonychus spider mites, where females that had mated with a conspecific after having mated with a heterospecific, did not produce female offspring7.
The observed heterospecific sexual interactions present an opportunity to explore a theoretical framework in which reproductive interference would play a key role in the population dynamics of each of the represented species, and interspecific competition. It is reasonable to believe that the presence of males from the dominant species – N. inaurata – on webs of the globally (N. komaci) or locally rare species (N. senegalensis) could decrease their reproductive rates. However, together with this, other factors such as climatic variation, individual species’ biology, inter- and intraspecific competition, annual fluctuations of natural enemies, amongst others, may all contribute to the considerable annual population fluctuations of each of these species in Maputaland. In the case of reproductive interference, the locally dominant species could also suffer from sperm depletion if too many males are failing to arrive at the correct/conspecific females, therefore also suffering a reduction in reproductive output. Such dynamics have been hypothesized to maintain the coexistence of ecologically identical species26.
A model of coexistence through reproductive interference26 applies to species using ephemeral patchy resources, which does not apply to our focal species. More inclusive population and ecological models may illuminate implications of reproductive interference for rare species like N. komaci, living in habitats of continuous resources (see Supplementary material 1). Coexistence could be possible if the rarer species has a greater impact on the reproductive output of the common species. On the contrary, if the asymmetry of the interference favors the dominant species, the rare species (N. komaci) would potentially become locally extinct. Since in most of the Nephila range, single species dominate, the here investigated coexistence of the four species is secondary, and may not be ecologically stable.
Methods
Specimen collection
We observed and collected specimens of N. fenestrata and N. inaurata in iSimangaliso Wetland Park (S28.15895, E32.28897), and of N. inaurata, N. komaci and N. senegalensis in Ndumo Game Reserve (S26.54217, E32.15957) in north-eastern KwaZulu-Natal, South Africa (southern Maputaland) for two weeks in February 2015. We counted all males present on each encountered Nephila web and the female’s ontogenetic stage. For subsequent body size measurements, we photographed each spider using a Canon Eos 7D camera equipped with a Canon 50 mm macro lens. For all photography of spiders, we used a standardized fixed focal length. The photos were used to calculate carapace width as a measure of male size, for 30 N. fenestrata, 70 N. inaurata, 22 N. komaci, and 16 N. senegalensis males (Fig. 2). After the field survey, we transferred live specimens of N. inaurata and N. komaci to the laboratory for additional experiments.
Spider husbandry
Adult females were kept in separate (60 × 60 × 12 cm) acrylic glass frames in the laboratory where light/dark cycles and temperature were controlled (LD 12:12 h, T = 20 °C). Males and small juveniles were kept in 200 mL plastic cups under the same conditions. All spiders were water-sprayed daily and fed twice a week. Large females were fed with 4–5 flies or one mealworm, while juveniles and males were fed with one blowfly or several fruit flies.
Mate choice experiments
We tested N. inaurata males for discriminating between a conspecific and a heterospecific female (n = 22). We placed a frame inhabited by female N. inaurata parallel to a frame with N. komaci female such that both frames shared a side opening (Fig. 3). We pulled a silk thread from each female web and attached it to a polystyrene platform placed in-between the two frames. We positioned the two silk threads in such a way that they crossed each other to allow the male to touch both threads simultaneously. We then gently placed a N. inaurata male on the polystyrene platform and positioned him to touch both threads. During a 60 min timeframe, we observed male choice for either of the two webs, the occurrence of courting, female aggression, and copulation. We determined choice when the male positioned himself on one of the females’ webs (n = 16, Fig. 3). We excluded those trials where males did not start climbing any thread or did not climb onto either web (n = 6). All females were mated with conspecifics prior to the choice experiments to control for their mating status.
Heterospecific mating trials
To confirm heterospecific mating, we performed mating trials between N. komaci males and N. inaurata females (n = 9). We placed a male on the attachment threads of the web of a heterospecific female for up to five hours to observe the occurrence of courtship and mating attempts. The female was given prey at the beginning of the mating trial in order to avoid sexual cannibalism due to hunger. We determined mating interaction when we observed that a male palpal insertion into female genital opening resulted in hematodocha swelling, which usually indicates sperm transfer25. However, actual sperm transfer to female spermathecae could not be confirmed. To test for hybridization, we collected egg-sacs produced by females that mated with heterospecific males. Egg-sacs were kept at 20 °C and sprayed daily with water for two months. Egg-sacs that failed to hatch within two months were considered unviable.
Statistical analyses
We tested whether male occupancy on female webs was random or biased towards conspecific/heterospecific females using Fisher’s Exact Test. Male mate choice was tested for randomness using a binomial test. We compared male size (i.e. carapace width) among species using one-way analysis of variance. All statistical tests were performed in R (R Development Core Team 2009).
Additional Information
How to cite this article: Quiñones-Lebrón, S. G. et al. Potential costs of heterospecific sexual interactions in golden orbweb spiders (Nephila spp.). Sci. Rep. 6, 36908; doi: 10.1038/srep36908 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Thum, R. A. Reproductive interference, priority effects and the maintenance of parapatry in Skistodiaptomus copepods. Oikos 116, 759–768 (2007).
Harper, J. L., Clatworthy, J. N., Mcnaughton, I. H. & Sagar, G. R. The evolution and ecology of closely related species living in the same area. Evolution 15, 209–227 (1961).
Valiente-Banuet, A. & Verdú, M. Temporal shifts from facilitation to competition occur between closely related taxa. J. Ecol. 96, 489–494 (2008).
Gröning, J. & Hochkirch, A. Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282 (2008).
Burdfield-Steel, E. R. & Shuker, D. M. Reproductive interference. Curr. Biol. 21, R450–R451 (2011).
Kyogoku, D. Reproductive interference: ecological and evolutionary consequences of interspecific promiscuity. Popul. Ecol. 57, 253–260 (2015).
Takafuji, A., Kuno, E. & Fujimoto, H. Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp. Appl. Acarol. 21, 379–391 (1997).
Ben-David, T., Gerson, U. & Morin, S. Asymmetric reproductive interference between two closely related spider mites: Tetranychus urticaev and T. turkestani (Acari: Tetranychidae). Exp. Appl. Acarol. 48, 213–227 (2009).
Schmitt, A. Conjectures on the origins and functions of a bridal veil spun by the males of Cupiennius coccineus (Araneae, Ctenidae). J. Arachnol. 20, 67–68 (1992).
Kronestedt, T. A case of heterospecific mating in wolf spiders (Araneae, Lycosidae). J. Arachnol. 22, 84–86 (1994).
Elgar, M. A. Sexual cannibalism, size dimorphism, and courtship behavior in orb-weaving spiders (Araneidae). Evolution 45, 444–448 (1991).
Kuntner, M., Arnedo, M. A., Trontelj, P., Lokovšek, T. & Agnarsson, I. A molecular phylogeny of nephilid spiders: Evolutionary history of a model lineage. Mol. Phylogenet. Evol. 69, 961–979 (2013).
Kuntner, M. & Agnarsson, I. Phylogeography of a successful aerial disperser: the golden orb spider Nephila on Indian Ocean islands. BMC Evol. Biol. 11, 119 (2011).
Kuntner, M. & Coddington, J. A. Discovery of the largest orbweaving spider species: The evolution of gigantism in Nephila. PLoS One 4, e7516 (2009).
Kuntner, M. & Cheng, R. C. In Evolutionary biology: Convergent evolution, evolution of complex traits, concepts and methods (ed. Pontarotti, P. ) 121–133 (Springer International Publishing, 2016).
Kuntner, M. & Elgar, M. A. Evolution and maintenance of sexual size dimorphism: Aligning phylogenetic and experimental evidence. Front. Ecol. Evol. 2, 1–8 (2014).
Constant, N., Valbuena, D. & Rittschof, C. C. Male contest investment changes with male body size but not female quality in the spider Nephila clavipes. Behav. Processes 87, 218–223 (2011).
Elgar, M. A., De Crespigny, F. E. C. & Ramamurthy, S. Male copulation behaviour and the risk of sperm competition. Anim. Behav. 66, 211–216 (2003).
Fromhage, L. & Schneider, J. M. Safer sex with feeding females: Sexual conflict in a cannibalistic spider. Behav. Ecol. 16, 377–382 (2005).
Neumann, R. & Schneider, J. M. Differential investment and size-related mating strategies facilitate extreme size variation in contesting male spiders. Anim. Behav. 101, 107–115 (2015).
Schneider, J. M. & Elgar, M. A. Sexual cannibalism and sperm competition in the golden orb-web spider Nephila plumipes (Araneoidea): female and male perspectives. Behav. Ecol. 12, 547–552 (2001).
Schneider, J. M. & Michalik, P. One-shot genitalia are not an evolutionary dead end - Regained male polygamy in a sperm limited spider species. BMC Evol. Biol. 11, 197 (2011).
Michalik, P. & Rittschof, C. C. A comparative analysis of the morphology and evolution of permanent sperm depletion in spiders. PLoS One 6, e16014 (2011).
Kuntner, M., Zhang, S., Gregorič, M. & Li, D. Nephila female gigantism attained through post-maturity molting. J. Arachnol. 40, 345–347 (2012).
Kuntner, M., Gregorič, M., Zhang, S., Kralj-Fišer, S. & Li, D. Mating plugs in polyandrous giants: Which sex produces them, when, how and why? PLoS One 7, e40939 (2012).
Ruokolainen, L. & Hanski, I. Stable coexistence of ecologically identical species: Conspecific aggregation via reproductive interference. J. Anim. Ecol. 85, 638–647 (2016).
Acknowledgements
Fieldwork was in agreement with iSimangaliso Wetland Park and Ezemvelo KZN Wildlife, permit num. OP 552/2015. We thank Nerosha Govender, Xander Combrink, and Scotty R. Kyle for their help. Slovenian Research Agency granted J1-6729 and P1-10236 to MK, National Research Foundation of South Africa granted #95569 to CH.
Author information
Authors and Affiliations
Contributions
Designed: S.Q., S.K.F., M.G., C.H., M.K. Performed experiments and analyzed data: S.Q., M.G., T.L., K.Č., C.H., M.K. Wrote paper: S.Q., S.K.F., M.G., C.H., M.K.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Quiñones-Lebrón, S., Kralj-Fišer, S., Gregorič, M. et al. Potential costs of heterospecific sexual interactions in golden orbweb spiders (Nephila spp.). Sci Rep 6, 36908 (2016). https://doi.org/10.1038/srep36908
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36908
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.