Abstract
The current standard treatment for ovarian cancer is aggressive surgery followed by platinum-based combination chemotherapy. Recurrence and chemotherapeutic drug resistance are the two main factors that account for the high mortality of most ovarian cancers. Liposomal doxorubicin is primarily used for the treatment of ovarian cancer when the disease has progressed after platinum-based chemotherapy. However, relatively little is known about the genomic changes that contribute to both cisplatin and doxorubicin resistance in high-grade serous ovarian cancer (HGSC) under the selective pressure of chemotherapy. Here, we found that protein tyrosine phosphatase PTPN3 gene expression was substantially increased in both cisplatin and doxorubicin-resistant ovarian cancer cells. Silencing of PTPN3 restored sensitivity to cisplatin and doxorubicin in resistant ovarian cancer cells. Down-regulation of PTPN3 also inhibited cell cycle progression, migration, stemness in vitro and the tumorigenicity of resistant ovarian cancer cells in vivo. Meanwhile, the expression of PTPN3 was found to be regulated by miR-199 in resistant ovarian cancer cells. These findings suggest that PTPN3 promotes tumorigenicity, stemness and drug resistance in ovarian cancer, and thus is a potential therapeutic target for the treatment of ovarian cancer.
Similar content being viewed by others
Introduction
Ovarian cancer, the most common cause of gynaecologic cancer-associated death, is responsible for approximately 14,000 deaths in the United States annually1. Patients with advanced-stage high-grade serous ovarian cancer (HGSC) have experienced little improvement in overall survival2. The standard treatment is aggressive surgery followed by platinum-based combination chemotherapy. Recurrence and chemotherapeutic drug resistance are the two main factors that account for the high mortality of most ovarian cancers. After chemotherapy, platinum resistance occurs in approximately 25% of patients within six months3. A report on the integrated genomic analyses of ovarian carcinoma by The Cancer Genome Atlas Research Network showed that HGSC is characterised by TP53 mutations in almost all tumours (96%). BRCA1 and BRCA2 are mutated in 22% of tumours, owing to a combination of germline and somatic mutations. Low prevalence mutations include RB1, NF1, FAT3, CSMD3, GABRA6 and CDK12. The commonly dysregulated signalling pathways, such as RB, RAS/PI3K, FOXM1 and Notch, may provide promising opportunities for the treatment of advanced ovarian cancer4.
A recent study on the whole-genome characterisation of chemoresistant ovarian cancer by The Australian Ovarian Cancer Study Group showed that CCNE1 amplification is common in primary resistant and refractory disease. Acquired chemotherapy resistance is mainly associated with the inactivation of tumour suppressors such as RB1, NF1, RAD51B and PTEN, germline BRCA1 or BRCA2 mutations, loss of BRCA1 promoter methylation, and overexpression of the drug efflux pump MDR12. Doxil, a pegylated (polyethylene glycol coated) liposome-encapsulated form of doxorubicin, is primarily used for the treatment of ovarian cancer where the disease has progressed or recurred after platinum-based chemotherapy5. Liposomal doxorubicin can be used together with platinum if the recurrence occurs after more than 6 months, or liposomal doxorubicin can be given alone if the recurrence occurs within 6 months of the initial or subsequent complete clinical response to platinum-containing chemotherapy6. However, relatively little is known of the genomic changes that contribute to both cisplatin and doxorubicin resistance of HGSC under the selective pressure of chemotherapy.
Here, we undertook a search for candidate genes that could confer both cisplatin and doxorubicin resistance in established resistant ovarian cancer cells. Using genomic analyses, we found that protein tyrosine phosphatase non-receptor type 3 (PTPN3) gene expression was substantially increased in both cisplatin and doxorubicin-resistant cells. Moreover, PTPN3 silencing restored the sensitivity of resistant ovarian cancer cells to cisplatin and doxorubicin. Silencing of PTPN3 also inhibited cell cycle progression, migration and stemness, and reduced the tumorigenicity of resistant ovarian cancer cells. These findings identify PTPN3 as a potential therapeutic target for ovarian cancer treatment and for overcoming cisplatin and doxorubicin resistance.
Results
PTPN3 is highly expressed in cisplatin and doxorubicin resistant ovarian cancer cells
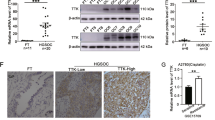
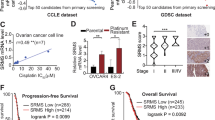
To identify the critical factors associated with both cisplatin and doxorubicin resistance in ovarian cancer, we compared the gene expression differences in A2780, A2780CIS and A2780ADR cells obtained from the European Collection of Cell Cultures (ECACC) using ovarian cancer cell line panel gene expression data7. Among the panel of significantly different genes, PTPN3 was significantly overexpressed in both A2780CIS and A2780ADR cells compared to A2780 cells (Table S1). The resistance of A2780CIS and A2780ADR to cisplatin and doxorubicin was confirmed by the MTS assay (Fig. 1A,B). The significantly increased expression of PTPN3 in both A2780CIS and A2780ADR was further validated by real-time quantitative reverse transcription PCR (qRT-PCR) and western blotting (Fig. 1C,D). Importantly, the highly increased expression of PTPN3 was also detected in approximately one third of the clinical ovarian cancer tissue samples, but not detectable in normal ovarian tissue samples (Fig. 1E). The high expression of PTPN3 was significantly associated with poor overall survival of ovarian cancer patients (Fig. 1F). These data suggest that overexpression of PTPN3 may play a critical role in mediating both cisplatin and doxorubicin resistance in ovarian cancer cells.
PTPN3 is highly expressed in cisplatin and doxorubicin resistant ovarian cancer cells.
(A,B) Comparison of the sensitivity of A2780 cells to that of A2780CIS and A2780ADR cells to cisplatin (A) and doxorubicin (B). *P < 0.05 (t test). (C,D) The expression of PTPN3 in cisplatin and doxorubicin resistant ovarian cancer cells was examined by qRT-PCR (C) and western blotting (D). PTPN3 was found to be highly expressed in cisplatin and doxorubicin resistant ovarian cancer cells. **P < 0.01 (t test). The gels were run under the same experimental conditions. Densitometric analysis was performed to quantify the bands. (E) Representative immunohistochemical (IHC) images of PTPN3 in ovarian cancer tissue samples. Scale bar = 50 μm. (F) The high expression of PTPN3 was significantly associated with poor overall survival of ovarian cancer patients.
Silencing of PTPN3 inhibits cell cycle progression in resistant ovarian cancer cells
Cell cycle progression has been shown to play important roles in drug resistance. To investigate the molecular mechanism of PTPN3-mediated cisplatin and doxorubicin resistance in ovarian cancer cells, cell cycle analysis was used to examine the effect of silencing PTPN3 on cell signalling pathways. After transfection with PTPN3 esiRNAs (esiPTPN3), an endoribonuclease-prepared siRNA pool comprised of a heterogeneous mixture of siRNAs that all target the same mRNA sequence of PTPN3, a significant silencing effect of PTPN3 in both cisplatin and doxorubicin resistant ovarian cancer cells was confirmed by qRT-PCR (Fig. 2A,B). Subsequent cell cycle analysis by flow cytometry indicated that silencing of PTPN3 significantly increased the G0-G1 phase cell population and decreased the S phase cell population in both cisplatin and doxorubicin resistant ovarian cancer cells. These data suggest that silencing PTPN3 inhibits cell cycle progression in resistant ovarian cancer cells.
Silencing of PTPN3 inhibits cell cycle progression in resistant ovarian cancer cells.
(A,B) The expression of PTPN3 was significantly silenced by PTPN3 esiRNA transfection using qRT-PCR analysis in both A2780CIS (A) and A2780ADR (B) cells. *P < 0.05 (t test). (C,D) Representative cell cycle analysis by flow cytometry shows that silencing PTPN3 significantly increased the G0-G1 phase cell population and decreased the S phase cell population in both A2780CIS (C) and A2780ADR (D) cells.
Silencing of PTPN3 inhibits resistant ovarian cancer cell growth, migration and drug resistance
To investigate the critical role of PTPN3 in ovarian cancer drug resistance and cell cycle progression, we examined the effects of silencing of PTPN3 on resistant ovarian cancer cell growth, migration and drug resistance. The cell growth curves indicated that cell growth was dramatically reduced by silencing PTPN3 in both cisplatin and doxorubicin resistant ovarian cancer cells (Fig. 3A,B). We next examined whether PTPN3 is a critical molecule for resistant ovarian cancer cell migration using the Transwell migration assay. Silencing PTPN3 significantly suppressed the migration rates of both cisplatin and doxorubicin resistant ovarian cancer cells (Fig. 3C,D). We then assayed drug sensitivity to cisplatin and doxorubicin. The results showed that silencing PTPN3 significantly contributed to increasing the sensitivity of resistant ovarian cancer cells to cisplatin and doxorubicin (Fig. 3E,F). These data collectively indicate that silencing of PTPN3 inhibits resistant ovarian cancer cell growth, migration and drug resistance.
Silencing of PTPN3 inhibits resistant ovarian cancer cell growth, migration and drug resistance.
(A,B) MTS assay results showing that cell growth was dramatically reduced by silencing PTPN3 in both A2780CIS (A) and A2780ADR (B) cells. (C,D) The Transwell migration assay showed that silencing PTPN3 significantly suppressed the migration rates of both cisplatin and doxorubicin resistant ovarian cancer cells. Scale bar = 100 μm. (E,F) MTS assay results showing that silencing PTPN3 significantly contributed to increasing the sensitivity of resistant ovarian cancer cells to cisplatin (E) and doxorubicin (F). *P < 0.05 (t test).
Stable silencing of PTPN3 inhibits colony formation and stemness in resistant ovarian cancer cells
Colony formation in soft agar is the most widely used assay to evaluate cellular anchorage-independent growth in vitro. Soft agar colony formation assays showed that stable silencing of PTPN3 significantly inhibited the colony forming ability of both cisplatin and doxorubicin resistant ovarian cancer cells, suggesting that stable silencing of PTPN3 inhibits cellular anchorage-independent growth of resistant ovarian cancer cells in vitro (Fig. 4A,B). Increasing evidence has suggested that the stemness of cancer cells is thought to be responsible for cancer initiation and drug resistance. Previous studies have shown that ALDH+ and CD133+ cells are enriched with ovarian cancer-initiating (stem) cells, and that ALDH and CD133 may be widely used as reliable markers to investigate ovarian cancer stem cell biology8. Flow cytometry analysis showed that stable silencing of PTPN3 significantly decreased the ALDH+, CD133+ and ALDH+ CD133+ cell populations (Fig. 4C,D). The tumour sphere formation assay showed that stable silencing PTPN3 significantly inhibited the sphere forming ability of both cisplatin and doxorubicin resistant ovarian cancer cells (Fig. 4E,F). These data suggest that stable silencing of PTPN3 inhibits colony formation and stemness in resistant ovarian cancer cells.
Stable silencing of PTPN3 inhibits colony formation and stemness in resistant ovarian cancer cells.
(A,B) Representative images of the soft agar assay and the quantification of colonies show that stable silencing of PTPN3 inhibited cellular anchorage-independent growth of resistant ovarian cancer cells in vitro. Scale bar = 50 μm. (C,D) Representative flow cytometry plots and the quantification of positive cells show that stable silencing of PTPN3 significantly decreased the ALDH+, CD133+ and ALDH+ CD133+ cell populations. (E,F) Representative images of tumour sphere formation and the quantification of spheres showed that stable silencing of PTPN3 significantly inhibited the sphere formation ability of both cisplatin and doxorubicin resistant ovarian cancer cells. Scale bar = 50 μm. *P < 0.05 (t test).
The expression of PTPN3 is regulated by miR-199 in resistant ovarian cancer cells
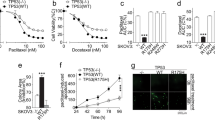
Previous studies have shown that at least one-third of human genes are regulated by miRNAs9. After demonstrating the important roles of PTPN3 in resistant ovarian cancer cells, we next investigated whether miRNAs regulate the expression of PTPN3. To identify the potential posttranscriptional regulation of PTPN3 by miRNAs, we used two online software resources, i.e. TargetScan and miRDB, for prediction. A panel of miRNAs was predicted to be potential regulators of PTPN3 by both miRNA target prediction programs (Table S3). We then used the luciferase reporter assay to validate that PTPN3 could be potentially regulated by miR-199. To validate whether miR-199 directly recognises the 3′-UTR of PTPN3 mRNA, we cloned a sequence containing the predicted target site and a mutated sequence with the predicted target sites downstream of the pGL3 luciferase reporter gene to generate pGL3-PTPN3-wt or pGL3-PTPN3-mut vectors (Fig. 5A). The vectors were then co-transfected with the miR-199 mimics or control into HEK293 cells. A Renilla luciferase vector (pRL-TK) was used to normalise the differences in transfection efficiency. Luciferase activity in cells co-transfected with the miR-199 mimics and the pGL3-PTPN3-wt vector was significantly decreased when compared with the control (Fig. 5B). Next, we further detected the protein expression of PTPN3 in resistant ovarian cancer cells after transfection with miR-199 mimics or control. The results show that overexpression of miR-199 decreased the expression of PTPN3 in resistant ovarian cancer cells (Fig. 5C). Consistently, overexpression of miR-199 also increased the sensitivity of resistant ovarian cancer cells (Fig. 5D,E). These data suggest that the expression of PTPN3 is regulated by miR-199 in resistant ovarian cancer cells.
The expression of PTPN3 is regulated by miR-199 in resistant ovarian cancer cells.
(A) The predicted binding sites of PTPN3 3′UTR and miR-199 by TargetScan. (B) Luciferase activity in cells co-transfected with miR-199 mimics and the pGL3-PTPN3-wt vector was significantly decreased when compared with the control. (C) Western blotting images showing that overexpression of miR-199 decreased the expression of PTPN3 in resistant ovarian cancer cells. The gels were run under the same experimental conditions. (D,E) The MTS assay showed that overexpression of miR-199 increased the sensitivity of resistant ovarian cancer cells to cisplatin (D) and doxorubicin (E). *P < 0.05 (t test).
Stable silencing of PTPN3 inhibits resistant ovarian cancer cell tumorigenicity in vivo
The effect of stable silencing of PTPN3 on the tumorigenicity of resistant ovarian cancer cells was further investigated in a mouse model in vivo. Immunodeficient Balb/C mice were subcutaneously injected with resistant ovarian cancer cells that had been previously stably transfected with PTPN3 shRNA or shScramble control. Throughout the tumorigenic period, the tumours that formed from shPTPN3 stably transfected cells grew significantly slower than those formed from shScramble control transfected cells, using both A2780CIS (Fig. 6A,B) and A2780ADR (Fig. 6C,D) cells. After 35 days, immunohistochemical (IHC) staining of tumour tissues showed that the expression of PTPN3 was significantly decreased in shPTPN3 stably transfected tumours (Fig. 6E). These data suggest that stable silencing of PTPN3 inhibits resistant ovarian cancer cell tumorigenicity in vivo.
Stable silencing PTPN3 inhibits resistant ovarian cancer cell tumorigenicity in vivo.
(A,B) Representative tumour images and tumour growth curved showing that the tumours formed from shPTPN3 stably transfected A2780CIS cells grew significantly more slowly than those formed from shScramble control transfected A2780CIS cells. *P < 0.05 (t test). (C,D) Representative tumour images and tumour growth curves showing that the tumours formed from shPTPN3 stable transfected A2780ADR cells grew significantly more slowly than those formed from shScramble control transfected A2780ADR cells. *P < 0.05 (t test). (E) Representative images of IHC staining of tumour tissues showing that the expression of PTPN3 was significantly decreased in shPTPN3 stably transfected tumours. Scale bar = 50 μm.
Discussion
The results reported here provide evidence that PTPN3 regulates sensitivity to cisplatin and doxorubicin in ovarian cancer cells. PTPN3 is a member of the protein tyrosine phosphatase (PTP) family, which is known to comprise signalling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitosis and oncogenic transformation10. Here, the expression of PTPN3 was observed to be substantially increased in both cisplatin and doxorubicin resistant ovarian cancer cells through the genomic analysis of cisplatin and doxorubicin resistant cells compared with the parental ovarian cancer cells. Silencing of PTPN3 restored sensitivity to cisplatin and doxorubicin in resistant ovarian cancer cells. These data suggest that PTPN3 may play a critical role in the development of ovarian cancer and contribute to chemotherapy resistance.
Platinum-based chemotherapy is currently the first-line treatment for advanced stage HGSC patients. However, resistance to platinum-based chemotherapy is the main obstacle in clinical practice11. Platinum resistant ovarian cancer is comprised of a heterogeneous and complex spectrum of diseases12. The mechanism of platinum therapy resistance has been reported, including changes in cisplatin transport and trafficking, disruption of apoptosis, increased tolerance to cisplatin-DNA adducts and increased DNA repair in response to cisplatin-DNA interactions13. Many ovarian tumours exhibit multiple resistance pathways simultaneously14. Patients with recurrent ovarian cancer are typically categorised as having either platinum resistant or platinum sensitive disease, based on a platinum-free interval of less than or greater than 6 months, respectively12. A range of second-line chemotherapeutic agents has been approved for the treatment of ovarian cancer, including doxorubicin, etoposide, topotecan, gemcitabine and trabectedin11. Liposomal doxorubicin can be used together with platinum if the recurrence occurs after more than 6 months, or liposomal doxorubicin can be given alone if the recurrence occurs within 6 months of the initial or subsequent complete clinical response to platinum-containing chemotherapy. These are mostly used as second-line drugs, but they have been poorly effective in trials involving large unselected populations of relapsed patients11. Therefore, it is interesting to identify the factors contributing to both cisplatin and doxorubicin resistance in HGSC under the selective pressure of chemotherapy.
Here, we found that overexpression of PTPN3 promotes both cisplatin and doxorubicin resistance. Previous studies on the mutational analysis of the tyrosine phosphatase gene superfamily in human cancers identified 83 somatic mutations in six PTPs (PTPRF, PTPRG, PTPRT, PTPN3, PTPN13, PTPN14), affecting 26% of colorectal cancers and a smaller fraction of lung, breast and gastric cancers15. Recently, activating mutations in PTPN3 have been shown to promote cholangiocarcinoma cell proliferation and migration and were associated with tumour recurrence in patients. Mutations in PTPN3 have been detected in 41.1% of cholangiocarcinomas. Transgenic expression of PTPN3 in cell lines increases cell proliferation, colony formation and migration16. However, the mutation rate of PTPN3 is relatively low in ovarian cancer, according to the TCGA dataset. Previous studies have suggested that at least one-third of human genes are estimated to be miRNA targets9. We found that the expression of PTPN3 was regulated by miR-199 in resistant ovarian cancer cells. Therefore, the miR-199/PTPN3 network likely contributes to both cisplatin and doxorubicin resistance of HGSC under the selective pressure of chemotherapy.
Protein tyrosine phosphatases (PTPs) have been found to function as tumour suppressors or oncogenes, depending on the substrate involved and the cellular context. PTPN3 has been reported to be an oncogene in gastric16, colon17 and breast cancer18. However, a recent study showed that PTPN3 inhibits lung cancer cell proliferation and migration by promoting the endocytic degradation of EGFR, indicating that PTPN3 may act as a tumour suppressor in lung cancer through its modulation of EGFR signalling19. In the present study, highly increased expression of PTPN3 was also detected in around one third of clinical ovarian cancer tissue samples, but was not detectable in normal ovarian tissue samples. Moreover, high expression of PTPN3 was significantly associated with poor overall survival in ovarian cancer patients. Silencing PTPN3 inhibits resistant ovarian cancer cell growth, cell cycle progression, migration, drug resistance, stemness and tumorigenicity. These data suggest that PTPN3 is an oncogene that promotes drug resistance and stem cell-like characteristics in ovarian cancer, and thus it is a potential therapeutic target for the treatment of ovarian cancer.
Materials and Methods
Cell lines and cell culture
The human epithelial serous ovarian cancer cell lines A2780 (cisplatin-sensitive) was purchased from the European Collection of Cell Cultures (ECACC, UK) and cultured in a humidified atmosphere at 37 °C, 5% CO2. They were maintained in RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin mixture (Thermo Fisher Scientific, USA). Cisplatin and doxorubicin were obtained from Sigma-Aldrich and dissolved in DMSO. Cisplatin and doxorubicin resistant cells were developed from their parental cell line A2780 by gradual incremental doses of cisplatin or doxorubicin administration in cell culture medium in subsequent passages for about 6 months. Thereafter cisplatin resistant A2780CIS and doxorubicin resistant A2780ADR cells were maintained in presence of 1 μg/ml cisplatin or doxorubicin in cultured medium for 72 h in every alternate passage in accordance with the ECACC guidelines.
Animal studies
The validated shRNA stable transfected A2780CIS and A2780ADR cells were re-suspended in PBS and implanted into the right and left flanks (5 × 106 cells per flank) of female BALB/c nude mice via subcutaneous injections. The study was approved by the Animal Ethics Committee of Yijishan Hospital Affiliated to Wannan Medical College, China. All animal related methods were performed in accordance with the relevant guidelines and regulations. Tumor volumes were determined each week by measuring their length (a) and width (b) using a vernier caliper. The tumor volume (V) was calculated according to the formula V = ab2/2. The statistical significance between tumor sizes in the shPTPN3 and shScramble control transfected groups was evaluated using the Student’s t test. The tumors were fixed in 10% neutral buffered formalin before being processed into paraffin blocks. The tissue sections were stained with PTPN3 primary antibody (Abcam) using standard IHC techniques.
Immunohistochemistry (IHC)
The tissue samples were collected and all experimental protocols were approved by the Committees for Ethical Review of Research Involving Human Subjects of Yijishan Hospital Affiliated to Wannan Medical College. Informed consent was obtained from all subjects. All experiments were performed in accordance with relevant guidelines and regulations. Paraffin embedded tissue samples from consented patients or xenograft mouse tissues derived from A2780CIS and A2780ADR transfected cells were cut into 5μm sections and placed on poly-lysine-coated slides. The slides were then deparaffinised in xylene and rehydrated using a series of graded alcohol. Antigen retrieval was performed by heat mediation in citrate buffer (pH 6; Dako). Samples were blocked with 10% goat serum before incubation with primary antibody. The samples were incubated overnight using following primary antibodies: rabbit anti-PTPN3 (1:100) or an isotype-matched IgG (Sigma) as a negative control in a humidified container at 4 °C. Immunohistochemical staining was performed with the Dako Envision plus System (Dako, Carpinteria, CA, USA) according to the manufacturer’s instructions. The intensity of staining was evaluated by digital image analysis on the scale of 0 to 4 according to the percentage of positive tumors (Low expression: 0, negative control; 1, 0–10%; 2, 10–25%; High expression: 3, 25–50% and 4, >50%).
The rest of materials and methods are provided in the online supplementary information.
Additional Information
How to cite this article: Li, S. et al. Protein tyrosine phosphatase PTPN3 promotes drug resistance and stem cell-like characteristics in ovarian cancer. Sci. Rep. 6, 36873; doi: 10.1038/srep36873 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA: A cancer journal for clinicians 66, 7–30 (2016).
Patch, A.-M. et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature 521, 489–494 (2015).
Pénzváltó, Z. et al. MEK1 is associated with carboplatin resistance and is a prognostic biomarker in epithelial ovarian cancer. BMC cancer 14, 1 (2014).
Network, C. G. A. R. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Gordon, A. N. et al. Phase II Study of Liposomal Doxorubicin in Platinum- and Paclitaxel-Refractory Epithelial Ovarian Cancer. Journal of Clinical Oncology 18, 3093–3100 (2000).
Kim, K. H. et al. Phase 1b safety study of farletuzumab, carboplatin and pegylated liposomal doxorubicin in patients with platinum-sensitive epithelial ovarian cancer. Gynecologic oncology 140, 210–214 (2015).
Beaufort, C. M. et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PloS one 9, e103988 (2014).
Kryczek, I. et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. International Journal of Cancer 130, 29–39 (2012).
Xiao, F. et al. miRecords: an integrated resource for microRNA–target interactions. Nucleic acids research 37, D105–D110 (2009).
Parker, E. J. The Molecular Basis for the Substrate Specificity of Protein Tyrosine Phosphatase PTPN3. Structure 23, 608–609 (2015).
Erriquez, J. et al. Xenopatients show the need for precision medicine approach to chemotherapy in ovarian cancer. Oncotarget (2016).
Davis, A., Tinker, A. V. & Friedlander, M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Gynecologic oncology 133, 624–631 (2014).
Galluzzi, L. et al. Molecular mechanisms of cisplatin resistance. Oncogene 31, 1869–1883 (2012).
Chowanadisai, W. et al. Cisplatin resistant spheroids model clinically relevant survival mechanisms in ovarian tumors. PloS one 11, e0151089 (2016).
Wang, Z. et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science 304, 1164–1166 (2004).
Gao, Q. et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 146, 1397–1407 (2014).
Hou, S.-W. et al. PTPH1 dephosphorylates and cooperates with p38γ MAPK to increase Ras oncogenesis through PDZ-mediated interaction. Cancer research 70, 2901–2910 (2010).
Zhi, H.-Y. et al. PTPH1 cooperates with vitamin D receptor to stimulate breast cancer growth through their mutual stabilization. Oncogene 30, 1706–1715 (2011).
Li, M. Y. et al. Protein tyrosine phosphatase PTPN3 inhibits lung cancer cell proliferation and migration by promoting EGFR endocytic degradation. Oncogene 34, 3791–3803, doi: 10.1038/onc.2014.312 (2015).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81402151, 81472366) and Anhui Provincial Education Natural Science Foundation (KJ2015A142).
Author information
Authors and Affiliations
Contributions
S.Q.L., H.P.X. and X.T. wrote the main manuscript; S.Q.L., J.C. W.Z. and H.P.X. performed the research and collected and analysed the data; F.Z., G.T.N., Q.L. and M.W. collected the samples and run the analysis; and all authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, S., Cao, J., Zhang, W. et al. Protein tyrosine phosphatase PTPN3 promotes drug resistance and stem cell-like characteristics in ovarian cancer. Sci Rep 6, 36873 (2016). https://doi.org/10.1038/srep36873
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36873
This article is cited by
-
Identification and verification of PTPN3 as a novel biomarker in predicting cancer prognosis, immunity, and immunotherapeutic efficacy
European Journal of Medical Research (2024)
-
PTPN3 expressed in activated T lymphocytes is a candidate for a non-antibody-type immune checkpoint inhibitor
Cancer Immunology, Immunotherapy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.