Abstract
Brain iron levels in patients of Parkinson’s disease (PD) are usually measured in postmortem samples or by MRI imaging including R2* and SWI. In this study we performed a meta-analysis to understand PD-associated iron changes in various brain regions, and to evaluate the accuracy of MRI detections comparing with postmortem results. Databases including Medline, Web of Science, CENTRAL and Embase were searched up to 19th November 2015. Ten brain regions were identified for analysis based on data extracted from thirty-three-articles. An increase in iron levels in substantia nigra of PD patients by postmortem, R2* or SWI measurements was observed. The postmortem and SWI measurements also suggested significant iron accumulation in putamen. Increased iron deposition was found in red nucleus as determined by both R2* and SWI, whereas no data were available in postmortem samples. Based on SWI, iron levels were increased significantly in the nucleus caudatus and globus pallidus. Of note, the analysis might be biased towards advanced disease and that the precise stage at which regions become involved could not be ascertained. Our analysis provides an overview of iron deposition in multiple brain regions of PD patients, and a comparison of outcomes from different methods detecting levels of iron.
Similar content being viewed by others
Introduction
Iron overload has been implicated in the pathology and pathogenesis of Parkinson’s disease (PD). The substantia nigra, where the selective loss of dopaminergic neurons occurs, is the primary region in the brain known to deposit iron. Additionally, aberrant iron concentrations have been observed in other brain regions such as red nuclei, globus pallidus and cortex of PD patients, despite of unknown pathology1,2,3. Spectroscopic analyses of postmortem brains display an increased iron levels in the substantia nigra, which has been suggested to correlate with the severity of PD2,4. In recent decades, advancements in imaging techniques, such as magnetic resonance imaging (MRI), have contributed to an enhanced understanding of the pathological progression and clinical diagnosis of PD. Consequently, iron load may be estimated in a non-invasive manner using R2/R2* relaxometry (with better results obtained using R2* 5,6,7) and, more recently, susceptibility-weighted imaging (SWI). Nonetheless, while largely consistent and reproducible results can be obtained in many experiments these techniques are not yet fully validated8.
In this study, we extracted results of iron analyses employing postmortem brains and R2* and SWI methods from the literature, and performed a systematical meta-analysis aiming to 1) confirm the iron overload observation in the substantia nigra, 2) explore other regions of the brain carrying different levels of iron, and 3) evaluate to what extent these two MRI methods correlate with the measurements of postmortem brains. Meanwhile, as detailed in the discussion section, several limitations are disclosed in an attempt to fully understand the scope of this meta-analysis, such that the disease severity was not differentiated due to insufficient information during data extraction that may affect outcomes of MRI imaging.
Results
Search Results
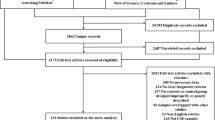
The initial search using the keywords as described in the method section returned a total of 4252 articles (Fig. 1). A subsequent screening of the titles and abstracts reduced the number to 257. Following an exhaustive examination of the contents, 224 articles were excluded according to the selection criteria detailed in the method section. Of the 33 articles being selected that report iron content (summarized in Table 1), 11 of them employed postmortem analyses2,4,9,10,11,12,13,14,15,16,17, 14 were measured by R2* 3,18,19,20,21,22,23,24,25,26,27,28,29,30 and 8 by MRI relaxometry SWI31,32,33,34,35,36,37,38. The disease comorbidity and diagnostic performance of the cohorts of these 33 studies are summarized in Table S1.
Quality Assessment
Quality assessment by Newcastle-Ottawa Scale suggested four-stars or above out of a maximum of nine for all of the 33 publications. The detailed quality assessment is listed in Table 1.
Postmortem comparison of iron concentration in defined brain regions
Eleven of the manuscripts examined iron concentration in seven regions of postmortem brains. The numbers of subjects for each region were 98 (frontal lobe), 44 (temporal lobe), 117 (nucleus caudatus), 104 (globus pallidus), 173 (substantia nigra), 100 (putamen), and 58 (cerebellum). Although iron concentration was significantly increased in the substantia nigra of PD patients (WMD = 39.85, 95% CI, 8.06–71.65, p = 0.01; Fig. 2E), significant heterogeneity was detected in these cohorts (I2 = 71%; p = 0.0006). Subsequent sensitivity analysis suggested that such heterogeneity was attributed to the study of Griffiths et al.11. Further analysis that eliminated this study (I2 = 12%; p = 0.33) also showed a significant increase of iron concentration in the substantia nigra (WMD = 23.60, 95% CI = 7.62–39.58, p = 0.004; Fig. 2F). Additionally, increased iron levels were observed in the putamen of PD subjects (WMD = 19.30, 95% CI = 7.24–31.36, p = 0.002, I2 = 4%; Fig. 2G). No significant differences were observed in other brain regions (Fig. 2). The funnel plots analyzing publication bias appeared to be symmetric by visual inspection (Fig. 3).
MRI comparison of iron concentration in defined brain regions
Fourteen articles were included in the R2* subgroup of meta-analyses in seven brain regions. The total subject numbers were 437 (nucleus candatus), 500 (globus pallidus), 631 (substantia nigra), 446 (putamen), 265 (red nucleus), 117 (white matter) and 182 (thalamus). In the substantia nigra of PD subjects, iron content was elevated (WMD = 3.81, 95% CI = 2.59–5.02, p < 0.00001) despite of a relatively high heterogeneity (I2 = 59%, p = 0.005; Fig. 4C). Results of a sensitivity analysis ascribed the heterogeneity to the studies of Ulla et al.25 and Gorell et al.18, as exclusion of them eliminated the heterogeneity (I2 = 0%, p = 0.49 ; Fig. 4D). Subsequent meta-analysis again demonstrated a significant increase of iron concentration in the substantia nigra (WMD = 3.91, 95% CI = 3.05–4.77, p < 0.00001; Fig. 4D). Iron concentration was significantly increased in the red nucleus (WMD = 1.93, 95% CI = 0.70–3.17, p = 0.002, I2 = 0%; Fig. 4F), but not in other brain regions (Fig. 4). The publication biases were acceptable as determined by funnel plots (Fig. 5).
Eight articles were included in the SWI subgroup of meta-analyses in seven brain regions. The total subject numbers were 431 (nucleus caudatus), 431 (globus pallidus), 431 (putamen), 306 (thalamus), 465 (substantia nigra), 465 (red nucleus) and 211 (white matter). A significant increase in iron concentration was observed in the substantia nigra (WMD = 6.5, 95% CI = 3.31–9.68, p < 0.0001) with high heterogeneity (I2 = 94%, p < 0.0001; Fig. 6D). Significant increases in iron concentration were also shown in the nucleus caudatus (WMD = 0.81, 95% CI = 0.37–1.25, p = 0.0003, I2 = 24%; Fig. 6A), putamen (WMD = 1.03, 95% CI = 0.06–2.01, p = 0.04, I2 = 60%; Fig. 6E), and red nucleus (WMD = 0.85, 95% CI = 0.15–1.54, p = 0.02, I2 = 44%; Fig. 6H). When the article of Wang et al.37 was removed based on sensitivity analysis, we still observed an increase of iron concentration in the putamen (WMD = 0.82, 95% CI = 0.33–1.30, p = 0.001, I2 = 0%; Fig. 6F). Significant heterogeneity (I2 = 87%, p < 0.00001) was detected in the globus pallidus group (Fig. 6B), which was attributed to Han et al.31 as determined by a sensitivity analysis. Meta-analysis after exclusion of this paper showed a significant increase of iron concentration in the globus pallidus (WMD = 1.76, 95% CI = 0.98–2.54, p < 0.0001, I2 = 0%; Fig. 6C). The publication biases were acceptable as determined by funnel plots (Fig. 7).
Structure by structure analyses of results from individual studies and meta-analyses
It is known that inferences can be particularly prone to Type-I error in studies based on a small number of papers, especially with a small sample size39. Therefore, we herein elaborated on the results reported in each study combining the results of meta-analyses and the methodological factors that could have contributed to discrepancies in a brain structure-based fashion.
Substantia nigra
As expected, an elevation of iron concentration was found in the substantia nigra in all the three types of measurements (Table 2). This was in line with the majority of the 29 articles we analyzed. Except for the three that did not show a change in postmortem samples10,12,16, the other 26 articles reported a trend toward or a statistically significant increase in iron content in the substantia nigra regardless of the type of measurement (postmortem, SWI or R2*). As a note, three postmortem iron analyses12,14,16 indicated that the pars compacta and reticulata were not discriminated during the measurement, while the other six studies did not state the relevant information to make this determination.
Putamen
Both postmortem and SWI meta-analyses showed an iron overload in PD patients. However, when individual articles describing postmortem samples were analyzed, we found that only one study reported a significant increase in iron content9, while the other five were completely negative with mixed trends2,4,11,12,13. Although the results of our meta-analysis suggested a significant increase in iron content in the putamen of PD patients in postmortem samples, caution should be taken in the interpretation of these results as one positive study9 dominated the other five negative ones in the analysis (Fig. 2G). For SWI, an iron overload was suggested in the putamen based on both random and fixed effects models. Results of two independent studies showed elevated iron content in this structure31,37, whereas the other five were not significantly different33,34,35,36,38. One of the positive studies37 was removed following a sensitivity analysis, and the remaining one31 drove half of the total effect size thereafter in the fixed effects model (Fig. 6F). Taken together, additional studies are needed to confirm iron accumulation in the putamen.
Globus pallidus
For SWI, results of six studies suggested a trend toward, or a significant, increase in the level of iron33,34,35,36,37,38, while one showed a decrease in iron content31, which was later removed based on a sensitivity analysis. The subsequent meta-analysis returned a significant increase of iron content in the globus pallidus. However, results of either postmortem or R2* meta-analyses did not display significant difference, which was in line with the mixed trends of changes in individual studies.
Nucleus caudatus
Similar to globus pallidus, both postmortem and R2* meta-analyses returned no significant difference with mixed trends in iron content in the individual studies. Results of pooled SWI analysis showed a significant increase of iron content in PD patients. There were six studies that showed a significant31,37 or a trend of increase33,34,35,36 in iron levels in the nucleus caudatus while only one study suggested a trend of decrease34.
Frontal lobe, temporal lobe and cerebellum
Although postmortem results of these structures were available, the pooled sample sizes were small (98, 44 and 58, respectively). All the four studies on frontal lobe2,11,12,14 and two on cerebellum2,14 reported negative results. Although one article reported a significant decrease of iron levels in the temporal lobe17, two studies showed no change11,13. Further studies were needed to clarify iron levels in these structures.
Red nucleus
No available studies using postmortem samples fit our criteria. Results of R2* and SWI pooled analyses suggested an increase of iron levels in the red nucleus. For the R2* analyses, four studies reported a significant increase3 or an increasing trend20,27,29, whereas one showed a decreasing trend30. For the SWI analyses, seven out of eight studies reported no remarkable changes, among which three showed a decreasing trend32,34,38 and four an increasing trend in iron content32,36,37,38. In comparison, the study that showed significantly elevated iron content in PD patients33 drove roughly half of the total effect size (Fig. 6H). Noteworthy, two PD groups (advanced and mild disease stage) were included in this study that had the same control group33. The advanced PD group was chosen for the current analysis to compare with postmortem samples that are usually obtained at late stage PD. When the mild group was included, results of SWI meta-analyses were not affected except in the red nucleus. There was no significant increase of iron content detected (Figs S1 and S2), suggesting that the severity of PD might be a factor affecting iron deposits in the red nucleus. As a note, the mild stage in this study33 was Hoehn and Yahr scale <1.5, which appeared milder than normally defined.
Thalamus and white matter
No qualified study using postmortem samples was available. Results of both R2* and SWI meta-analyses suggested no association of iron levels with PD in the thalamus and white matter of the brain. Furthermore, all of the selected individual studies31,34,35,36,37 returned negative results.
Discussion
Iron dysregulation is frequently associated with neurodegenerative disorders, including Huntington disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and frontotemporal lobar degeneration40,41. Nonetheless, it remains unclear whether such defect is a cause or a consequence of neurodegeneration. A large body of evidence suggests abnormal iron levels in the brains of PD patients and a role for iron dysregulation in PD pathogenesis42,43,44. Our study represents the first meta-analysis that systematically assesses iron levels in various brain regions of PD patients by postmortem measurements and by MRI (R2* and SWI). Our analysis confirms a perturbed iron homeostasis in the substantia nigra and suggests that an increase in iron levels may also occur in the putamen and red nucleus (Table 2).
Some caveats in regard to the scope of this meta-analysis must be taken into account. First, in the postmortem analyses different iron quantification methods (SPH, AA, COL, ICP and MS) have been used. The differential sensitivity and specificity of these methods may contribute to an elevated heterogeneity. Second, disease stage and age may be two influencing factors when evaluating iron concentration in the brain40,45,46, which unfortunately is not addressed in the current study due to incomplete information and limited sample size. For example, the inclusion of a sub-group of mild-stage PD patients results in a loss of significance in iron levels in the red nucleus of SWI meta-analysis.
It is well recognized that iron overload contributes to oxidative stress through Fenton reaction, promoting the death of dopaminergic neurons in the substantia nigra47. Such iron accumulation is known to be associated with increased ferritin and neuromelanin iron loads48,49, as well as increased expression of divalent metal transporter 1 that may contribute to PD pathogenesis via its capacity of transporting ferrous iron47. Furthermore, aggregation of α-synuclein can be accelerated when bound with free iron50. However, it remains unclear whether iron deposit triggers or accelerates neurodegeneration, or if they are a secondary event due to neuronal degeneration. Therefore, it is important to determine the timing of iron deposit in substantia nigra during the pathogenesis of PD. Because postmortem measurements are usually made in a very late stage of PD, future longitudinal studies of iron contents are warranted47. Consistent results obtained from postmortem, R2*, and SWI measurements suggest that longitudinal evaluation of iron content in the substantia nigra can be appropriately made by MRI methods.
It appears that the MRI methods of R2* and SWI do not completely match the postmortem results, presumably the latter being the standard. Iron deposit is detected by SWI in the globus pallidus and nucleus caudatus, but these are inconsistent with the postmortem observations. Results from R2* studies also suggest an inconsistency in the putamen as both postmortem and SWI effects show an iron overload. Loss of striatal dopamine in PD is most prominent in sub-regions of the putamen51, which may be associated with an increase in iron levels. However, this may be a weak argument considering that the postmortem iron increase in this structure is driven by a single study as noted in the Results. It has previously been proposed that SWI is more specific and precise than other methods to estimate brain iron content52. Our results suggest that both methods have weakness in measuring iron content. The iron signal determined by R2* may be disrupted by calcification53 and lipid content54, and the output value is a weighted summation of magnetic properties from both local and surrounding tissues28. Intrinsic defects of SWI include a difficulty in distinguishing diamagnetic and paramagnetic susceptibility owning to the convoluting effect of the dipole fields55. There are also limitations of MRI per se, such that myelin, especially small myelinated fibers, cannot be easily distinguishable from iron deposition46, and the phase value of MRI reflects not only non-heme iron deposited in the tissue but also the heme iron in hemosiderin or in circulating blood56. Microbleeds may also be a confounding factor especially when brain iron content is estimated in older adults57. Given the MRI phase’s nonlocal behavior, one should pay attention to the signal interference of adjacent structures. For example, the red nucleus lies adjacent to substantia nigra in the midbrain and is likely high in iron levels due to its proximity58. In other words, the differences detected in iron levels in the red nucleus may arise from the adjacent substantia nigra, instead of from the structure itself. Increased iron levels in red nucleus are associated with levodopa-induced dyskinesia of PD3. Future postmortem studies are warranted to confirm iron deposit in this structure. This is also the case for the putamen and globus pallidus, due to their relative proximity. Recently, quantitative susceptibility mapping (QSM), a potentially superior method to measuring iron content in vivo, has been applied to measure PD-related iron deposition and progression28. By this method, Guan et al.59 have recently reported a distinct pattern of iron accumulation according to disease stage, with iron spreading from the substantia nigra in early stages to the substantia nigra, red nucleus and globus pallidus in later stages. This could explain the aforementioned discrepancy in the red nucleus when the mild PD group is included, as well as provide a potential explanation for inconsistent findings between neuropathology and MRI techniques.
In conclusion, the current meta-analysis corroborates iron overload in substantia nigra and suggests such iron homeostasis defect in the putamen (by postmortem and SWI, but not R2*) and the red nucleus (by R2* and SWI; no data by postmortem) of PD patients. Both the R2* or SWI techniques may not authentically reflect iron changes in brain regions other than substantia nigra. Our results offer a comprehensive understanding of iron loads in different brain regions in association with PD, and contribute to the evaluation of measuring accuracy of iron concentration by MRI methods.
Methods
Literature Search Strategy
Literature related to iron and Parkinson’s disease were searched in four databases including Medline via PubMed, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase via OVID, dated till 19th November 2015. The keywords for iron and Parkinson’s disease are “iron” or “Fe” and “Parkinson disease”, “Parkinson’s disease”, “Parkinsons disease” or “Parkinsonian”, respectively.
Study Selection
Based on the keywords, titles and abstracts of the identified publications were screened. Following an exhaustive examination of the literature contents, articles were included according to our selection criteria: population (idiopathic PD patients), comparators (individuals free of neurological disorders), outcome measurement (iron content in brain regions), and language (articles written in English or Chinese). Review articles, qualitative and semi-quantitative studies were excluded.
Data Extraction
The literature search and data extraction were conducted by two researchers (Qing-Qing Zhuang and Jian-Yong Wang) independently. In the case of a dispute, a third investigator was included to discuss and reach an agreement. The following data was extracted: sample size, age, sex, PD diagnosis, iron detection methods, the type of samples, clinical scores, and iron content or R2* value or phase value in brain regions. Assessment of the detailed information was listed in Table 1. As shown in this table, the disease severity (Hoehn and Yahr scale) was not provided by all the included studies and the provided else information was also varied in forms including UPDRS score, UPDRS motor score, and/or disease duration. Therefore, we did not include the disease severity as a source of variance in the analysis.
Iron quantification methods employed in the postmortem study of brain samples included spectrophotometry (SPH), atomic absorption (AA), colorimetry (COL), inductively coupled plasma spectroscopy (ICP) and Mössbauer spectroscopy (MS). To be consistent in brain weights, a conversion of dry weight to wet weight was applied based on a dry/wet ratio as suggested in previous studies60,61. The SWI signal phase is orientation-dependent and nonlocal55. As a result, the phase value appears to be either positively or negatively correlated with iron concentration depending on the orientation relative to the Bo field62. Thus, a conversion from SWI phase value to iron concentration was applied based on formulas suggested in previous studies35,36; that is, concentration = 397.72 × (phase value) + 3.4097 (extracted from Fig. 1 of ref. 36) for the studies of positive setting31,34,36, and concentration = −128.23 × (phase value) + 3.1897 (extracted from Fig. 2 of ref. 35) for the studies of negative setting32,33,35,37,38.
Quality Assessment
The Newcastle-Ottawa Scale63 was employed to assess the quality of the chosen studies. This tool classified studies in three broad perspectives: selection of the study groups, comparability of the groups, and ascertainment of either exposure or outcome of interest for the studies. Semi-quantitative measurement using a star system assesses the quality of study. The highest quality studies can get a maximum of nine stars.
Statistical Analysis
Eleven postmortem analysis and 22 MRI analysis articles were eventually selected for our meta-analysis. Means, standard deviations (or standard errors), and the number of samples were extracted in each study. Meta-analyses were conducted within the studies of the same brain region after sorting into their respective quantitative groups of postmortem analysis, R2* and SWI. In the case that the same data appeared in multiple studies, the data were used only once. All of the analyses were performed using Review Manager 5.2 for Windows (http://ims.cochrane.org/recman). A two-tailed p value <0.05 was considered statistically significant. Weighted mean difference (WMD) was regarded as an effect size. Q-statistics and I2 were used for assessing the heterogeneity64,65. A random effects model was applied when heterogeneity was found by Q-statistics or when I2 > 50%. A fixed effects model was applied otherwise.
Additional Information
How to cite this article: Wang, J.-Y. et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci. Rep. 6, 36669; doi: 10.1038/srep36669 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Dexter, D. T. et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114 (Pt 4), 1953–1975 (1991).
Dexter, D. T. et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52, 1830–1836 (1989).
Lewis, M. M. et al. Higher iron in the red nucleus marks Parkinson’s dyskinesia. Neurobiol Aging 34, 1497–1503 (2013).
Riederer, P. et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52, 515–520 (1989).
Hardy, P. A. et al. Correlation of R2 with total iron concentration in the brains of rhesus monkeys. J Magn Reson Imaging 21, 118–127 (2005).
Langkammer, C. et al. Quantitative MR Imaging of Brain Iron: A Postmortem Validation Study. Radiology 257, 455–462 (2010).
Ordidge, R. J., Gorell, J. M., Deniau, J. C., Knight, R. A. & Helpern, J. A. Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 Tesla. Magn Reson Med 32, 335–341 (1994).
Pyatigorskaya, N., Gallea, C., Garcia-Lorenzo, D., Vidailhet, M. & Lehericy, S. A review of the use of magnetic resonance imaging in Parkinson’s disease. Ther Adv Neurol Disord 7, 12–26 (2014).
Chen, J. C. et al. MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. Am J Neuroradiol 14, 275–281 (1993).
Galazka-Friedman, J. et al. Iron in parkinsonian and control substantia nigra–a Mossbauer spectroscopy study. Mov Disord 11, 8–16 (1996).
Griffiths, P. D. & Crossman, A. R. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson’s disease and Alzheimer’s disease. Dementia 4, 61–65 (1993).
Loeffler, D. A. et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem 65, 710–724 (1995).
Sofic, E. et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74, 199–205 (1988).
Uitti, R. J. et al. Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can J Neurol Sci 16, 310–314 (1989).
Visanji, N. P. et al. Iron deficiency in parkinsonism: region-specific iron dysregulation in Parkinson’s disease and multiple system atrophy. J Parkinsons Dis 3, 523–537 (2013).
Wypijewska, A. et al. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord 16, 329–333 (2010).
Yu, X. et al. Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology 80, 492–495 (2013).
Gorell, J. M. et al. Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45, 1138–1143 (1995).
Graham, J. M., Paley, M. N., Grunewald, R. A., Hoggard, N. & Griffiths, P. D. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 123 Pt 12, 2423–2431 (2000).
Martin, W. R. W., Wieler, M. & Gee, M. Midbrain iron content in early Parkinson disease - A potential biomarker of disease status. Neurology 70, 1411–1417 (2008).
Du, G. et al. Serum iron and ferritin level in idiopathic Parkinson. Pak J Biol Sci 15, 1094–1097 (2012).
Bunzeck, N. et al. Motor phenotype and magnetic resonance measures of basal ganglia iron levels in Parkinson’s disease. Parkinsonism Relat Disord 19, 1136–1142 (2013).
Lee, M. F. et al. N-acetylcysteine (NAC) inhibits cell growth by mediating the EGFR/Akt/HMG box-containing protein 1 (HBP1) signaling pathway in invasive oral cancer. Oral Oncol 49, 129–135 (2013).
Rossi, M., Ruottinen, H., Soimakallio, S., Elovaara, I. & Dastidar, P. Clinical MRI for iron detection in Parkinson’s disease. Clin Imaging 37, 631–636 (2013).
Ulla, M. et al. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One 8, e57904 (2013).
Rossi, M. E., Ruottinen, H., Saunamaki, T., Elovaara, I. & Dastidar, P. Imaging brain iron and diffusion patterns: a follow-up study of Parkinson’s disease in the initial stages. Acad Radiol 21, 64–71 (2014).
Barbosa, J. H. O. et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging 33, 559–565 (2015).
Du, G. et al. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov Disord 31, 317–324 (2016).
He, N. et al. Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Hum Brain Mapp 36, 4407–4420 (2015).
Murakami, Y. et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. Am J Neuroradiol 36, 1102–1108 (2015).
Han, Y. H. et al. Topographical differences of brain iron deposition between progressive supranuclear palsy and parkinsonian variant multiple system atrophy. J Neurol Sci 325, 29–35 (2013).
Wang, C. et al. Application of quantitative measurement on midbrain in Parkinson disease with MR susceptibility-weighted imaging. Chin J Med Imaging Technol 27, 1129–1133 (2011).
Wu, S. F. et al. Assessment of cerebral iron content in patients with Parkinson’s disease by the susceptibility-weighted MRI. Eur Rev Med Pharmacol Sci 18, 2605–2608 (2014).
Zhang, J. et al. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: an in vivo MR study. Brain Res 1330, 124–130 (2010).
Jin, L. et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain 134, 50–58 (2011).
Kim, T. H. & Lee, J. H. Serum uric acid and nigral iron deposition in Parkinson’s disease: A pilot study. Mov Disord 29, S88–S88 (2014).
Wang, Y. et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. Am J Neuroradiol 33, 266–273 (2012).
Huang, X. M., Sun, B., Xue, Y. J. & Duan, Q. Susceptibility-weighted imaging in detecting brain iron accumulation of Parkinson’s disease. Zhonghua Yi Xue Za Zhi 90, 3054–3058 (2010).
Manor, O. & Zucker, D. M. Small sample inference for the fixed effects in the mixed linear model. Comput Stat Data An 46, 801–817 (2004).
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R. & Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13, 1045–1060 (2014).
Biasiotto, G., Di Lorenzo, D., Archetti, S. & Zanella, I. Iron and Neurodegeneration: Is Ferritinophagy the Link? Mol Neurobiol 53, 5542–5574 (2015).
Oshiro, S., Morioka, M. S. & Kikuchi, M. Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv Pharmacol Sci 2011, 378278 (2011).
Perry, G. et al. The role of iron and copper in the aetiology of neurodegenerative disorders: therapeutic implications. CNS Drugs 16, 339–352 (2002).
Sayre, L. M., Perry, G., Atwood, C. S. & Smith, M. A. The role of metals in neurodegenerative diseases. Cell Mol Biol (Noisy-le-grand) 46, 731–741 (2000).
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5, 863–873 (2004).
Daugherty, A. & Raz, N. Age-related differences in iron content of subcortical nuclei observed in vivo: A meta-analysis. Neuroimage 70, 113–121 (2013).
Hadzhieva, M., Kirches, E. & Mawrin, C. Review: iron metabolism and the role of iron in neurodegenerative disorders. Neuropathol Appl Neurobiol 40, 240–257 (2014).
Ben-Shachar, D., Riederer, P. & Youdim, M. B. Iron-melanin interaction and lipid peroxidation: implications for Parkinson’s disease. J Neurochem 57, 1609–1614 (1991).
Jellinger, K., Paulus, W., Grundke-Iqbal, I., Riederer, P. & Youdim, M. B. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J Neural Transm Park Dis Dement Sect 2, 327–340 (1990).
Wolozin, B. & Golts, N. Iron and Parkinson’s disease. Neuroscientist 8, 22–32 (2002).
Kish, S. J., Shannak, K. & Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. New Engl J Med 318, 876–880 (1988).
Ogg, R. J., Langston, J. W., Haacke, E. M., Steen, R. G. & Taylor, J. S. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging 17, 1141–1148 (1999).
Naderi, S., Colakoglu, Z. & Luleci, G. Calcification of basal ganglia associated with pontine calcification in four cases: a radiologic and genetic study. Clin Neurol Neurosurg 95, 155–157 (1993).
He, X. & Yablonskiy, D. A. Biophysical mechanisms of phase contrast in gradient echo MRI. Proc Natl Acad Sci USA 106, 13558–13563 (2009).
Liu, C., Li, W., Tong, K. A., Yeom, K. W. & Kuzminski, S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42, 23–41 (2015).
Anderson, C. M. et al. Brain T2 relaxation times correlate with regional cerebral blood volume. MAGMA 18, 3–6 (2005).
Penke, L. et al. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 33, 510–517 e512 (2012).
Drayer, B. et al. MRI of brain iron. Am J Roentgenol 147, 103–110 (1986).
Guan, X. et al. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed, doi: 10.1002/nbm.3489 (2016).
Schrag, M., Mueller, C., Oyoyo, U., Smith, M. A. & Kirsch, W. M. Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol 94, 296–306 (2011).
House, M. J. et al. Correlation of proton transverse relaxation rates (R2) with iron concentrations in postmortem brain tissue from alzheimer’s disease patients. Magn Reson Med 57, 172–180 (2007).
Yablonskiy, D. A. & Haacke, E. M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32, 749–763 (1994).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010).
Cochran, W. G. The combination of estimates from different experiments. Biometrics 10, 101–129 (1954).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002).
Calne, D. B., Snow, B. J. & Lee, C. Criteria for diagnosing Parkinson’s disease. Ann Neurol 32 Suppl, S125–S127 (1992).
Hughes, A. J., Daniel, S. E., Blankson, S. & Lees, A. J. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 50, 140–148 (1993).
Gibb, W. R. & Lees, A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51, 745–752 (1988).
Acknowledgements
The authors appreciate Drs Jennifer Harr and Wen-Hsing Cheng for critical help improving readability and accuracy of the manuscript. This work was supported by funding from Zhejiang Provincial Natural Science Foundation (LY16H250003, LY16H260003, and LR13H020002), National Natural Science Foundation of China (81571087), and Wenzhou Science and Technology Bureau (Y20150005).
Author information
Authors and Affiliations
Contributions
J.Y.W. and Q.Q.Z. performed the data collection, extraction and analyses, L.B.Z., H.Z., T.L., R.L. and S.F.C. contributed to partial data extraction and interpretation, X.Z., C.P.H. and J.H.Z. designed and supervised the study, J.Y.W., Q.Q.Z. and J.H.Z. wrote the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, JY., Zhuang, QQ., Zhu, LB. et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci Rep 6, 36669 (2016). https://doi.org/10.1038/srep36669
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36669
This article is cited by
-
Mitochondrial dysfunction in Parkinson’s disease – a key disease hallmark with therapeutic potential
Molecular Neurodegeneration (2023)
-
A defect in mitochondrial fatty acid synthesis impairs iron metabolism and causes elevated ceramide levels
Nature Metabolism (2023)
-
Genome-wide screening in pluripotent cells identifies Mtf1 as a suppressor of mutant huntingtin toxicity
Nature Communications (2023)
-
The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson’s disease
Journal of Neural Transmission (2023)
-
Spatiotemporal patterns of brain iron-oxygen metabolism in patients with Parkinson’s disease
European Radiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.