Abstract
Cucurbit chlorotic yellows virus (CCYV) (genus Crinivirus, family Closteroviridae) is an emerging plant virus, and is now spreading and causing severe economic losses to cucurbit crops in many Asian countries. CCYV is believed to be transmitted specifically by the sweetpotato whitefly, Bemisia tabaci, in a semipersistent manner. In the present study, we provide direct evidence for the semipersistent transmission of CCYV by Mediterranean (MED) cryptic species of B. tabaci complex. We investigated CCYV transmission characteristics, and immunofluorescently labeled and localized the virus retention site within the vector by laser confocal microscopy. Whiteflies required ≥1 h of acquisition access period (AAP) to successfully acquire CCYV, and the proportion of RT-PCR positive whitefly individuals reached to 100% at 48 h of AAP. CCYV virons could be retained within vectors as long as 12 d, but the proportion of RT-PCR positive whiteflies dropped to 55% by 3 d. Groups of thirty whiteflies given a 24 h of inoculation access period (IAP) to inoculate CCYV on cucumber plants showed a transmission efficiency rate of 72.73%. The retention site of CCYV virons was located in the foregut of virion-fed vectors. These results definitely indicated the semipersistent transmission mode of CCYV by B. tabaci MED.
Similar content being viewed by others
Introduction
Due to a strong cell wall boundary and immobility of plants, most plant viruses need vectors for the transmission to new host plants or to a new habitat1. Hemipterans, because of their piercing-sucking mouthparts and special feeding behaviors, are the most common vectors for transmission of plant viruses2,3. Insect vectors transmit plant viruses in different ways depending on the combination of characteristics of the viruses, vectors and plants. These transmission types are generally categorized into nonpersistent, semipersistent and persistent modes, according to the length of the period the vector can harbor infectious particles, which can range from minutes to hours (nonpersistent), to days (semipersistent) and to life-time and even inheritance by the insect progeny (persistent-propagative)4,5,6.
Virus retention sites within vectors vary depending on type of virus, vector and transmission modes. Viruses that are transmitted in a nonpersistent manner are retained in the stylet4,7, while viruses transmitted in a persistent manner are circulative-non-propagative or circulative-propagative, i.e., they move with plant sap into the vector gut, from the gut lumen into the hemolymph or other tissues and finally back into the salivary glands from which they are introduced back into the plant during insect feeding6, or even can pass directly through the sheath of the filter chamber and be readily transmitted to healthy plants, as the case of Wheat dwarf virus (WDV) in its leafhopper vector, Psammotettix alienus8. Compared to the viruses having nonpersistent or persistent transmission relationships, transmission characteristics and retention sites of viruses transmitted in a semipersistent manner are less understood7. It is generally believed that viruses transmitted in a semipersistent manner are retained in the foregut of the vector, as experimentally shown for Lettuce infectious yellows virus (LIYV, genus Crinivirus) with unique membrane feeding and immunofluorescent localization9,10. But Hogenhout et al.6 pointed out that biological transmission characteristics are not always correlated with retention site of virus within the insect vector, so the retention sites within vectors and strategies still need to be identified for some nonpersistent or semipersistent viruses, especially for the newly reported viruses7.
Cucurbit chlorotic yellows virus (CCYV) is a newly identified virus species of the genus Crinivirus within the family Closteroviridae11, and is transmitted specifically by cryptic species Middle East-Asia Minor 1 (MEAM1, previously known as B biotype) and Mediterranean (MED, previously known as Q biotype) of the sweetpotato or tobacco whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae)12. CCYV was firstly identified in melon plants in Japan, and is now widespread throughout China, Sudan, Lebanon, Iran and Egypt12,13,14,15,16,17,18,19. CCYV can cause chlorotic leaf spots and complete yellowing of leaves in cucumber, melon, watermelon, pumpkin, loofah plants and Nicotiana benthamiana, resulting in yield and economic losses12. Moreover, it was reported that CCYV and CYSDV (Cucurbit yellow stunting disorder virus) showed mixed infections in Lebanon20. Studies on CCYV were mainly on the detection methods and plant resistance breeding12,21,22, but the details of the transmission process and the virus retention site within its vectors are still unclear20.
In this study, we investigated CCYV transmission characteristics by B. tabaci MED cryptic species, i.e., transmission efficiency, acquisition access period (AAP), retention time within the vector, and inoculation access period (IAP). Meanwhile, we used a membrane feeding and immunofluorescent labeling9 to localize the specific retention of virions within the whitefly vector. These results provide direct evidence for the semipersistent transmission of CCYV and establish the basis for further research on vector-virus interactions and for implementation of effective strategies for control of the virus and insect vectors.
Results
Detection of CCYV in individual whiteflies
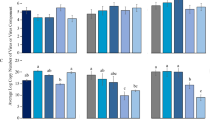
We used real-time RT-PCR to detect CCYV RNA copy numbers of individual whiteflies having fed on virus-infected cucumber plants for 3 d. The results showed that 100% whitefly individuals after 3 d feeding on CCYV-infected plants had acquired CCYV with amounts ranging from 1.34 × 104 to 1.09 × 107 copies with 95% of individual whiteflies carrying 104–106 copies (Fig. 1). No CCYV RNA was detected in control group of individual whiteflies feeding on non-infected cucumber plants (data not shown in Fig. 1).
Copies of CCYV RNA in individual whiteflies of Bemisia tabaci MED.
Whiteflies were allowed to feed on CCYV-infected cucumber plants for 3 d, and 60 whiteflies were individually subjected to real- time RT-PCR test. Data (0 copies) for non-viruliferous whiteflies (control) were not shown. In the Y-axis, 1.00E + 05 = 105.
Transmission efficiency
After feeding on CCYV-infected cucumber plants for 3 days, groups of 5, 15 and 30 whiteflies of B. tabaci MED cryptic species were respectively placed on non-infected cucumber leaves (3–4 leaf stage) with clip cages for 24 h. After 30 days, plant symptoms were checked and the infection status was analyzed with RT-PCR. The results showed that the efficiency of CCYV transmission was positively related to the number of whiteflies used for inoculation. After 24 h of inoculation on healthy cucumber plants, groups of 30 whiteflies transmitted CCYV with the efficiency of 72.73%, while groups of 15 and 5 whiteflies only transmitted CCYV to 40% and 8% plants, respectively (Fig. 2).
The effects of B. tabaci MED number on the proportion of CCYV-infected plants.
After feeding on CCYV-infected cucumber plants for 3 days, groups of 5, 15 and 30 whiteflies were respectively placed on non-infected cucumber leaves (3–4 leaf stage) with clip cages for 24 h. Twenty four plants were used for each whitefly group with 3 replicates, and plants were analyzed by RT-PCR after 30 days. Data shown are mean ± SE.
Acquisition access period
Newly emerged whitefly adults of B. tabaci MED cryptic species were allowed to feed on CCYV-infected cucumber plants for 0.5, 1, 2, 6, 12, 24 and 48 h, respectively, and 24 whiteflies from each group were collected for real-time RT-PCR tests. The proportion of RT-PCR positive whiteflies increased with the time of acquisition access period (AAP) when whiteflies fed on CCYV-infected cucumber plants, so AAP significantly affected the percentage of RT-PCR positive whiteflies (F = 33.66, df = 6, 147, P < 0.0001). Whiteflies required ≥1 h of AAP to acquire CCYV virons successfully, and the proportion of RT-PCR positive whiteflies reached to ca. 55% at 6 h AAP, and to 100% at 48 h of AAP (Fig. 3).
The effects of acquisition access period (AAP) on the proportion of RT-PCR positive adults of B. tabaci MED.
Newly emerged whiteflies were allowed to feed on CCYV-infected cucumber plants for 0.5, 1, 2, 6, 12, 24 and 48 h, respectively, and 24 whiteflies from each group were collected for real-time RT-PCR tests. Three replicates were used. Data shown are mean ± SE.
Retention time
After 3 d feeding on CCYV-infected plants, adults of B. tabaci MED were transferred to cotton plants (cotton is the host plant for whiteflies, but non-host plant for CCYV) for determination of the retention time. Whiteflies collected at time intervals of 1, 3, 6, 9, 12 and 15 d, respectively, were subject for real-time RT-PCR tests. The results showed that the proportions of RT-PCR positive whiteflies decreased with time (F = 19.36, df = 5, 130, P < 0.0001) (Fig. 4). CCYV could be retained in B. tabaci as long as 12 d, but the proportion of RT-PCR positive whiteflies dropped to 55% on 3rd d and even lower level on 6th d or later.
The effects of retention time on the proportion of RT-PCR positive B. tabaci MED.
Whiteflies having fed on CCYV-infected cucumber plants for 3 days were transferred to cotton plants (non-host plants for CCYV but host plant for whiteflies). Twenty-four whiteflies were collected at time intervals of 1, 3, 6, 9, 12 and 15 d, respectively, for real-time RT-PCR tests. Data shown are mean ± SE. Three replicates were used for each collection.
Inoculation access period
Thirty whiteflies, having fed on CCYV-infected cucumber plants for 3 days, were placed on non-infected cucumber leaves (3–4 leaf stage) with clip cages for inoculation access period (IAP) of 1, 6, 12, 24 and 48 h, respectively. After 30 days, 24 plants used for each inoculation time treatment were detected with RT-PCR. We found that CCYV transmission efficiency of B. tabaci was positively correlated with IAP (F = 7.50, df = 4, 51, P < 0.0001) (Fig. 5). Groups of 30 whiteflies were unable to transmit CCYV within 1 h IAP, but CCYV was transmissible after 6 h inoculation, though the transmission efficiency was still low. The transmission efficiency for groups of 30 whiteflies after 24 h and 48 h of IAP was 70.00% and 81.82%, respectively.
The effects of inoculation access period (IAP) of the vector B. tabaci MED on the proportion of CCYV-infected cucumber plants.
After feeding CCYV-infected cucumber plants with 3 days, 30 whiteflies were placed on non-infected cucumber leaves (3–4 leaf stage) with clip cages for 1, 6, 12, 24 and 48 h, respectively. Twenty-four plants were used for each treatment and detected with RT-PCR after 30 days. Data shown are mean ± SE. Three replicates were used.
Localization of CCYV virions within whitefly vectors
To determine the retention site of CCYV virions within its whitefly vector, we used a membrane feeding approach to give viruliferous whiteflies sequential access to basal artificial liquid diet containing anti-CCYV-CP IgG and goat anti-rabbit IgG conjugated with Alexa Fluor 4889. Then we observed the dissected heads or foreguts of whiteflies under confocal laser scanning microscopy (Leica, TCS SP8). The results revealed the presence of bright green fluorescent signals distributed in the cibarium, anterior pharynx and posterior esophagus (generically referred to as the foregut) within vectors, showing that the retention site of CCYV virions within the whitefly is the foregut (Fig. 6).
Retention site localization of CCYV virions in the foregut and cibarium of B. tabaci MED under confocal laser scanning microscopy (Leica, TCS SP8).
(A) Confocal view of the head of viruliferous B. tabaci adult, with background transimitted light blocked. (B) Transmitted light view of A. (C) Confocal view of the head of non-viruliferous B. tabaci adult, with background transimitted light blocked. (D) Transmitted light view of C. Under confocal laser scanning microscopy, we observed non-viruliferous (having fed on healthy cucumber plants) or viruliferous whiteflies (having fed on CCYV-infected cucumber plants) after sequential membrane feeding of the following solutions: (i) basal diet, (ii) diet containing anti-CCYV-CP IgG, and (iii) diet containing a goat anti-rabbit IgG conjugated with Alexa Fluor 488. The presence of fluorescent signals showing the retention site within the vector was indicated with the arrow (Fg). The eye, foregut (Fg), cibarium (Cb), and stylets (S) are indicated. (Scale bars, 50 μm).
Discussion
As a member of the genus Crinivirus, CCYV is believed to be transmitted by whitefly vectors in a semipersistent mode12, but the transmission details have so far been lacking. In this study, we provided direct evidence for the semipersistent transmission of CCYV by investigating transmission characteristics and localizing the virus retention site within the vector.
Transmission efficiency is variable with virus species and their vectors. Criniviruses occur at low titer and are restricted to the phloem of infected plants9, therefore whiteflies take some time to reach the phloem to acquire viruses and inoculate viruses to new host plants, and the transmission efficiency is vector-number dependent. Whitefly numbers less than 30 generally are not efficient for Crinivirus transmission. In our study, the efficiency of CCYV transmission by 30 B. tabaci MED whiteflies after a 24 h of IAP was 72.73% (Fig. 2). Similarly, 40 whiteflies B. tabaci A biotype had efficiency of 74.30% for LCV (Lettuce chlorosis virus) transmission23. But transmission of CYSDV (Cucurbit yellow stunting disorder virus) by 30 adults of B. tabaci A biotype, SqVYV (Squash vein yellowing virus) by 30 adults of B. tabaci B biotype (MEAM1), and LCV by 40 adults of B. tabaci B biotype had only efficiency of 47.00%24, 57.00%25 and 57.5%23, respectively.
In our study, B. tabaci MED whiteflies required a minimum 1 h AAP to acquire detectable CCYV, while 1 h AAP for LCV23 and 2 h for CYSDV24, but only 10 min for LIYV23. In our experiments, whiteflies within 1 h IAP were unable to transmit CCYV. The minimum IAP for inoculation of CCYV to new host plants by 30 whitefly was 6 h, much longer than those for SqVYV (30 min)25, LIYV (Lettuce infectious yellows virus) (1 h), LCV (1 h), and CYSDV (2 h). For these viruses above, 48 h IAP was enough for the vector whiteflies to obtain 80% or more transmission efficiency.
It was reported that criniviruses like CYSDV, LIYV and LCV can be harbored in the vectors for ca. 10 days26. Our results showed that B. tabaci MED can harbor CCYV as long as 12 d when analyzed using real-time RT-PCR. The vectors of CYSDV, LIYV and LCV retained the ability to transmit the respective virus to plants for 3 or 4 days, and it can be presumed that B. tabaci MED whiteflies can not transmit CCYV after 3 or 5 days because the percentage of RT-PCR positive whiteflies was ca. 20% on 6th day.
Semipersistently transmitted viruses can be retained in the stylet or foregut of insect vectors9,10,27. Viruses of the genus Crinivirus, by virtue of their semipersistent transmission characteristics, have been postulated to be retained in the foreguts, and clearly the work of Chen et al.9 showed this to be the case for LIYV. By contrast, Cauliflower mosaic virus (CaMV) was shown to be retained at the stylet tip of their aphid vectors28, but CaMV differs from most viruses that are transmitted in a semipersistent manner in that it readily moves through mesophyll tissues of infected plants while criniviruses are limited to the phloem, and CaMV can be acquired from and inoculated to plants by probing, vs. feeding as the means for criniviruses. While the retention sites for many criniviruses remains to be identified7, here we found that CCYV is retained in the foregut within the whitefly vector, B. tabaci MED cryptic species, by using the membrane feeding and immunofluorescent localization approach (Fig. 6). Our results are very similar to those of Chen et al.9 for LIYV.
In conclusion, our results have shown that CCYV transmission characteristics and retention within the vector are consistent with a semipersistent type of transmission. Future work includes comparison of transmission capacity between two cryptic species (MED and MEAM1) of B. tabaci and molecular mechanisms. In the previous experiment we found that MED is more effective in transmission of CCYV than MEAM1 (unpublished data), and studies of protein-protein interactions between virus and vector may reveal the mechanisms of difference in transmission ability9,29.
Materials and Methods
Maintenance of plants, whiteflies, and virus clones
The colony of B. tabaci Mediterranean (MED) cryptic species (formerly referred to as Q biotype) was maintained on tobacco plants (Nicotiana tabacum cv. Zhongyan-100) in cages (60 cm × 40 cm × 80 cm) in a greenhouse at 28 ± 0.5 °C, 16:8 h LD and 75 ± 0.5% relative humidity. The genetic purity of B. tabaci MED cultures was monitored every 3–5 generations using the random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) technique combined with the sequencing of mtCO1 gene30. Plants of cucumber (Cucumis sativus cv. Youlu-10) were used for virus clone maintenance and transmission assays. Cotton (Gossypium hirsutum L. cv. Yinshan-1) is the suitable host plants of B. tabaci but not of CCYV, so it was used for the retention time experiment in transmission assays (see below). Viruliferous B. tabaci MED adults were collected from CCYV-infected melon plants and transferred to cucumber plants, and cucumber plants were inoculated with CCYV by viruliferous B. tabaci MED adults and maintained in growth chambers under above-mentioned conditions.
Detection of CCYV virions in the whitefly vectors
RNA extraction and synthesis of cDNA
Total RNA of individual adult whiteflies from infected cucumber plants was extracted using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. RNA concentration and purity were measured in a NanoDropTM spectrophotomer (Thermo Scientific) and stored at −20 °C for subsequent analysis. Total RNA (1 μg) from each sample was reverse transcribed to generate the first-strand cDNA using the PrimeScriptTM RT reagent Kit (Takara).
Primer design
Primers were designed based on CCYV coat protein coding sequences by using primer premier 5 software and the nucleotide sequence in GenBank (accession no. HM581658.1). The forward primer (CCYV-F: 5′-GCGACCATCATCTACAGGCA-3′; nucleotide positions 548–567) and the reverse primer (CCYV-R: 5′-CCGACTTGTTCCTTTCAGAGC-3′; nucleotide positions 679–699) were found to have an optimal annealing temperature of 60 °C. The primer pair generates a 152 base pair fragment. Subsequent Primer-BLAST searches showed that they had a high specificity towards CCYV.
SYBR Green real-time RT-PCR
The real-time RT-PCR assays were performed using SYBR Premix Ex TaqTM II (Takara). All reactions were carried out in a final volume of 20 μl each containing: 10 μl of SYBR Premix Ex TaqTM II (Takara), 1 μl of cDNA or plasmid dilutions and 0.8 μl of each primer (CCYV-F and CCYV-R). Amplification reactions were performed as follows: 94 °C for 2 min; 40 cycles of 94 °C for 15 s, 60 °C for 20 s, 72 °C for 20 s.
For generation of standard quantification curves, serial 10-fold dilutions of plasmid (3.79 × 102 to 3.79 × 108 copies/μl) were used as templates for real-time RT-PCR. Standard curves were obtained by linear regression analysis of the threshold cycle (Ct) value of each of three replicates over the logarithm of the copy numbers present in each sample. To determine the sensitivity and the reliability of the real-time RT-PCR, three individual assays were undertaken using the 10-fold serial dilutions of plasmid.
Assays for virus transmission
Transmission efficiency
Thousands of whiteflies were placed into the insect-proof cage containing two CCYV-infected cucumber plants. Three days after feeding, groups of 5, 15 and 30 whiteflies were transferred in clip cages31 onto the underside of healthy leaves of cucumber plants (3–4 leaf stage), respectively. Twenty-four plants were used for each whitefly density treatment with 3 replicates. After 24-h IAP, clip cages were removed and whiteflies were collected for CCYV detection, while the plants were treated with insecticide (imidacloprid) and maintained in whitefly- free greenhouse to test with RT-PCR after 30 days.
Acquisition access period (AAP)
Ca. 500 non-viruliferous whiteflies were placed on two CCYV-infected cucumber plants in the insect-proof cage. Twelve whiteflies (12 per plant) were collected at time intervals following introduction of CCYV-infected cucumber plants: 0.5, 1, 2, 6, 12, 24, 48 h. Whiteflies feeding on non-infected cucumbers served as negative controls. Three replicates were used. The total RNA of individual whiteflies was extracted using Trizol reagent, and detected by real-time RT-PCR.
Retention time
Hundreds of whiteflies were released into the insect-proof cage containing two CCYV-infected cucumber plants for feeding. Three days after feeding, about 500 whiteflies were collected and starved for 2–3 h before putting into another cage containing two cotton plants (CCYV non-host plants) for feeding. Whiteflies (12 per plant) were collected at time intervals of 1, 3, 6, 9, 12 and 15 d after feeding. Whiteflies feeding on healthy cotton plants for 3 days were served as negative controls. The total RNA of individual whiteflies was extracted using Trizol reagent, and analyzed by real-time RT-PCR. Twenty-four whiteflies were tested for each collection with 3 replicates.
Inoculation access period (IAP)
Thousands of whiteflies were put into the insect-proof cage containing two CCYV-infected cucumber plants for feeding. Three days after feeding, 30 whiteflies which were starved for 2–3 hours, were placed in clip cages clipped in the back of non-infected cucumber leaves (3–4 leaf stage). Clip cages were removed at time intervals of 1, 6, 12, 24 and 48 hours after feeding, and whiteflies were collected for CCYV detection, while the plants were treated with insecticide (imidacloprid) and maintained in the whitefly-free greenhouse. Twenty-four plants were used for each treatment with 3 replicates. Whiteflies feeding on non-infected cucumber plants served as negative controls.
Location of virion retention site within the vector
Two groups of B. tabaci MED adults were used: one group fed on CCYV-infected cucumber plants whereas the other group fed on healthy cucumber plants. Adult whiteflies were placed in membrane feeding cages, with approximately 50–100 whiteflies per cage. Whiteflies were first fed with basal artificial liquid diet [15% sucrose and 1% BSA in 1× TE (10 mM Tris-HCl, 1 mM EDTA, pH 7.4)] to remove unbound or nonspecifically bound virions, and then fed with basal artificial liquid diet containing anti-CCYV-CP polyclonal IgG raised in rabbit (1/500-fold dilution of 0.74 mg/mL), followed by basal artificial liquid diet containing a 1/200-fold dilution of goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen). Whiteflies were allowed to feed on each of the above solutions for 10- to 12-h. To remove unbound or nonspecifically bound antibodies present in the ingested solutions, whiteflies were fed with a basal artificial diet for several hours after feeding of antibody-containing solutions9. We observed the dissected heads or foreguts of whiteflies under confocal laser scanning microscopy (Leica, TCS SP8).
Statistical analyses
In experiments of AAP and retention time, numbers of RT-PCR positive whiteflies and copies of CCYV were recorded and calculated, and proportions of RT-PCR positive whiteflies were calculated by numbers of RT-PCR positive over test whiteflies which did not include the failure ones for RNA extraction. In experiments of efficiency of transmission and IAP, numbers of infected plants were recorded and proportions of infected plants were calculated by numbers of infected plants over test plants which did not include the dead ones in the process of growth. Based on these data, variance analysis with Duncan’s new multiple range method was carried out using IBM Statistical SPSS software 20.0.
Additional Information
How to cite this article: Li, J. et al. Direct evidence for the semipersistent transmission of Cucurbit chlorotic yellows virus by a whitefly vector. Sci. Rep. 6, 36604; doi: 10.1038/srep36604 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Blanc, S., Uzest, M. & Drucker, M. New research horizons in vector transmission of plant viruses. Microbiology. 14, 483–491 (2011).
Nault, L. R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 90, 521–541 (1997).
Mitchell, P. L. Heteroptera as vectors of plant pathogens. Neotrop. Entomol. 33, 519–545 (2004).
Ng, J. C. K. & Falk, B. W. Virus-vector interactions mediating non-persistent and semi-persistent transmission of plant viruses. Annu. Rev. Phytopathol. 44, 183–212 (2006).
Hohn, T. Plant virus transmission from the insect point of view. Proc. Natl. Acad. Sci. USA 104(46), 17905–17906 (2007).
Hogenhout, S. A. et al. Phytoplasmas: Bacteria that manipulate plants and insects. Mol. Plant. Pathol. 9, 403–423 (2008).
Ng, J. C. K. & Zhou, J. S. Insect vector–plant virus interactions associated with non-circulative, semi-persistent transmission: current perspectives and future challenges. Curr. Opin. Virol. 15, 48–55 (2015).
Wang, Y. et al. Localization and distribution of Wheat dwarf virus in its vector leafhopper, Psammotettix alienus. Phytopathology. 104, 897–904 (2014).
Chen, A. Y. S., Walker, G. P., Carter, D. & Ng, J. C. K. A virus capsid component mediates virion retention and transmission by its insect vector. Proc. Natl. Acad. Sci. USA 108(40), 16777–16782 (2011).
Ng, J. C. K. A quantum dot-immunofluorescent labeling method to investigate the interactions between a crinivirus and its whitefly vector. Front Microbiol. 4, 77 (2013).
Gyoutoku, Y. et al. Chlorotic yellows disease of melon caused by Cucurbit chlorotic yellows virus, a new crinivirus. Jpn. J. Phytopathol. 75, 109–111 (2009).
Okuda, M., Okazaki, S., Yamasaki, S., Okuda, S. & Sugiyama, M. Host range and complete genome sequence Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology. 100, 560–566 (2010).
Huang, L. H., Tseng, H. H., Li, J. T. & Chen, T. C. First report of Cucurbit chlorotic yellows virus infecting Cucurbits in Taiwan. Plant Dis. 94, 1168 (2010).
Gu, Q. S. et al. First report of Cucurbit chlorotic yellows virus in cucumber, melon, and watermelon in China. Plant Dis. 95, 73 (2011).
Zeng, R., Dai, F. M., Chen, W. J. & Lu, J. P. First report of Cucurbit chlorotic yellows virus infecting melon in China. Plant Dis. 95(3), 354 (2011).
Hamed, K., Menzel, W., Dafalla, G., Gadelseed, A. M. A. & Winter, S. First report of Cucurbit chlorotic yellows virus infecting muskmelon and cucumber in Sudan. Plant Dis. 95, 1321 (2011).
Abrahamian, P. E., Sobh, H. & Abou-Jawdah, Y. First report of Cucurbit chlorotic yellows virus on cucumber in Lebanon. Plant Dis. 96(11), 1704–1705 (2012).
Bananej, K., Menzel, W., Kianfar, N., Vahdat, A. & Winter, S. First report of Cucurbit chlorotic yellows virus infecting cucumber, melon, and squash in Iran. Plant Dis. 97(7), 1005 (2013).
Mahmoud, A. A. Serological and molecular characterization of Cucurbit chlorotic yellows virus affecting cucumber plants in Egypt. Int. J. Virol. 11(1), 1–11 (2015).
Abrahamian, P. E. & Abou-Jawdah, Y. Whitefly-transmitted croniviruses of cucurbits: current status and future prospects. Virus Dis. 25(1), 26–38 (2014).
Abrahamian, P. E., Seblani, R., Sobh, H. & Abou-Jawdah, Y. Detection and quantification of two cucurbit criniviruses in mixed infection by real-time RT-PCR. J. Virol. Methods. 193, 320–326 (2013).
Okuda, S. et al. Resistance in melon to Cucurbit chlorotic yellows virus, a whitefly-transmitted crinivirus. Eu. J. Plant Pathol. 135, 313–321 (2013).
Duffus, J. E., Larsen, R. C. & Liu, H. Y. Lettece infectious yellows virus: a new type of whitefly-transmitted virus. Phytopathology. 76, 97–100 (1996).
Celix, A., Lepez-Sese, A., Almarza, N., Gomez-Guillamon, M. L. & Rodriguez-Cerezo, E. Characterization of Cucurbit yellow stunting disorder virus, a Bemisia tabaci-transmitted closterovirus. Phytopathology. 86, 1370–1376 (1996).
Webb, S. E., Adkins, S. & Reitz, S. R. Semipersistent whitefly transmission of Squash vein yellowing virus, causal agent of viral watermelon vine decline. Plant Dis. 96, 839–844 (2012).
Wisler, G. C., Duffus, J. E., Liu, H. Y. & Li, R. H. Ecology and epidemiology of whitefly-transmitted Closteroviruses. Plant Dis. 82, 270–280 (1998).
Childress, S. A. & Harris, K. F. Localization of virus-like particles in the foreguts of viruliferous Graminella nigrifrons leafhoppers carrying the semi-persistent Maize chlorotic dwarf virus. J. Gen. Virol. 70, 247–251 (1989).
Uzest, M. et al. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc. Natl. Acad. Sci. USA 104, 17959–17964 (2007).
Liu, W. W. et al. Proteomic analysis of interaction between a plant virus and its vector insect reveals new functions of hemipteran cuticular protein. Mol. Cell. Proteomics. 14(8), 2229–2242 (2015).
De Barro, P. J., Liu, S. S., Boykin, L. M. & Dinsdale, A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011).
Zang, L. S., Liu, Y. Q. & Liu, S. S. A new type of small leaf clip-cage for rearing whiteflies in experimental studies. Chinese Bulletin of Entomology. 42(3), 329–331 (2005). (in Chinese with English abstract).
Acknowledgements
Authors are grateful to Dr. Xin-Cheng Zhao for his help in confocal microscopy and 3 anonymous reviewers for their critical comments on the manuscript. This research was financially supported by the National Natural Foundation of China (Project No. 31471776).
Author information
Authors and Affiliations
Contributions
F.M.Y. and B.W.F. designed the experiments. J.J.L., X.Z.L. and X.L.W. conducted experiments. J.J.L., X.Z.L. and X.L.W. analyzed and interpreted the data under supervision of F.M.Y. and Y.W.K. Y.S and Q.S.G. contributed materials and supervised methods. F.M.Y., J.J.L., Y.W.K. and B.W.F. wrote the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, J., Liang, X., Wang, X. et al. Direct evidence for the semipersistent transmission of Cucurbit chlorotic yellows virus by a whitefly vector. Sci Rep 6, 36604 (2016). https://doi.org/10.1038/srep36604
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36604
This article is cited by
-
Molecular basis of mutual benefits between Cucurbit chlorotic yellows virus (CCYV) transmission and imidacloprid resistance in Bemisia tabaci
Journal of Pest Science (2023)
-
Can biological control be a strategy to control vector-borne plant viruses?
Journal of Pest Science (2023)
-
Widely targeted analysis of metabolomic changes of Cucumis sativus induced by cucurbit chlorotic yellows virus
BMC Plant Biology (2022)
-
Changes in Bemisia tabaci feeding behaviors caused directly and indirectly by cucurbit chlorotic yellows virus
Virology Journal (2019)
-
Transcriptome analysis of Cucumis sativus infected by Cucurbit chlorotic yellows virus
Virology Journal (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.