Abstract
Liver kinase B1 (Lkb1) plays crucial roles in development, metabolism and survival. As constitutive knockout of Lkb1 in mice leads to embryonic lethality, whether Lkb1 is required for the growth and survival of adult mice is unclear. Here we address this question using a tamoxifen-inducible Lkb1 knockout (KO) mouse model: Rosa26-CreER: Lkb1flox/flox (abbreviated as Rosa-Lkb1). The Rosa-Lkb1 mice exhibited body weight reduction and died within 6 weeks after tamoxifen induction. The body weight reduction was due to reduced weight of various tissues but the brown and white adipose tissues underwent much more pronounced weight reduction relative to the overall body weight reduction. Accordingly, the Rosa-Lkb1 mice had increased blood glucose levels and were intolerant to glucose challenge. Expression levels of adipogenic and lipogenic genes in adipose tissues were also dramatically reduced by Lkb1 deletion. Additionally, Lkb1 deletion reduced lipid deposition and increased expression of mitochondrial (Pgc1a, Cox5b and Cox7a) and hepatic gluconeogenesis related genes (Pepck) in liver. Finally, the Rosa-Lkb1 mice had much reduced oxygen consumption, carbon dioxide production, and energy expenditure. These results demonstrate that Lkb1 plays an important role in maintaining body weight, liver and adipose tissue function, blood glucose homeostasis and survival in adult mice.
Similar content being viewed by others
Introduction
Liver kinase B1 (Lkb1), also known as serine/threonine protein kinase 11 (Stk11), plays crucial roles in embryo and tissue development, energy metabolism and survival1,2,3,4,5. Lkb1 acts as a central metabolic sensor of glucose and lipid metabolism by phosphorylating and regulating the AMP-activated protein kinase (AMPK) signaling pathway5,6. In addition, Lkb1 is a ‘master’ protein kinase that functions upstream of 13 other kinases of the AMPK subfamily6.
Previous studies have shown that constitutive knockout of Lkb1 causes severe developmental defects and the KO embryos die at embryonic day 8.5–111. Cardiac-specific deletion of Lkb1 causes early defects in atrial channel expression, atrial fibrillation, hypertrophy and heart dysfunction7,8,9. Liver-specific deletion of Lkb1 in mice results in defective canaliculi and bile duct formation, hyperglycemia, body weight reduction and death2,10,11,12. Skeletal muscle-specific deletion of Lkb1 regulates muscle development, lipid oxidation, glucose homeostasis, and insulin sensitivity13,14,15,16. In addition, tissue-specific deletions of Lkb1 in adipose tissue17,18, pancreas19,20,21 and brain22,23 further demonstrate that Lkb1 plays important roles in these tissues. Moreover, loss of Lkb1 regulates haematopoietic stem cells’ metabolism and survival3,4,5, muscle stem cells’ proliferation and differentiation16,24, and pancreatic β cells’ polarity20. However, due to the embryonic lethality of global knockout of Lkb1 in mice1, the role of Lkb1 in tissue homeostasis and survival of adult mice is unclear.
Therefore, in the current study, we used the Cre-LoxP system to generate a tamoxifen (TMX)-inducible whole-body Lkb1 knockout mouse model (Rosa26-CreER:Lkb1flox/flox, abbreviated as Rosa-Lkb1) by crossing Lkb1flox/flox mice with Rosa26-CreER mice. We found several metabolic consequences in the Rosa-Lkb1 mice that eventually lead to lethality within 6 weeks after TMX induced deletion of Lkb1 in adult mice.
Results
Generation of TMX-inducible whole-body Lkb1 knockout mice
To directly investigate the effects of global Lkb1 knockout in adult mice, we used the Cre-LoxP recombination system. We generated TMX-inducible whole-body Lkb1 knockout mice Rosa-Lkb1 by crossing the Lkb1flox/flox mice with the Rosa26-CreER mice (Fig. 1A). In adult Rosa-Lkb1 mice, TMX injection activates Cre in all cells due to the ubiquitous expression of the Rosa26 gene. Indeed, real-time PCR analysis showed a dramatic reduction of Lkb1 in brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), soleus muscle (Sol), heart (Hrt), kidney (Kid), spleen (Spl), and brain (Bra) (Fig. 1B). Consistently, western blot results confirmed the efficient reduction of Lkb1 in various tissues (Fig. 1C). Notably, the Lkb1 gene was robustly deleted in adult liver (Liv) (Fig. 1B,C). These results demonstrate the utility of the Rosa-Lkb1 mouse model for temporally controlled deletion of the Lkb1 gene after TMX induction.
Global deletion of Lkb1 leads to body weight reduction and lethality.
(A) Targeting strategy. (B,C) Relative mRNA (B) and protein (C) levels of Lkb1 in different tissues (n = 4). (D) TMX induction strategy. (E,F) Body weight (E, n = 7–18 ) and survival index (F, n = 24–28) of Lkb1f/f and KO mice. Error bars: S.E.M.; *means P < 0.05, **means P < 0.01, and ***means P < 0.001 unless otherwise indicated in figure legends.
Global deletion of Lkb1 in adult mice causes body weight reduction and lethality
To examine the role of Lkb1 in adult mice, TMX (300 μl per mouse, 10 mg/ml) was injected intraperitoneally (I. P.) to 10-wk-old mice once a day for 5 consecutive days (Fig. 1D). After TMX injection, we examined the body weight changes of the mice (Fig. 1D). Although the body weights of the Lkb1flox/flox and Rosa-Lkb1 mice were similar before TMX injection, the body weight of the Rosa-Lkb1 mice began to decrease from 2 weeks after TMX induction (Fig. 1E). At 5 weeks after TMX injection, the body weight of the Rosa-Lkb1 mice was only 18.30 ± 0.34 g, about 40% lighter than that of the control Lkb1flox/flox littermates (30.75 ± 0.74 g) (Fig. 1E). Notably, we also found that the Rosa-Lkb1 mice began to die from 1 week after TMX injection (Fig. 1F). At 4 weeks, more than 57% KO mice died, and no KO mice survived beyond 6 weeks after TMX injection (Fig. 1F). In contrast, all Lkb1flox/flox mice survived after TMX treatment (Fig. 1F). These results indicate that Lkb1 plays an important role in maintaining body weight and survival of adult mice.
Global deletion of Lkb1 in adult mice decreases the ratio of WAT to body weight
To examine how global deletion of Lkb1 decreased the body weight of adult mice, we investigated the weight of various tissues at 3 weeks after the start of TMX administration. At that time, the body weights of the KO mice were also less than that of the Lkb1flox/flox mice, though the body weights were similar before TMX treatment (Fig. 2A). Similar food intake was found between Lkb1flox/flox and KO mice at 2- and 3-weeks after TMX injection (Fig. 2B). Upon examination of various tissues, we found that global deletion of Lkb1 significantly decreased the weights of iWAT, BAT, TA, EDL and Gas muscles, as well as various organs including spleen, kidney, liver and lung (Fig. 2C–E). When normalized to the body weight, only the relative weights of iWAT, BAT and spleen were lower in KO compared to control mice, but the relative weights of muscle and other organs including liver, heart and lung to body weight were unchanged or even higher in KO mice than that of Lkb1flox/flox mice (Supplemental Fig. 1A–C). As the spleen only accounts for a very small fraction of body weight, these results indicate that global deletion of Lkb1 in adult mice leads to decreases in body weight mainly due to the reduction of adipose tissues.
Lkb1 deletion reduces body weight and tissue mass.
(A) Body weight of Lkb1f/f (n = 8) and KO (n = 12) mice at 3 weeks after TMX induction. (B) Food intakes of Lkb1f/f and KO mice at 2- and 3-week after TMX injection (n = 6). (C–E) The weight of fat (C), muscle (D) and organs (E) from Lkb1f/f (n = 6) and KO (n = 9) mice.
To further examine the effects of global deletion of Lkb1 on fat mass and to determine if the lethality in KO mice is due to reduction of fat mass, we used high-fat diet (HFD) to promote fat deposition prior to induced-deletion of Lkb1. After 10 weeks of HFD treatment, both Lkb1flox/flox and Rosa-Lkb1 mice were obese and then treated with TMX to delete Lkb1. We found that the HFD-fed mice still died within 6 weeks after TMX induced deletion of Lkb1 (data not shown). Though the body weights of the Lkb1flox/flox and Rasa-Lkb1 mice were similar before TMX, the body weights of KO mice (17.16 ± 0.62 g) were ~50% lighter than that of Lkb1flox/flox mice (34.6 ± 2.18 g) at 4 weeks after TMX injection (Fig. 3A). Notably, the WAT mass of the KO mice was dramatically decreased (Fig. 3B). Furthermore, we found that the weights of the other tissues including TA, Gas, Kid, Spl, and Liv were also significantly decreased (Fig. 3C,D). However, the ratios of iWAT, asWAT and eWAT to body weight were lower, but the ratios of kidney, liver, heart and lung to body weight were unchanged or even higher in KO mice than that of Lkb1flox/flox mice (Supplemental Fig. 2A–C). Therefore, HFD fails to prevent from body weight loss and lethality in the Lkb1 KO adult mice.
At the gene expression level, the relative protein levels of the adipocyte differentiation related PPARγ2 were decreased in iWAT and eWAT of the KO mice compared to that of the control mice (Fig. 4A). Real time PCR results also showed that the mRNA levels of the fat deposition related gene Fas and the mature adipocytes markers Adipoq and Leptin were significantly decreased in adipose tissues of HFD-fed (Fig. 4B,C) or from normal fat diet (NFD)-fed (Fig. 4D,E) Lkb1flox/flox and KO mice after TMX induction. Taken together, these results showed that reduction of adipose tissues (both brown and white) is correlated to changes in the expression of adipogenic genes in the Lkb1 KO adult mice.
Deletion of Lkb1 affects glucose metabolism
As Lkb1 KO in adult mice affects both BAT and WAT mass, we next determined if the changes in fat mass are associated with alterations in thermoregulation and energy metabolism. We first measured the rectal temperatures, as an indication of heat production by BAT, of the Lkb1flox/flox and KO mice before and after TMX injection. We found the rectal temperature of the KO mice was lower compared to the control mice at 3 weeks after TMX injection, though similar rectal temperature was found between Lkb1flox/flox and KO mice before TMX (Fig. 5A,B). The blood glucose levels of the KO mice were higher than that of Lkb1flox/flox mice at 3 weeks after TMX injection (Fig. 5C,D). Furthermore, compared to the Lkb1flox/flox littermates, Rosa-Lkb1 mice had higher blood glucose levels after intraperitoneal (IP) injection of glucose (Fig. 5E) and lower blood glucose levels at 60 minutes after insulin injection (Fig. 5G). The area under the curve (AUC) of the GTT, but not the AUC of the ITT, showed significant difference between Lkb1f/f and KO mice (Fig. 5F,H). In addition, the Rosa-Lkb1 mice had lower rates of oxygen consumption and carbon dioxide production and expended less energy compared to the Lkb1flox/flox mice (Fig. 6). Taken together, these results suggest that global deletion of Lkb1 in adult mice leads to glucose intolerance and decreases energy metabolism.
Global deletion of Lkb1 results in glucose intolerance.
(A,B) Rectal temperature of Lkb1f/f and KO mice before (A, n = 6) or after (B, n = 14) TMX induction. (C,D) Blood glucose before (C) or after fasting16 h (D) (n = 8–10). (E–H) Blood glucose and the area under curve (AUC) during GTT (E,F; n = 8–10) and ITT (G,H; n = 5–6).
Deletion of Lkb1 affects liver function and glucose homeostasis
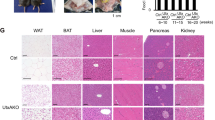
We found that the expression of Lkb1 was nearly abolished in liver tissues of the Rosa-Lkb1 mice (Fig. 1). To examine the effects of Lkb1 deletion on liver tissues, we injected Evans Blue (EB) dye to Lkb1flox/flox and KO mice at 3 weeks after TMX injection. We found that EB uptake was more pronounced in the liver tissues of KO mice compared to control mice (Supplemental Fig. 3A), indicating Lkb1 deletion may induce liver degeneration. Staining results also showed that deletion of Lkb1 reduced lipid deposition in liver (Supplemental Fig. 3B). Indeed, quantification results showed that deletion of Lkb1 dramatically decreased triglyceride content in the liver from the KO mice compared to that of Lkb1flox/flox mice (Supplemental Fig. 3C). Moreover, we checked the gene expression and found that the mitochondrial related genes including Pgc1a, Cox5b and Cox7a were significantly upregulated in the KO livers (Supplemental Fig. 3D). Deletion of Lkb1 also increased the expression of Pepck gene, a rate-determining enzyme in hepatic gluconeogenesis, in the liver (Supplemental Fig. 3D), indicating defective liver glucose homeostasis. The expression of Fas and Srebp1c, however, was similar between Lkb1flox/flox and KO mice (Supplemental Fig. 3D). These results suggested that deletion of Lkb1 affects liver function and liver glucose homeostasis.
Discussion
In this study, we generated a TMX-inducible whole-body Lkb1 knockout mice model and examined the effects of global Lkb1 ablation on adult mice. We found that global deletion of Lkb1 in adult mice reduces body weight and leads to lethality within 6 weeks after TMX induction. Moreover, the absolute and relative masses of adipose tissues are dramatically decreased in the KO mice compared to the Lkb1flox/flox mice. Furthermore, global deletion of Lkb1 in adult mice affects the glucose metabolism, liver function and liver glucose homeostasis. These results indicate that Lkb1 is necessary for maintaining adult mice body weight, liver and adipose tissue function, blood glucose homeostasis, and animal survival.
Lkb1 plays a major role in regulating of metabolism and controlling physiological functions of many tissues2,9,16,17. Here we found that TMX-inducible whole-body deletion of Lkb1 in adult mice reduces fat mass and body weight under chow diet or HFD diet. Likewise, several tissue-specific Lkb1 deletion mice models also exhibited a similar phenotype in body weight reduction. For example, FABP4-Cre induced adipose-specific Lkb1 knockout mice (FABP4-Lkb1) exhibited a reduced amount of WAT and postnatal growth retardation17. However, Adiponectin-Cre mediated adipocyte-specific deletion of Lkb1 resulted in normal WAT mass and body weight but enhanced BAT mass and induced browning of WAT18. As Fabp4-Cre is expressed in mature adipocytes, preadipocytes, and non-adipose tissues including endothelial cells25,26, the body weight reduction and premature death of the Fabp4-Lkb1 KO mice may be due to the role of Lkb1 in preadipocytes or non-adipose effects25,26. Muscle-specific deletion of Lkb1 caused skeletal muscle dysfunction and reduced body weight in the old mutant mice27. In our previous study, we deleted Lkb1 in muscle progenitors and muscle tissues using MyoD-Cre and found the MyoD-Lkb1 mice were born smaller and grew more slowly than their WT littermates16. But the lower body weight of the MyoD-Lkb1 mice was mainly due to the reduction of muscle mass. Mice lacking Lkb1 in the liver were smaller than WT mice and rapidly began to lose weight from around 15 days of age11. RIP2-Cre mediated deletion of Lkb1 in pancreatic β-cell decreased body weight and daily food intake, improved glycemia but did not alter insulin sensitivity21. However, other studies showed that deletion of Lkb1 in all pancreatic lineage cells28,29 did not affect body weights. Here we also found that the heart of KO mice appeared larger than that of the Lkb1flox/flox mice. Consistently, cardiac-specific deletion of Lkb1 resulted in the hypertrophy and dysfunction of heart9 but did not affect the body weight7,8. Taken together, the reduction of body weight in the TMX-inducible Lkb1 knockout mice may be mainly driven by the Lkb1 deficiency in liver or endothelial cells, and the reduction of fat mass may be a secondary effect of altered systemic energy metabolism.
We found that TMX-inducible whole-body deletion of Lkb1 in adult mice causes severe hyperglycemia and glucose intolerance. Likewise, higher fasting blood glucose levels were found in liver-specific Lkb1 deletion mice2. Mice lacking hepatic Lkb1 had glucose intolerance after injection of glucose, and normal reduction in blood glucose in response to insulin injection2. Hepatocyte-specific deletion of Lkb1 in adult mice demonstrated its critical role in control of hepatic glucose homeostasis2,10. Liver-specific ablation of Lkb1 causes increased glucose production in hepatocytes in vitro and hyperglycaemia in fasting mice in vivo30. In contrast, deletion of Lkb1 in all pancreatic lineages show marked improvements in glucose tolerance but did not affect body weight29. The blood glucose levels of FABP4-Lkb1 mice were markedly lower than those of WT mice17. However, the Adipoq-Lkb1 mice have improved glucose tolerance and insulin sensitivity18. In addition, skeletal muscle-specific deletion of Lkb1 improves glucose tolerance and affects muscle glucose uptake13,14,15. Taken together, our current and previous data suggest that the expression of Lkb1 in liver is beneficial for maintaining normal blood glucose concentrations.
We found that TMX-inducible whole-body Lkb1 knockout adult mice died within 6 weeks after TMX induction. It has been reported that Lkb1 global knockout embryos die at E8.5–11 with severe developmental defects, such as defective vasculature, and mesenchymal tissue cell death1. Likewise, Tie1-Cre or Tie2-Cre mediated ablation of Lkb1 in hematopoietic lineages and neurological tissues also results in embryonic lethality31,32. Recently, Zhang et al. reported that the endothelial cell-specific deletion of Lkb1 causes endothelial dysfunction and only about 5.6% of the KO mice survived into adulthood33. Deletion of Lkb1 in the haematopoietic system impairs haematopoietic stem cell quiescence, survival and metabolism, which results in rapid ultimately animal death4,5. The KO mice died within 30 days post completing tamoxifen injection4,5. Lkb1 plays important roles in the cardiovascular system1. Cardiac-specific deletion of Lkb1 using MHC-Cre leads to hypertrophy cardiac dysfunction and the KO mice died within 6 months of age7,9. FABP4-Lkb1 mice began to die at day 3 after birth and typically died within 3 weeks after birth17. However, Adipoq-Cre medicated deletion of Lkb1 does not affect the survival of young mice. Moreover, we found that some MyoD-Lkb1 mice began to die after weaning, and reach up to 45% mortality within 6 months16. Consistent with our findings, liver-specific knockout mice exhibited severe metabolic defects, substantial weight loss, and died within 6 weeks11,12. In addition, Lkb1 is required for hepatic canalicular membrane integrity and liver function11,12. Liver-specific deletion of Lkb1 leads to defective bile duct and canalicular network formation11,12. More recently, Porat-Shliom et al. directly demonstrated that Lkb1 plays a key role in maintaining the functional tight junction and paracellular permeability34, which may explain why there were more EB uptake in liver tissues of Rosa-Lkb1 mice compared to control mice. These findings combined with our results indicated that the lethality of Rosa-Lkb1 mice maybe mainly due to the ablation of Lkb1 in liver or haematopoietic stem cells. However, more studies are needed to examine the exact reason for the lethality of the Rosa-Lkb1 adult mice.
In conclusion, we generated a TMX-inducible whole-body Lkb1 knockout mice model and used this model to show that global deletion of Lkb1 reduces body weight, leads to hyperglycaemia and glucose intolerance, accompanied by morphological, functional and gene expression changes in the liver. Our results demonstrate that Lkb1 plays important roles in maintaining body weight, liver and adipose tissue function, blood glucose homeostasis and survival in adult mice.
Material and Methods
Animals
The Rosa26-CreER (stock number: 008463) and Lkb1flox/flox (stock number: 014143) mouse strains were bought from Jackson Laboratory (Bar Harbor, ME). Mice were housed in the animal facility with free access to water and diet. All procedures involving mice were performed in accordance with Purdue University Animal Care and Use Committee. Additionally, all experimental protocols were approved Purdue University Animal Care and Use Committee.
Indirect calorimetry study
The oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratios (RER) and heat production were measured by using indirect calorimetry system (Oxymax, Columbus Instruments) as previously described35.
Glucose tolerance tests (GTT) and insulin tolerance tests (ITT)
At 3 weeks after TMX induction, the glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were measured as previously described35. Briefly, for GTT, mice were fasted for 16 h before intraperitoneal injection of 100 mg/ml D-glucose (2 g/kg body weight). For ITT, mice were given intraperitoneal injection of human insulin (Santa Cruz) (0.75 U/kg body weight) after fasting for 4 h. After injection, the tail blood glucose concentrations were monitored.
Hematoxylin-eosin (H&E) and Oil Red O staining
Frozen livers were cut into 10 μm thick cross sections using a Leica CM1850 cryostat for H&E and Oil Red O (ORO) staining. For HE staining, liver sections were stained as previously described16. For ORO staining, liver sections were stained using Oil red O work solutions containing 6 ml stock solution (5 g/L in isopropanol) and 4 mL ddH2O for 30 min.
Total RNA extraction, cDNA synthesis and real-time PCR
Total RNA was extracted using Trizol Reagent and the concentration of total RNA was measured by Nanodrop 3000 (Thermo Fisher) at 260 nm and 280 nm. Then 5 μg of total RNA were reverse transcribed using MMLV reverse transcriptase and real-time PCR was carried out. The 18S rRNA was used as internal control and the relative expression of related genes was analyzed using 2−∆∆CT method.
Western blot analysis
Total protein was extracted using RIPA buffer16. Total proteins were separated by SDS-PAGE, transferred to PVDF membrane (Millipore Corporation) and determined with specific primary antibodies. The PPARγ2, Lkb1, FABP4 and GAPDH antibodies are from Santa Cruz Biotechnology (Santa Cruz). The horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit IgG, 111-035-003 or anti-mouse IgG; 115-035-003, Jackson ImmunoResearch) were diluted 1: 10,000. Immunodetection was performed using enhanced chemiluminescence western blotting substrate (Pierce Biotechnology) and detected with FluorChem R System (ProteinSimple).
Data Analysis
All experimental data are presented as means ± SEM. Comparisons were made by unpaired two-tailed Student’s t-tests or one-way ANOVA, as appropriate. Effects were considered significant at P < 0.05.
Additional Information
How to cite this article: Shan, T. et al. Deletion of Lkb1 in adult mice results in body weight reduction and lethality. Sci. Rep. 6, 36561; doi: 10.1038/srep36561 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ylikorkala, A. et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 293, 1323–1326, doi: 10.1126/science.1062074 (2001).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646, doi: 10.1126/science.1120781 (2005).
Nakada, D., Saunders, T. L. & Morrison, S. J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 468, 653–658, doi: 10.1038/nature09571 (2010).
Gan, B. Y. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704, doi: 10.1038/nature09595 (2010).
Gurumurthy, S. et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 468, 659–663, doi: 10.1038/nature09572 (2010).
Lizcano, J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J 23, 833–843, doi: 10.1038/sj.emboj.7600110 (2004).
Kim, G. E. et al. LKB1 deletion causes early changes in atrial channel expression and electrophysiology prior to atrial fibrillation. Cardiovascular research 108, 197–208, doi: 10.1093/cvr/cvv212 (2015).
Ozcan, C., Battaglia, E., Young, R. & Suzuki, G. LKB1 Knockout Mouse Develops Spontaneous Atrial Fibrillation and Provides Mechanistic Insights Into Human Disease Process. J Am Heart Assoc 4, doi: ARTN e001733. 10.1161/JAHA.114.001733 (2015).
Ikeda, Y. et al. Cardiac-specific Deletion of LKB1 Leads to Hypertrophy and Dysfunction. J Biol Chem 284, 35839–35849, doi: 10.1074/jbc.M109.057273 (2009).
Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120, 2355–2369, doi: 10.1172/JCI40671 (2010).
Woods, A. et al. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. Biochem J 434, 49–60, doi: 10.1042/Bj20101721 (2011).
Homolya, L. et al. LKB1/AMPK and PKA Control ABCB11 Trafficking and Polarization in Hepatocytes. Plos One 9, doi: ARTN e91921. 10.1371/journal.pone.0091921 (2014).
Koh, H. J. et al. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves, glucose homeostasis, and decreases TRB3. Mol Cell Biol 26, 8217–8227, doi: 10.1128/Mcb.00979-06 (2006).
Sakamoto, K. et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. Embo J 24, 1810–1820, doi: 10.1038/sj.emboj.7600667 (2005).
Jeppesen, J. et al. LKB1 Regulates Lipid Oxidation During Exercise Independently of AMPK. Diabetes 62, 1490–1499, doi: 10.2337/db12-1160 (2013).
Shan, T. Z. et al. Lkb1 Is Indispensable for Skeletal Muscle Development, Regeneration, and Satellite Cell Homeostasis. Stem cells 32, 2893–2907, doi: 10.1002/stem.1788 (2014).
Zhang, W. C., Wang, Q. L., Song, P. & Zou, M. H. Liver Kinase B1 Is Required for White Adipose Tissue Growth and Differentiation. Diabetes 62, 2347–2358, doi: 10.2337/db12-1229 (2013).
Shan, T. Z. et al. Lkb1 controls brown adipose tissue growth and thermogenesis by regulating the intracellular localization of CRTC3. Nat Commun 7, doi: Artn 12205. 10.1038/Ncomms12205 (2016).
Swisa, A. et al. Loss of Liver Kinase B1 (LKB1) in Beta Cells Enhances Glucose-stimulated Insulin Secretion Despite Profound Mitochondrial Defects. J Biol Chem 290, 20934–20946, doi: 10.1074/jbc.M115.639237 (2015).
Granot, Z. et al. LKB1 Regulates Pancreatic beta Cell Size, Polarity, and Function. Cell Metab 10, 296–308, doi: 10.1016/j.cmet.2009.08.010 (2009).
Sun, G. et al. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol-Endoc M 298, E1261–E1273, doi: 10.1152/ajpendo.00100.2010 (2010).
Courchet, J. et al. Terminal Axon Branching Is Regulated by the LKB1-NUAK1 Kinase Pathway via Presynaptic Mitochondrial Capture. Cell 153, 1510–1525, doi: 10.1016/j.cell.2013.05.021 (2013).
Barnes, A. P. et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549–563, doi: 10.1016/j.cell.2007.03.025 (2007).
Shan, T. Z., Zhang, P. P., Bi, P. P. & Kuang, S. H. Lkb1 Deletion Promotes Ectopic Lipid Accumulation in Muscle Progenitor Cells and Mature Muscles. J Cell Physiol 230, 1033–1041, doi: 10.1002/jcp.24831 (2015).
Lee, K. Y. et al. Lessons on Conditional Gene Targeting in Mouse Adipose Tissue. Diabetes 62, 864–874, doi: 10.2337/db12-1089 (2013).
Urs, S., Harrington, A., Liaw, L. & Small, D. Selective expression of an aP2/Fatty Acid Binding Protein4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res 15, 647–653, doi: 10.1007/s11248-006-9000-z (2006).
Thomson, D. M. et al. Skeletal muscle dysfunction in muscle-specific LKB1 knockout mice. J Appl Physiol 108, 1775–1785, doi: 10.1152/japplphysiol.01293.2009 (2010).
Sun, G. et al. LKB1 and AMPKalpha1 are required in pancreatic alpha cells for the normal regulation of glucagon secretion and responses to hypoglycemia. Molecular metabolism 4, 277–286, doi: 10.1016/j.molmet.2015.01.006 (2015).
Hezel, A. F. et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol 28, 2414–2425, doi: 10.1128/MCB.01621-07 (2008).
Patel, K. et al. The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat Commun 5, doi: Artn 453510.1038/Ncomms5535 (2014).
Ohashi, K., Ouchi, N., Higuchi, A., Shaw, R. J. & Walsh, K. LKB1 deficiency in Tie2-Cre-expressing cells impairs ischemia-induced angiogenesis. J Biol Chem 285, 22291–22298, doi: 10.1074/jbc.M110.123794 (2010).
Londesborough, A. et al. LKB1 in endothelial cells is required for angiogenesis and TGFbeta-mediated vascular smooth muscle cell recruitment. Development 135, 2331–2338, doi: 10.1242/dev.017038 (2008).
Zhang, W. C. et al. Endothelial Cell-Specific Liver Kinase B1 Deletion Causes Endothelial Dysfunction and Hypertension in Mice In Vivo. Circulation 129, 1428–1439, doi: 10.1161/Circulationaha.113.004146 (2014).
Porat-Shliom, N. et al. Liver kinase B1 regulates hepatocellular tight junction distribution and function in vivo. Hepatology 64, 1317–1329, doi: 10.1002/hep.28724 (2016).
Bi, P. et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nature medicine 20, 911–918, doi: 10.1038/nm.3615 (2014).
Acknowledgements
We thank Jun Wu for mouse colony maintenance and technical support, and members of the Kuang Laboratory for comments. Thanks to Dr. Ruth O’connor, Purdue University Student Health Center, West Lafayette, Indiana, for English corrections. The project was partially supported by funding from NIH (R01AR060652) and an incentive grant from Purdue University Office of Vice President for Research (OVPR) to SK; and the “Thousand (Young) Talents Plan” and the “Hundred Talents Program” funding from Zhejiang University to TS.
Author information
Authors and Affiliations
Contributions
T.S. and S.K. conceived the research; T.S. and Y.X. performed the experiments; T.S. and Y.X. analyzed the data; T.S. and S.K. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shan, T., Xiong, Y. & Kuang, S. Deletion of Lkb1 in adult mice results in body weight reduction and lethality. Sci Rep 6, 36561 (2016). https://doi.org/10.1038/srep36561
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36561
This article is cited by
-
Microtubule affinity-regulating kinases are potential druggable targets for Alzheimer’s disease
Cellular and Molecular Life Sciences (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.