Abstract
Emergence agitation preventive medicine should be combined with pediatric anesthesia because of the high frequency of emergence agitation. However, it is challenging to determine the most appropriate medication that can be introduced into pediatric anesthesia for the sake of emergence agitation prevention. We reviewed and retrieved the data from PubMed and Embase. Various medications were assessed based on several endpoints including Emergence agitation outcomes (EA), postoperative nausea and vomiting (PONV), the number of patients who required analgesic (RA), pediatric anesthesia emergence delirium (PAED), the extubation time, the emergency time and the duration of post-anesthesia care unit (PACU) stay. Both traditional and network meta-analysis were carried in this study. A total of 45 articles were complied with the selection criteria and the corresponding articles were reviewed. Fentanyl demonstrated the highest cumulative ranking probability which was followed by those of ketamine and dexmedetomidine with respect to EA and PAED. When PONV and RA were concerned together, clonidine exhibited the highest cumulative ranking probability compared to other medications. Our study suggested that dexmedetomidine perhaps is the most appropriate prophylactic treatment which can be introduced into anesthesia for preventing emergence agitation.
Similar content being viewed by others
Introduction
Sevoflurane has been introduced into clinical practices as an inhaled volatile anesthetic since 1992. This medication is particularly effective for inhalation induction and maintaining the effects of general anesthesia on pediatric patients due to its inherent stability, minimal respiratory pungency and minimal blood-gas partition coefficient1. Another advantage of sevoflurane is its ability to rapidly induce anesthetic effects in a controllable manner once injected.
Unfortunately, postoperative behavioral disturbance was predominantly observed in patients who received pediatric surgeries accompanied by sevoflurane as anesthetic. Another major issue caused by sevoflurane is the significant increase in the incidence of emergence agitation (EA). For instance, the incidence of emergence agitation was increased from 12–13% to 56% when sevoflurane was introduced as the main agent2,3.
Emergence agitation resulted from general anesthesia is usually characterized by either disorientation or abnormal excitation during the early stage of patient recovery. However, more severe symptoms such as sympathetic activation and arrhythmia are likely to be observed, which may further impede the recovery of patients. Some researches argued that the toxicity of sevoflurane may affect the central nervous system and trigger EA, while others suggested that other factors including age may contribute to EA4. Since sevoflurane is likely to induce EA in certain circumstances, prophylactic medicine has been introduced into sevoflurane in order to enhance the recovery of patients and reduce the risk of postoperative behavioral disturbance. Conventional prophylactic medicine includes sedative-hypnotic, opioid receptor agonist and narcotic analgesic and they have been introduced into sevoflurance in clinical practices. On the other hand, treatments for preventing EA include midazolam, dexmedetomidine, clonidine, ketamine, propofol and fentanyl and they appear to have significant difference in pharmacological characteristics. As a result, the effectiveness and safety of these treatments should be verified in clinical practices.
This study enabled us to compare the effectiveness and safety of placebo, midazolam, dexmedetomidine, clonidine, ketamine, propofol and fentanyl which are commonly introduced as prophylactic treatments. We incorporated various endpoints in our study so that both direct and indirect comparison can be comprehensively achieved.
Materials and Methods
Two phases were involved in this study. Phase one was collecting all the articles about the efficacy and safety of seven auxiliary medications that are introduced into pediatric sevoflurane anesthesia. Phase two was meta-analysis on a select group of these techniques.
Search strategy
Articles complied with the selection criteria were thoroughly searched, including PubMed, Embase and other databases. The following keywords and searching terms including their corresponding synonyms were used to retrieve the corresponding articles according to standard PICOS (population, intervention, comparison, outcome, study design) criteria: pediatric anesthesia (population), clonidine, dexmedetomidine, fentanyl, ketamine, midazolam, propofol (intervention and comparison) and randomized controlled trial (study design), emergence agitation (primary outcome).
Inclusion and exclusion criteria
Literature inclusion criteria: (1) researching type: randomized controlled trials; (2) researching objects: children between the age of six months and fourteen years who received sevoflurane as anesthetic (3) interventions: single or mixed clonidine, dexmedetomidine, fentanyl, ketamine, midazolam, propofol; (4) outcomes contain at least one of the followings: EA, postoperative nausea and vomiting (PONV), the number of patients who required analgesic, pediatric anesthesia emergence delirium (PAED), the extubation time, the emergency time and the duration of PACU stay. Literature exclusion criteria: (1) non-randomized controlled trials; (2) research objects were not complied with the inclusion criteria; (3) literatures which were not written in English; (4) duplicated literatures which were published by the same author; (5) literatures in which data integrity cannot be guaranteed. A Jadad Scale table concerning randomization, blinding and withdraw was used as an appendix to qualify the included papers (Table S1).
Outcome measures and data extraction
Data extraction was performed using a standard approach: two researchers (W. C. Wang and P. Huang) extract the corresponding data from literatures independently including the sample size and data integration was also carried out for each study. The number of paper included varied between researchers, and difference in data extraction was used for correction. Any disagreement or different opinions with respect to data extraction and integration was resolved by a third researcher (X. L. Guo).
Statistical analysis
First, we accomplished a conventional meta-analysis on the selected data. Odds ratios (OR) were selected as the appropriate statistics for comparing binary outcomes whereas standardized mean difference (SMD) were selected for comparing continuous outcomes. Apart from that, the 95% CI were also obtained in order to assess the precision of the corresponding statistics. Heterogeneity across studies was assessed by the statistic of I2 and significant heterogeneity was presented if I2 > 50%. The fixed-effect model was implemented if studies are homogeneous in nature (P-value of heterogeneity >0.05). By contrast, the random-effects were chosen in the case of significant heterogeneity (P-value for heterogeneity <0.05).
Moreover, the network meta-analysis was conducted in the same manner and the surface under the cumulative ranking curve (SUCRA) in order to rank the corresponding interventions. SUCRA, a transformation of the mean rank, provides a hierarchy of treatments and accounts for the location and variance of clinical outcomes. Higher accumulative SUCRA values indicate better treatment ranks, which is equal to 1 when the treatment is certain to be the best.
Results
Literature search results
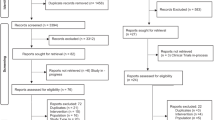
We identified a total of 1,598 publications and 537 of them were removed since they are either duplicated literatures, comments, letters and case reports. Another 605 publications were removed since they were not related to the research topic and 411 of the remaining articles contain incomplete data. As a result of this, 45 articles published from 1999 to 2015 were complied with the selection criteria (Fig. 1)5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49. A total of 4,032 cases were included and the detailed baseline characteristics of the included studies were displayed in Table 1. A Jadad Scale table concerning randomization, blinding and withdraw was used as an appendix to qualify the included papers (Table S1).
Conventional meta-analysis
We carried out conventional meta-analysis to compare the efficacy and safety of seven auxiliary medications that are introduced into pediatric sevoflurane anesthesia (Table 2). Clonidine, dexmedetomidine, fentanyl and ketamine and propofol significantly reduced the risk of EA (Fig. 2). The same approach was adopted to evaluate the relative safety of these auxiliary medications compared to placebo. Both clonidine and dexmedetomidine were associated with a decrease in the risk of PONV. Furthermore, patients with dexmedetomidine experienced a reduced risk of sedative. Fentanyl exhibited less favorable results than the placebo with respect to PONO, the emergency time and the duration of PACU stay. However, Fentanyl showed compelling results with respect to RA and PAED. Ketamine exhibited convincing results in both PAED and the emergency time. We also observed that patients treated with clonidine, dexmedetomidine, fentanyl and midazolam and propofol exhibited significantly longer emergency response time compared to placebo. Patients treated with propofol were associated with a downward trend of RA and PAED.
Network meta-analysis
We also carried out pair wise comparisons among these medications through network meta-analysis Table 3: patients treated with clonidine, dexmedetomidine, fentanyl, ketamine and propofol were less likely to have EA. Fentanyl exhibited the least favorable results with respect to PONV compared to the other six auxiliary medications whereas clonidine and dexmedetomidine exhibited more compelling results than placebo. Additionally, dexmedetomidine, fentanyl, ketamine and midazolam were less likely to result in sedatives use compared to placebo. Our study also demonstrated that dexmedetomidine, fentanyl and ketamine significantly reduced the average PAED in comparison to placebo and dexmedetomidine appeared to be more effective than clonidine with respect to PAED.
Besides, we compared the average extubation and emergency time for determine the overall safety of these medications. Patients treated with dexmedetomidine exhibited significantly longer extubation time compared to those who were given placebo. On the other hand, patients treated with clonidine, dexmedetomidine, fentanyl and ketamine and midazolam exhibited significantly shorter emergency time compared to those treated with propofol, and ketamine group had significantly shorter average emergency time compared to the midazolam group. The comparison of duration for the corresponding treatments revealed that both clonidine and fentanyl demonstrated relatively longer duration of PACU stay compared to placebo whereas such a figure in the propofol group is significantly shorter than that in the clonidine group.
The corresponding SUCRA values of seven pediatric sevoflurane anesthesia auxiliary medications with respect to each efficacy and safety endpoint were illustrated in Table 4, Fig. 3 and Figs S1a–S6a. Fentanyl had the highest cumulative ranking probability with respect to EA and PAED (EA, 88.8%; PAED, 83.9%) whereas both ketamine and dexmedetomidine demonstrated robust results with respect to EA (70.5% and 66.7%, respectively); clonidine exhibited the most compelling SUCRA values with respect to PONV and RA (PONV, 91.6%, RA, 75.0%) and ketamine ranked the best with respect to the emergency time (96.0%). More importantly, placebo exhibited the highest cumulative ranking probability with respect to the extubation time and PACU, therefore other medications may trigger several adverse effects which are reflected by longer extubation time and PACU (Extubation Time, 80.7%; the PACU, 92.2%).
Discussion
In current study, we conducted a network meta-analysis to compare the relative efficacy and safety of six prophylactic treatments including clonidine, dexmedetomidine, fentanyl, ketamine, midazolam and propofol. Our results showed that fentanyl, ketamine, and dexmedetomidine are significantly associated with a lower risk of EA and PAED together with enhanced effectiveness compared to the placebo. It appears that dexmedetomidine is more appropriate than others and such a conclusion is supported by Fang et al. reporting that dexmedetomidine was the most appropriate medication with respect to EA prevention50.
One potential explanation for above conclusion is that dexmedetomidine is an α(2)-adrenoceptor agonist with several analgesic, anxiolytic and sedative properties. It is suspected that these properties may enhance the hemodynamic stability, hence contributing to risk reduction of EA51,52. It is acknowledged that pain relief medicine is able to reduce anesthesia-related EA effectively23,29,53. However, some researchers argued that the use of general analgesic is not effective in reducing the risk of EA54. Dahmani et al. demonstrated that the sedation triggered by dexmedetomidine played a key role in reducing the risk of EA during the recovery period55. Therefore, we suspect that the reduction in the risk of EA is likely to be triggered by the analgesic and anxiolytic roles of dexmedetomidine. Apart from that, dexmedetomidine has somehow neuroprotective effects which are able to reduce neurocognitive impairment resulted from anesthetics56. Meanwhile, Robert et al. reported that the neuroprotective effect of dexmedetomidine resulted from the increase of expression levels of Mdm2 and Bcl-2, up-regulating the neurotrophic factor-Cyclic AMP response element-binding protein (BDNF-CREB) and activating the ERK signaling pathways57,58,59.
This study demonstrates that fentanyl is particularly more effective than dexmedetomidine in reducing the risk of EA and PAED. As suggested by Fenmei et al., fentanyl is able to reduce the risk of EA in a non-specific way regardless of its undiscovered relationship with postoperative pain and EA60,61,62. This may be explained by the fact that fentanyl has a durable analgesic and sedative effect. However, fentanyl has excitatory effects on the gastrointestinal smooth muscle and both patients in the fentanyl group are more likely to experience PONV and RA compared to those in the dexmedetomidine group. Furthermore, the effect of ketamine on risk reduction of PAED and EA is almost equal to that contributed by dexmedetomidine which is consistent with a study conducted by Dahmani et al.55 ketamine is an aspartate receptor antagonist which not only exhibits similar sedative and hypnotic effects to those of dexmedetomidine but also contain strong analgesic effects63,64,65,66.
This study is a network meta-analysis which compares different types of prophylactic treatments including clonidine, dexmedetomidine, fentanyl, ketamine, midazolam, and propofol. However, some limitations should be further addressed by future researchers due to the nature of network meta-analysis. For instance, there may be significant variations with respect to design, sample size and patient selection which cannot be incorporated by our network meta-analysis. Apart from that, the unequal number of interventions for each endpoint did not enable us to carry out a cluster analysis. In summary, our findings suggested that dexmedetomidine should be considered as the most appropriate prophylactic treatment that can be introduced into sevoflurane anesthesia. We recommend researchers to carry out specific following-up studies so that the long-term effects of these interventions can be discovered.
Additional Information
How to cite this article: Wang, W. et al. Efficacy and Acceptability of Different Auxiliary Drugs in Pediatric Sevoflurane Anesthesia: A Network Meta-analysis of Mixed Treatment Comparisons. Sci. Rep. 6, 36553; doi: 10.1038/srep36553 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Klastersky, J. et al. A randomized study comparing cisplatin or carboplatin with etoposide in patients with advanced non-small-cell lung cancer: European Organization for Research and Treatment of Cancer Protocol 07861. J Clin Oncol 8, 1556–1562 (1990).
Steinmetz, J. et al. Hemodynamic differences between propofol-remifentanil and sevoflurane anesthesia for repair of cleft lip and palate in infants. Paediatr Anaesth 17, 32–37 (2007).
Nakayama, S., Furukawa, H. & Yanai, H. Propofol reduces the incidence of emergence agitation in preschool-aged children as well as in school-aged children: a comparison with sevoflurane. J Anesth 21, 19–23 (2007).
Costi, D. et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 9, CD007084 (2014).
Abu-Shahwan, I. Effect of propofol on emergence behavior in children after sevoflurane general anesthesia. Paediatr Anaesth 18, 55–59 (2008).
Abu-Shahwan, I. & Chowdary, K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth 17, 846–850 (2007).
Akin, A. et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth 22, 871–876 (2012).
Almenrader, N. et al. Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatr Anaesth 17, 1143–1149 (2007).
Al-Zaben, K. R. et al. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgery. Eur J Anaesthesiol 27, 247–252 (2010).
Aouad, M. T. et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology 107, 733–738 (2007).
Bergendahl, H. T. et al. Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiol Scand 48, 1292–1300 (2004).
Binstock, W. et al. The effect of premedication with OTFC, with or without ondansetron, on postoperative agitation, and nausea and vomiting in pediatric ambulatory patients. Paediatr Anaesth 14, 759–767 (2004).
Bock, M. et al. Comparison of caudal and intravenous clonidine in the prevention of agitation after sevoflurane in children. Br J Anaesth 88, 790–796 (2002).
Bortone, L. et al. The effect of fentanyl and clonidine on early postoperative negative behavior in children: a double-blind placebo controlled trial. Paediatr Anaesth 24, 614–619 (2014).
Breschan, C. et al. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth 17, 347–352 (2007).
Chen, J. Y. et al. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth 60, 385–392 (2013).
Costi, D. et al. Transition to propofol after sevoflurane anesthesia to prevent emergence agitation: a randomized controlled trial. Paediatr Anaesth 25, 517–523 (2015).
Cravero, J. P., Beach, M., Thyr, B. & Whalen, K. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg 97, 364–367, table of contents (2003).
Dalens, B. J. et al. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth Analg 102, 1056–1061 (2006).
Demirbilek, S. et al. Effects of fentanyl on the incidence of emergence agitation in children receiving desflurane or sevoflurane anaesthesia. Eur J Anaesthesiol 21, 538–542 (2004).
Finkel, J. C. et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth Analg 92, 1164–1168 (2001).
Galinkin, J. L. et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology 93, 1378–1383 (2000).
Ghosh, S. M., Agarwala, R. B., Pandey, M. & Vajifdar, H. Efficacy of low-dose caudal clonidine in reduction of sevoflurane-induced agitation in children undergoing urogenital and lower limb surgery: a prospective randomised double-blind study. Eur J Anaesthesiol 28, 329–333 (2011).
Guler, G. et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 15, 762–766 (2005).
Ibacache, M. E., Munoz, H. R., Brandes, V. & Morales, A. L. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg 98, 60–63, table of contents (2004).
Inomata, S. et al. Effects of fentanyl infusion on tracheal intubation and emergence agitation in preschool children anaesthetized with sevoflurane. Br J Anaesth 105, 361–367 (2010).
Isik, B., Arslan, M., Tunga, A. D. & Kurtipek, O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth 16, 748–753 (2006).
Kain, Z. N. et al. Family-centered preparation for surgery improves perioperative outcomes in children: a randomized controlled trial. Anesthesiology 106, 65–74 (2007).
Kim, M. S., Moon, B. E., Kim, H. & Lee, J. R. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth 110, 274–280 (2013).
Kim, N. Y., Kim, S. Y., Yoon, H. J. & Kil, H. K. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J 55, 209–215 (2014).
Kulka, P. J., Bressem, M. & Tryba, M. Clonidine prevents sevoflurane-induced agitation in children. Anesth Analg 93, 335–338, 332nd contents page (2001).
Lankinen, U., Avela, R. & Tarkkila, P. The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesth Analg 102, 1383–1386 (2006).
Lee, C. J. et al. The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean J Anesthesiol 59, 75–81 (2010).
Lee, Y. S. et al. The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J Anesthesiol 58, 440–445 (2010).
Lili, X., Jianjun, S. & Haiyan, Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth 26, 556–561 (2012).
Lundblad, M., Marhofer, D., Eksborg, S. & Lonnqvist, P. A. Dexmedetomidine as adjunct to ilioinguinal/iliohypogastric nerve blocks for pediatric inguinal hernia repair: an exploratory randomized controlled trial. Paediatr Anaesth 25, 897–905 (2015).
Meng, Q. T. et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol 76, 1036–1041 (2012).
Ozcengiz, D., Gunes, Y. & Ozmete, O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth 25, 184–188 (2011).
Patel, A. et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg 111, 1004–1010 (2010).
Pestieau, S. R. et al. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr Anaesth 21, 1128–1135 (2011).
Rampersad, S. et al. Two-agent analgesia versus acetaminophen in children having bilateral myringotomies and tubes surgery. Paediatr Anaesth 20, 1028–1035 (2010).
Saadawy, I. et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand 53, 251–256 (2009).
Sato, M. et al. Effect of single-dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. J Anesth 24, 675–682 (2010).
Sheta, S. A., Al-Sarheed, M. A. & Abdelhalim, A. A. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth 24, 181–189 (2014).
Shukry, M., Clyde, M. C., Kalarickal, P. L. & Ramadhyani, U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth 15, 1098–1104 (2005).
Tazeroualti, N. et al. Oral clonidine vs midazolam in the prevention of sevoflurane-induced agitation in children. a prospective, randomized, controlled trial. Br J Anaesth 98, 667–671 (2007).
Tesoro, S., Mezzetti, D., Marchesini, L. & Peduto, V. A. Clonidine treatment for agitation in children after sevoflurane anesthesia. Anesth Analg 101, 1619–1622 (2005).
Tsai, P. S. et al. Ketamine but not propofol provides additional effects on attenuating sevoflurane-induced emergence agitation in midazolam premedicated pediatric patients. Paediatr Anaesth 18, 1114–1115 (2008).
Viitanen, H., Annila, P., Viitanen, M. & Tarkkila, P. Premedication with midazolam delays recovery after ambulatory sevoflurane anesthesia in children. Anesth Analg 89, 75–79 (1999).
Fang, X. Z. et al. Network Meta-Analysis on the Efficacy of Dexmedetomidine, Midazolam, Ketamine, Propofol, and Fentanyl for the Prevention of Sevoflurane-Related Emergence Agitation in Children. Am J Ther (2015).
Su, F. & Hammer, G. B. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf 10, 55–66 (2011).
Farag, E. et al. The use of dexmedetomidine in anesthesia and intensive care: a review. Curr Pharm Des 18, 6257–6265 (2012).
Kim, D. et al. Effect of ketorolac on the prevention of emergence agitation in children after sevoflurane anesthesia. Korean J Anesthesiol 64, 240–245 (2013).
Cravero, J., Surgenor, S. & Whalen, K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth 10, 419–424 (2000).
Dahmani, S. et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 104, 216–223 (2010).
Sun, L., Guo, R. & Sun, L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand 58, 642–650 (2014).
Sanders, R. D. et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 110, 1077–1085 (2009).
Engelhard, K. et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg 96, 524–531, table of contents (2003).
Wang, Q., Lu, R., Zhao, J. & Limbird, L. E. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem 281, 25948–25955 (2006).
Cohen, I. T. et al. The effect of fentanyl on the emergence characteristics after desflurane or sevoflurane anesthesia in children. Anesth Analg 94, 1178–1181, table of contents (2002).
Veyckemans, F. Excitation phenomena during sevoflurane anaesthesia in children. Curr Opin Anaesthesiol 14, 339–343 (2001).
Shi, F. et al. Effects of Fentanyl on Emergence Agitation in Children under Sevoflurane Anesthesia: Meta-Analysis of Randomized Controlled Trials. PLoS One 10, e0135244 (2015).
Warncke, T., Stubhaug, A. & Jorum, E. Ketamine, an NMDA receptor antagonist, suppresses spatial and temporal properties of burn-induced secondary hyperalgesia in man: a double-blind, cross-over comparison with morphine and placebo. Pain 72, 99–106 (1997).
Honarmand, A., Safavi, M. R. & Jamshidi, M. The preventative analgesic effect of preincisional peritonsillar infiltration of two low doses of ketamine for postoperative pain relief in children following adenotonsillectomy. A randomized, double-blind, placebo-controlled study. Paediatr Anaesth 18, 508–514 (2008).
Dahmani, S. et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth 21, 636–652 (2011).
White, P. F., Way, W. L. & Trevor, A. J. Ketamine–its pharmacology and therapeutic uses. Anesthesiology 56, 119–136 (1982).
Author information
Authors and Affiliations
Contributions
W.W., W.G. and L.C. Literature search, data extraction and manuscript writing; P.H. and F.L. Literature search and data extraction; M.Y. and X.G. Statistical analysis; W.W., F.C. and L.C. Manuscript revision and experimental design. W.W. and P.H. are responsible for the overall content as the guarantor. All authors have read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, W., Huang, P., Gao, W. et al. Efficacy and Acceptability of Different Auxiliary Drugs in Pediatric Sevoflurane Anesthesia: A Network Meta-analysis of Mixed Treatment Comparisons. Sci Rep 6, 36553 (2016). https://doi.org/10.1038/srep36553
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36553
This article is cited by
-
Comparing the effects of propofol and ketamine on the emergence agitation of male children undergoing circumcision
Annals of Pediatric Surgery (2022)
-
Efficacy and safety of intranasal ketamine compared with intranasal dexmedetomidine as a premedication before general anesthesia in pediatric patients: a systematic review and meta-analysis of randomized controlled trials
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.