Abstract
Previous studies have indicated that engineered nanomaterials can be transferred through the food chain. However, their potential ecotoxicity to the environment is not fully understood. Here, we systematically evaluated the physiological behavior and toxicity of polyvinylpyrrolidone (PVP)-coated silver nanoparticles (AgNPs) using a food chain model from Escherichia coli (E. coli) to Caenorhabditis elegans (C. elegans). Our results demonstrated that AgNPs accumulated in E. coli could be transferred to the C. elegans, and AgNPs were clearly distributed in the gut lumen, subcutaneous tissue and gonad. After being transferred to C. elegans through the food chain, the accumulated AgNPs caused serious toxicity to the higher trophic level (C. elegans), including effects on germ cell death, reproductive integrity and life span. Relative to larger particles (75 nm), small AgNPs (25 nm) more easily accumulated in the food chain and exhibited a stronger toxicity to the higher trophic level. More importantly, both the AgNPs that had accumulated in C. elegans through the food chain and the resulting impairment of germ cells could be transferred to the next generation, indicating that AgNP can cause genetic damage across generations. Our findings highlight that nanomaterials pose potential ecotoxicity to ecosystems via transport through the food chain.
Similar content being viewed by others
Introduction
Silver nanoparticles (AgNPs) and related products have been widely used in medicine and in commercialized products for their antimicrobial property1,2. Owing to the large-scale production and application of AgNP-related products, however, AgNPs will inevitably enter the environment3,4, and pose potential risks to the environment as well as to human health5,6. Many studies have demonstrated the toxic effects of AgNPs in a variety of models, such as in bacteria, mammalian cells and mice7,8,9. In a related aspect, the potential ecotoxicity of nanomaterials has become a principal object of research in recent years. For example, Roh et al. have investigated the ecotoxicity of AgNPs on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics10; by comparing the ecotoxicity of bare and coated AgNPs in the aquatic midge Chironomus riparius11, they found that the surface coating generally mitigated the toxicity of the nanoparticles. Meanwhile, Hund et al. obtained ecotoxicological data for AgNPs with the modified OECD test guidelines12.
Food chain transport is an important consideration for evaluations of the ecological toxicity of engineered nanomaterials (ENMs). There is evidence that ENMs can be transferred between trophic levels of different food chains, such as those of bacteria-protozoa13, bacteria-ciliate-rotifer14, and plant-worm15, and their distribution in predators has been confirmed using various methods. In addition, the trophic transfer of quantum dots has been demonstrated in a simplified invertebrate food web13,14. Another study reported the trophic transfer and biomagnification of gold nanoparticles from tobacco plants to tobacco hornworms15. These results demonstrate the potential trophic transfer of ENMs and raise the likelihood of human exposure through dietary uptake. Moreover, a few studies have focused on the toxicity of ENMs in food webs. For example, McTeer et al. assessed the toxicity and transfer of AgNPs in two model food chain organisms, from green algae to grazing Daphnia16. Chen et al. reported that nano-TiO2 was mildly toxic to Scenedemus obliquus, and could be transferred along the aquatic food chain with a biomagnification effect17. Chae et al. suggested the potential toxicological effects of silver nanowires in an aquatic food chain18. However, the behavior and toxicity of ENMs through food chains is not fully understood, especially the effects of specific nanomaterial properties, or their effects on subsequent generations.
C. elegans is a broadly distributed nematode species in soil ecosystems and plays a key role in nutrient cycling. Importantly, it has been widely employed as a test model in toxicology studies from the whole-animal level down to the single-cell level10,19. Moreover, C. elegans has a translucent body that can be exploited to observe the distribution of AgNPs in the organ system. Therefore, it is an excellent food chain model to elucidate the potential ecotoxicological effects of AgNPs. Here, we systematically studied the ecotoxicity of AgNPs by following their transfer from Escherichia coli to C. elegans. We found that a significant number of AgNPs that had accumulated in E. coli could be transferred to organisms at a higher trophic level (C. elegans). Moreover, the AgNP-treated E. coli cells were notably toxic to C. elegans in that they disturbed germ cell death and decreased the brood size and lifespan of the nematode. Importantly, we demonstrated that AgNPs accumulated in C. elegans through the food chain and that the impairment of germ cells could be transferred to the next generation. Our findings illustrate the potential ecotoxicity of AgNPs that are released into the environment onto food webs.
Results
Characterization of the AgNPs
The two different-sized AgNPs (25 nm and 75 nm) used in our studies were first characterized using transmission electron microscopy (TEM), and hydrodynamic diameter and zeta potential analyses, under normal and test conditions. The AgNPs were monodispersed in distilled water, with a narrow size distribution (Fig. 1a). However, after incubation in the test medium (Luria Bertani, LB) for 12 h, TEM images showed that the AgNPs had aggregated greatly and that the hydrodynamic diameter had changed to 100–1000 nm. Although AgNP aggregation was extensive in the test medium, there were still some individual nano-sized particles in solution. The AgNP zeta potential was negative in both water and the medium, indicating that the nanoparticles could resist aggregation to some extent (Fig. 1c).
Characterization of AgNPs in water or LB medium.
(a) Transmission electron microscopy (TEM) images of dispersed AgNPs in water or cultured in LB for 12 h. Scale bars equal 200 nm in water and 100 nm in LB. (b) The size distribution of AgNPs dispersed in water or LB medium as determined using dynamic light scattering (DLS). (c) Zeta potential of AgNPs in water or LB medium.
Induction of mutigenerational C. elegans germ cell death by AgNPs through the food chain
The toxicity of AgNPs to C. elegans through the food chain was first evaluated using a germ cell death assay, which represents an excellent end-point for toxicity assessment20,21. We first measured the impact of AgNPs on E. coli. As shown in Fig. S1a, E. coli growth was not significantly inhibited by either size of AgNPs at the concentrations of 1 and 5 μg/mL, but at the higher concentration of 25 μg/mL, the 25 nm nanoparticles could inhibit bacterial cell growth for up to 6 h post-treatment. However, all AgNP-treated E. coli cells reached the same stationary growth after 12 h. There was no significant reduction of colony number in response to AgNP exposure for 12 h (Fig. S1b). We further investigated the toxicity of AgNPs and Ag+ ions on E. coli using the flow cytometric method, which indicated that the AgNPs-treated E. coli cells used in our trophic transfer studies were mostly alive at the time of feeding to C. elegans (Fig. S1c). When the C. elegans worms were fed AgNP-treated E. coli, germ cell death of the nematode increased significantly in a dose-dependent manner. The 25 nm AgNPs were more toxic than the 75 nm nanoparticles (Fig. 2a), inducing significant germ cell death even at the lowest dose (1 μg/mL). Relative to the 25 nm AgNPs, the 75 nm nanoparticles did not induce obvious germ cell death until an exposure dose of up to 25 μg/mL. These results confirm that AgNPs could induce C. elegans germ cell death, and the smaller AgNPs were more toxic to germ cells than the larger nanoparticles.
AgNPs induced germ cell death in C. elegans for all generations through the food chain.
(a) Germ cell death in parental C. elegans induced by 25 nm and 75 nm AgNPs at concentrations of 1, 5 and 25 μg/mL through the food chain for 24 h. (b) Germ cell death in parental C. elegans induced by two size AgNPs at 25 μg/mL through the food chain transferred to subsequent generations (F1–F3). Only F0 was fed AgNPs-treated E. coli for 24 h. Subsequently, that F0 was moved to AgNPs-free NGM with fresh E. coli and allowed to reproduce next generations (F1 to F4). For the germ cell death assay in each generation, more than 20 C. elegans were screened in more than three independent experiments. The error bars indicate SD. Significant difference (**p < 0.01) from control, significant difference (##p < 0.01) between 25 nm and 75 nm AgNPs-treated group.
The parental generation fed AgNPs-treated E. coli showed significant differences in germ cell death compared with the control group. We also performed a post-generation assay to study the toxic effects on subsequent generations that were incubated under AgNPs-free conditions. As shown in Fig. 2b, after removal of the AgNPs from the food chain, we confirmed that damaged F0 generations could transfer these adverse effects to subsequent generations. The gonads of the F1 generation were seriously impaired, and the germ cell death of generations F2 and F3 were higher than that in the control worms. The germ cell death receded gradually up to the F4 generation in the 25 nm AgNPs-treated group, whereas the recovery of germ cell death to normal levels was confirmed in the F3 generation in the 75 nm AgNPs-treated group. The transgenerational effects of the AgNPs may result from the impairment of germ cells caused by the nanoparticles to the parental generation. These results suggest that AgNPs in the food chain are potentially toxic to subsequent generations.
AgNPs induction of germ cell death in C. elegans through the food chain is dependent on the accumulation and exclusion of AgNPs by E. coli
We have thus further demonstrated that AgNPs could induce germ cell death in C. elegans through the food chain. We hypothesized that this effect was dependent on the accumulation of AgNPs by E. coli. To confirm our hypothesis, we first exposed E. coli to AgNPs for different times before feeding to C. elegans. As expected, AgNPs-treated E. coli of early exposure times (≤2 h) had no germ cell death effect for either size of the nanoparticles. E. coli cells that had been exposed to AgNPs for 3 h resulted in a significant increase in germ cell death, but only for the 25 nm AgNPs. In the case of 75 nm AgNPs treated group, there was no significant difference in germ cell death relative to the control group up to the exposure time of 12 h (Fig. 3a). However, the toxicity of the two types of AgNPs in C. elegans reached a plateau after 12 h, indicating that the amount of AgNPs accumulated in E. coli had achieved saturation. After exposure to the AgNPs for 12 h, the E. coli cells were separated from the nanoparticles and allowed a clearance period (0–10 h) before being fed to C. elegans. There was a depuration-dependent decrease in AgNP toxicity to germ cells, where the 75 nm AgNPs did not induce germ cell death increase after a clearance period of 4 h, whereas the 25 nm AgNPs-treated group needed 8 h of depuration. No obvious increase in germ cell death was observed after 10 h of depuration for both AgNPs groups (Fig. 3b). These results indicate that the 25 nm AgNPs were taken up more easily by the E. coli cells, and depuration of the 25 nm AgNPs was slower than that of the 75 nm AgNPs in the food chain.
(a) Duration of E. coli exposure to AgNPs (25 μg/mL) on the induction of germ cell death in C. elegans through the food chain. At the indicated times, AgNPs-treated E. coli were collected to remove free AgNPs and were fed to C. elegans for 24 h. Then, the germ cell death in C. elegans was counted. (b) Depuration of AgNP-treated (25 μg/mL) E. coli cultured in NPs-free medium on the induction of germ cell death in C. elegans through the food chain. E. coli were cultured in LB containing 25 μg/mL AgNPs for 12 h. Then, the E. coli were collected to remove the AgNPs and re-cultured in AgNPs-free LB medium for the indicated times. Next, the E. coli were collected and fed to C. elegans for 24 h. Then, the germ cell death in C. elegans was counted. Significant difference (**p < 0.01) from control, significant difference (##p < 0.01) between 25 nm and 75 nm AgNPs-treated groups.
AgNPs affect the reproduction and life cycle of C. elegans through the food chain
To further confirm that AgNPs are toxic to C. elegans through the food chain, we also used brood size, population size and life span as endpoints. Brood size and population size provide an indication of the effects of a potential toxicant on development. As shown in Fig. 4a,b, C. elegans fed AgNPs-treated E. coli showed a decreased brood size. After removal of the offspring to the AgNPs-free condition, the brood size of the F1 generation (produced from F0 parents fed 5 and 25 μg/mL AgNPs-treated E. coli) was also significantly decreased compared with that of the control. Moreover, the population size, which is a consequence of both survival and all the factors that contribute to reproduction at any given time, showed a dose-dependent decrease when C. elegans worms were fed AgNP-treated E. coli (Fig. 4c). The ability of eliciting a brood size and population size decrease was in the order of 25 nm AgNPs >75 nm AgNPs, which was similar to the trend for germ cell death. These results indicate that AgNPs, with size-dependent, transferred to C. elegans through E. coli could induce a serious decrease in the reproduction rate of the parental C. elegans and F1 generation.
Comparison of the toxicological effects of AgNP to C. elegans through the food chain.
(a) Brood size assay in F0 generation. The F0 generation was fed with AgNPs-treated E. coli. At least 20 C. elegans were used in each group, and they were transferred to new plates every 48 h until reproduction ceased. The number of larvae was counted. This assay was performed in three independent experiments. (b) Brood size assay in F1 generation. F1 generation was treated under AgNPs-free condition. (c) Population growth. The population size on days 5 to 9 of worms fed E. coli treated with different sized AgNPs; 20 worms were used in this assay. (d) Life span assays. Worms were grown on NGM plates with AgNPs-treated E. coli. The mortality of each group was determined by counting the numbers of live and dead animals daily. The number of C. elegans used in each group was 60, and three independent experiments were performed. (e) The mean life span of the C. elegans in each group was calculated from the survival curves in d. Significant difference (**p < 0.01) from control, significant difference (##p < 0.01) between 25 nm and 75 nm AgNPs-treated group.
To investigate the transfer of the toxic effects of AgNPs to the lifespan of C. elegans, nematodes were fed E. coli exposed to varying concentrations of nanoparticles. As shown in Fig. 4d, size- and dose-dependent effects of the AgNPs on longevity were evident. The sharpest decrease in mean lifespan was observed in worms treated with 25 nm AgNP through the food chain compared with the control worms (8 vs. 19 days) (Fig. 4e). Our results reveal that AgNPs accumulating in species of lower trophic levels are a potential threat to higher trophic levels in the environment.
Accumulation of AgNPs in E. coli
The accumulation of different sizes of AgNPs in the food chain was studied to elucidate the possible mechanism of their toxicity. E. coli was cultivated in LB medium with AgNPs for 12 h. TEM analyses showed AgNP uptake into the E. coli cells (Fig. 5a). Moreover, energy-dispersive X-ray spectroscopy (EDS) clearly detected Ag in the E. coli cells treated with AgNPs. In addition, only small particles or aggregates of AgNPs were found in the cells. Larger aggregates were not internalized in E. coli. This was supported by the presence of large aggregates of AgNPs in the surrounding environment of the bacteria (Fig. S2). To further confirm and quantify the accumulation of AgNPs in E. coli, the total Ag content in the cells were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). To avoid the influence of large aggregates precipitating with E. coli during the centrifugation washing steps, flow cytometry was used to separate the E. coli cells from the large AgNP aggregates. After exposure to 25 μg/mL AgNPs for 12 h, 3 × 107 E. coli cells were collected, and the cellular dose of AgNPs in the E. coli was found to be 14.25 and 2.95 pg per 103 cells for the 25 nm and 75 nm AgNPs, respectively (Fig. 5b, Table S1). However, in the AgNO3 exposure control (2.5 μg/mL), ICP-AES did not detect any Ag content in the E. coli cells (data not shown). The results demonstrate that bacteria accumulate AgNPs from the surrounding medium, leading to a total cellular Ag content that is significantly higher than that in the medium with a volumetric concentration factor13 of 877 and 182 for 25 nm and 75 nm AgNPs, respectively (Table S1). These data also confirm that the small AgNPs are accumulated more easily than the larger nanoparticles in E. coli.
Accumulation of AgNPs in E. coli.
(a) TEM analysis of the intracellular distribution of 25 nm AgNPs within E. coli treated with 25 μg/mL for 12 h (Left). Scale bar equals 400 nm. EDS spectra for the AgNPs in E. coli (Right). (b) Uptake of AgNPs by E. coli. After exposure to 25 μg/mL AgNPs for 12 h, 3 × 107 E. coli cells were collected by flow cytometry, and the cellular dose of AgNPs in the E. coli was measured using ICP-AES.
Imaging and quantitation of AgNPs in C. elegans through the food chain
Since E. coli could accumulate AgNPs from the aqueous medium, we next investigated whether the AgNPs could be transferred to C. elegans through the food chain. C. elegans worms at the L4 stage (L4-larvae) were fed with AgNPs-exposed E. coli. To detect the food uptake and distribution in specific tissues, the fed worms were first examined using darkfield hyperspectral microscopy. It was obvious that the major uptake site of the AgNPs was the intestinal tract, but some AgNPs had also entered adjacent cells, including surface epithelial cells, intestinal cells, gonad cells, and eggs (Fig. S3). We further confirmed the intracellular localization of the AgNPs using TEM coupled with EDS. The TEM images revealed that some of the small AgNPs had clearly remained in the digestive lumen, subcutaneous tissue and gonad (Fig. 6a), and EDS verified the AgNPs uptake at the intracellular level. The total Ag uptake of whole C. elegans organisms was also measured using ICP-AES analysis. After collecting ~500 C. elegans worms fed 25 μg/mL AgNP-treated E. coli and digesting them in strong acids, we determined the amount of Ag accumulated in the organisms to be in the range of ~1233 and 346 pg per worm for the 25 nm and 75 nm AgNPs, respectively (Fig. 6b, Table S1). We also calculated the volume-based and dry-mass-based cellular Ag concentrations, which allowed us to calculate the trophic transfer factor (TTF) (Table S1). The TTF was defined as the ratio of the Ag concentration in C. elegans at final trophic transfer to that in E. coli at initial trophic transfer. The TTF values were <1, indicating that AgNP biomagnification by the C. elegans had not occurred.
Uptake and accumulation of AgNPs in C. elegans through the food chain.
(a) TEM images of intracellular localization of AgNPs in C. elegans fed AgNPs-treated E. coli included the subcutaneous tissue, gut lumen, and gonad. The red arrows indicate AgNPs. (b) Accumulation of AgNPs in C. elegans through the food chain. After being fed AgNP-treated E. coli for 10 d, 500 worms were selected, and the cellular dose of AgNPs in C. elegans was measured using ICP-AES. (c) AgNPs that had accumulated in C. elegans through the food chain were passed down from parent to the offspring. Data are shown for the F0 generation fed AgNP-treated E. coli and generations F1 to F4 fed E. coli not treated with AgNPs. On average, more than 500 C. elegans were collected from generations F0 to F4, and total Ag content was determined using ICP-AES in two independent experiments. The error bars indicate SD.
Notably, after removal of subsequent generations to the AgNPs-free condition, a small amount of Agwas still detected in the F1 generation that had been produced from parents exposed to 25 nm AgNP-treated E. coli (Fig. 6c); this may be explained by the presence of AgNPs in the eggs, found using darkfield hyperspectral microscopy (Fig. S3). This result revealed that the smaller AgNPs had bioconcentrated more easily in C. elegans, which was consistent with the results obtained in E. coli. These combined data provide strong evidence for the trophic transfer of AgNPs from E. coli to C. elegans and suggest that differences in the accumulation of the two sizes of AgNPs by C. elegans is a likely cause of the observed size-dependent toxicity in this nematode through the food chain.
Discussion
Up to now, there is no doubt that AgNPs exert toxicity to bacteria and other organisms, but little is known of their potential ecotoxicity to the environment, especially of the effects of specific nanomaterial properties. In the present study, we established a simplified experimental food chain using a predator (C. elegans) and a bacterial prey (E. coli) to investigate the ecotoxicological effects of AgNPs. The exposure concentrations of AgNPs were in the range of 1–25 μg/mL, based primarily on previous studies of AgNPs as antibacterial agents22,23. Our results demonstrated that AgNPs transferred to C. elegans through the food chain clearly induced toxicity in the gonad cells, rendering deleterious effects on the reproduction rate and life span of the worms. More importantly, the damage caused by AgNPs could transfer to subsequent generations, affected either directly by the nanoparticles themselves or through impairment of germ cells in the parental generation, highlighting the potential genetic toxicity of AgNPs in the food chain.
The characteristics of AgNPs within the test medium are important factors influencing their toxic exposure in the environment. The dissolution and aggregation behaviors of AgNPs are dependent on surface coating. Polyvinylpyrrolidone (PVP) is an environmentally friendly polymer that stabilizes AgNPs via steric repulsion. PVP-AgNPs are very stable in the environment and are therefore less toxicity to ecological systems24. Our data showed that AgNP aggregation was extensive after incubation in the test medium for 12 h, but there were still some individual nano-sized particles in solution. We also found only the small-sized AgNPs could be more easily accumulated in E. coli (Fig. 5 and Fig. S2). There is evidence that small AgNPs can more easily penetrate gram-negative bacteria than larger AgNPs or AgNPs aggregates. For example, a study by Chamakura et al. using TEM combined with elemental analysis showed that AgNPs within a size range of 10–50 nm were not found in gram-negative E. coli after 2 h of exposure25. Using the same method, Yuan et al. suggested that AgNPs with sizes between 4 and 10 nm had the ability to gain access beyond the lipid bilayer and enter gram-negative bacteria26. Although these studies suggested that particle size was a contributing factor to the ability of AgNPs to enter E. coli, they have focused mainly on the direct interactions of AgNPs with the organisms. There is little information and only a few reports on the transport of nanoparticles through the food chain27. In our study, trophic transfer of AgNPs between the bacterial prey and C. elegans was observed. Interestingly, only small nanoparticles were found in the subcutaneous tissue, gut lumen and gonad (Fig. 6). Our results demonstrated that the small (25 nm) AgNPs easily penetrated cells of various tissues through the intestinal wall. The tissue-specific distribution of nanomaterials in C. elegans has been reported in some direct exposure studies28,29. We have also provided direct evidence of the trophic transfer and intracellular localization of AgNPs. More importantly, the AgNPs that had accumulated in C. elegans through the food chain could be transferred to the next generation (Fig. 6c), indicating that AgNP exposure through the food chain can cause toxicity to subsequent generations. However, as shown in Table S1, AgNPs biomagnification by C. elegans is unlikely to occur, with the TTF values being < 1. Using the blood-worm as a food chain model, Yoo-iam et al. have also demonstrated that the food chain transfer of AgNPs occurred only in the lower trophic groups and there was no evidence of biomagnification from food sources to consumers in their simple trophic food chain model30. As in our model, this may because the depuration rates of C. elegans are much greater, or perhaps the period of AgNPs exposure to C. elegans may not have been long enough for biomagnification to occur though the food chain, a fact that needs to be further clarified.
Although several studies have examined the short-term acute toxic effects of AgNPs over the past decades, work on the chronic toxicity of AgNPs within lower trophic organisms is limited. In C. elegans, aspects of the life cycle, including germ cell death, brood size, population growth rate, and lifespan, all vary as a consequence of food quantity and quality or conditions of environmental stress31. In order to explore the genotoxicity of AgNPs for C. elegans in the food chain, we used germ cell death, brood size, population growth rate, and lifes pan to evaluate two sizes of AgNPs. We showed that 1 μg/mL of 25 nm AgNPs, rather than 75 nm AgNPs, could cause germ cell death and reproduction inhibition and consequently affect the population growth of C. elegans (Figs 2 and 4). Many direct-exposure studies have reported that small AgNPs were more toxic than large particles. For example, Liu et al. found that 5 nm AgNPs were more toxic than 20 and 50 nm AgNPs to human cells32. Wang et al. also found that 20 nm PVP-AgNPs induced more cellular toxicity than 110 nm AgNPs33. Moreover, Gliga et al. reported that 10 nm AgNPs were much more cytotoxic than 40 and 75 nm AgNPs to human lung cells. However, there was no difference in toxicity between the 10 nm citrate- and 10 nm PVP-coated AgNPs34. These results indicated that the toxicity of AgNPs, not only in direct exposure but also through the food chain, was dependent on size. In addition, there was a time effect of AgNPs exposure to E. coli in the food chain. We found that germ cell death was induced drastically with the exposure time extended, where the 25 nm AgNPs exerted toxicity to C. elegans only after they had been exposed to E. coli for 3 h. With regard to the depuration period, the 25 nm AgNPs-treated group also needed the longest time to eliminate the toxic effects of AgNPs, which indicated that it was more difficult for the small-sized AgNPs to be excluded from the E. coli cell (Fig. 3). Indeed, the accumulation and depuration of nanoparticles in lower species are more variable, and are related to the surface chemistry and aggregate size of the nanoparticles14. These results suggest that the exposure and depuration durations should be considered as potentially important factors in the toxicity of AgNPs to C. elegans through the food chain.
We also investigated the generational transfer and multigenerational toxicity of AgNPs in C. elegans, where we found that only 25 nm AgNPs could be detected in F1 generations. The germ cell death caused by the 25 nm AgNPs was clearly increased in the F2 and F3 generations but then gradually recovered in the F4 generation. Previous reports have suggested that AgNPs could distribute to embryos and modify the embryonic development of vertebrates35,36. There was evidence that nanoparticles, which permeated the gonad of C. elegans, could be passed to the next generation and exert potentially toxic effects on their postembryonic development19,28. A recent study has revealed that AgNPs (10 nm) could significantly reduce the lifespan of parent nematodes (F0) as well as those of 3 subsequent generations (F1–F3)37. These phenomena may be due to the AgNPs that had accumulated in C. elegans through the food chain and the resulting transfer of impaired germ cell.
Although there is no doubt that the Ag ions can be released from AgNPs, there is no conclusive answer to date on the exact source of the AgNPs-mediated toxicity: that is whether the toxicity is due to particle-specific effects or released Ag ion effects. Previous studies have shown that Ag ions govern the toxicity of AgNPs towards bacteria22. Yang et al. reported that AgNPs toxicity was dependent on released Ag ions and AgNPs with higher Ag release were more toxic to C. elegans38. In our experiment, the amount of Ag released into solution after 12 h incubation of AgNPs in the test medium was 1 μg/mL, which is 4% of that released from 25 μg/mL AgNPs added (Fig. S4a). In order to investigate whether the released Ag ionic species could cause toxicity in the food chain, the E. coli was exposed to 2.5 μg/mL AgNO3 (representing 10% of Ag released from 25 μg/mL of AgNPs) and then fed to C. elegans. There was no significant effect on the germ cell death of C. elegans through the food chain (Fig. S4b), suggesting that the toxic effects were not related to Ag ion release. We also observed that large aggregates of AgNPs that had formed in the LB medium would coprecipitate during E. coli collection. These large aggregates may be toxic to C. elegans through direct interactions. However, we found that germ cell death was not significantly different between large-aggregate-treated C. elegans and the controls (Fig. S4c). Therefore, we confirmed that all toxic effects towards C. elegans were directly due to the AgNPs transferred by E. coli through the food chain. Overall, our results clearly showed that AgNPs could sizd-dependently be accumulated though the food chain and cause toxicity to high trophic levels.
Methods
Preparation and characterization of AgNPs
Two different sized AgNPs were investigated in our study. The 25 and 75 nm PVP BioPureTM AgNPs were obtained from NanoComposix, Inc. (San Diego, CA, USA) in the form of stock dispersions (5 mg/mL, PVP coated). The morphology of the AgNPs was observed using transmission electron microscopy (TEM, JEM-2011, Japan). The hydrodynamic diameter and zeta potential of the AgNPs were characterized using a Zetasizer (Malvern Nano series, Malvern, UK).
C. elegans culture conditions
Wild-type N2 C. elegans worms maintained in our laboratory were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, USA) and were maintained at 20 °C on a standard nematode growth medium (NGM) with living Escherichia coli OP50 bacteria as a food source. All manipulations of C. elegans were carried out according to the standard procedures.
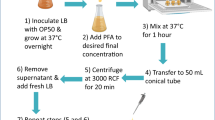
E. coli exposed to AgNPs and fed to C. elegans in the food chain experiment
To investigate the uptake and transfer of AgNPs in a food chain, we examined a simplified invertebrate food chain using C. elegans, and E. coli strain OP50 was chosen as the food source. First, E. coli OP50 was cultured in 5 ml of LB medium overnight (37 °C, 200 rpm). Then, 200 μL of the E. coli that had been cultured overnight was added to 10 mL of LB with different concentrations of AgNPs (0, 1, 5, 25 μg/mL) and was incubated at 37 °C for 12 h at 200 rpm. After being exposed for 12 h, treated E. coli were collected at 5000 rpm for 5 min at 4 °C and were then washed 3 times with deionized water. The final pellets were resuspended into 1 mL of deionized water. Then, an aliquot (100 μL) of the collected E. coli was dropped onto the surface of NGM plates (35 mm dishes). The plates were dried at 20 °C to evaporate the moisture from the NGM medium. Then, worms were placed onto NGM plates seeded with treated E. coli and were incubated at 20 °C until the toxicity assays were performed.
Germ cell death, brood size, population size and lifespan assays for the assessment of AgNP toxicity Germ cell death assay
Germ cell death was measured using acridine orange (AO, Sigma)39. L4 larvae (50–60) were fed AgNP-treated E. coli for 24 h. Then, the treated worms were strained into 500 μL of AO (25 μg/mL) for 1 h in the dark at 20 °C. They were then transferred to a new NGM plate and allowed to recover for 40 min on bacterial lawns. Dead cells stained positive for AO were counted using an Olympus IX71 fluorescence microscope (Olympus, Japan).
Brood size assay
The procedures for brood size assay were conducted as described previously40. Wild-type L1 larvae were fed AgNP-treated E. coli and were subsequently transferred onto a new plate with treated E. coli every day. The number of newly hatched larvae was counted. This procedure was repeated until the mother worms stopped laying eggs. The brood size was calculated by combining the numbers of hatched larvae.
Population size assay
Population growth was measured according to a method described in a previous report31. In brief, 20 randomly selected worms were individually placed on an NGM plate seeded with AgNP-treated E. coli. The population that grew from these individual worms was assessed from 5 to 9 days.
Life span assay
The same AgNP-treated E. coli feeding procedures were followed for the life span assay. Every day, worms were transferred to a new AgNP-treated E. coli plate and until it could be confirmed that they were dead, i.e., when they would not respond to being tapped with a pick.
Observation of AgNP distribution in the food chain
C. elegans with AgNPs were imaged using hyperspectral imagery with enhanced darkfield microscopy (CytoViva, Inc.) as previously described41,42. A high-resolution darkfield condenser was directly positioned on a glass slide upon the work stage with an immersion solution and was focused until a donut ring-type light pattern was obtained. All the images were captured using a CCD camera (Olympus BX51, Japan).
AgNP distribution in the E. coli and C. elegans was also confirmed using TEM equipped with energy dispersive spectroscopy (EDS). After being exposed to AgNPs, E. coli cells and C. elegans were collected and fixed with 2.5% glutaraldehyde. Then, the samples was washed 3 times with 0.1 M phosphate buffer and postfixed with 1% osmium tetroxide in phosphate buffer. The fixed samples were then dehydrated in ethanol and embedded in Epon 812 resin. The resin specimens were sectioned using an ultramicrotome with a diamond knife. The thin sections were observed using TEM equipped with EDS.
Calculations of the concentration of the total Ag used in the food chain experiment
To avoid the influence of large aggregates precipitating with E. coli during the centrifugation washing steps, flow cytometry was used to separate the E. coli from the AgNP suspensions. E. coli OP50 expressing a GFP (OP50-GFP, from CGC) were used in the AgNP treatment. After exposure, the OP50-GFP cells were washed 3 times with deionized water. The final pellets were resuspended in deionized water. The flow cytometry procedure was modified according to a previous description43. In brief, a laser output power of 0.5 W was used for measuring forward scatter (FSC), side scatter (SSC) and green fluorescence intensity (FL1). For sorting out the OP50-GFP cells, a 488 nm argon laser was used for excitation. The drop drive frequency was set to ~26 kHz, and the system threshold rate was kept under 8000 s−1. Finally, 3 × 107 E. coli were collected for each sample. The collected samples were acidified with 10% HNO3 and allowed to digest at 37 °C overnight prior to the quantification of silver concentration by inductively coupled plasma mass spectrometry (ICP-AES, PlasmaQuad3, VG Elemental). For quantitation of the total Ag in the C. elegans, the worms were fed AgNP-treated E. coli OP50. After the indicated times, 500 worms were selected and analyzed using ICP-MS.
The method used for the calculation of the trophic transfer factor (TTF) was modified according to a previous study13. The total masses of Ag out of the mass of the counted organisms, the E. coli and worms, were measured using ICP-AES. Ag mass per cell or worm was determined by dividing these values by the organism count in each sample. Volume-based cellular Ag concentrations were then calculated by dividing the Ag mass per organism by the organism volume. The volumes of the E. coli and worms were 0.65 μm3 and 5 × 106 μm3, respectively, as reported in the literature44,45. E. coli dry mass (0.71 pg) was calculated by multiplying the cell volume (0.65 μm3) by cell density (1.09 mg/μL)46. For the worms, a cellular dry mass of 5 μg was estimated from 1000 worms using a microbalance. TTF was determined as the worm: E. coli ratios of cellular Ag concentrations on a volume basis and on a dry mass basis, where cellular Ag concentration of C. elegans was Ag in C. elegans at final trophic transfer experiment (C.ele 5d in Table S1 indicated Ag concentration in worms which fed with AgNPs-treated E. coli for 5 days), and cellular Ag concentration of E. coli was Ag in E. coli at initial trophic transfer experiment (E. coli exposed to AgNPs for 12 h and fed to C. elegans to start trophic transfer experiment was defined E. coli 0 h in Table S1). The two TFFs (volume and dry mass based) for each treatment differed by less than 10%, and an average was obtained.
Statistical analysis
All the results are expressed as the mean ± SD. One-way analysis of variance (ANOVA) was used to analyze the mean differences among groups compared with the control groups, and two-way ANOVA was used to compare the mean differences between groups. The criterion for statistical significance was p < 0.05.
Additional Information
How to cite this article: Luo, X. et al. Insights into the Ecotoxicity of Silver Nanoparticles Transferred from Escherichia coli to Caenorhabditis elegans. Sci. Rep. 6, 36465; doi: 10.1038/srep36465 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahamed, M., Alsalhi, M. S. & Siddiqui, M. K. Silver nanoparticle applications and human health. Clin. Chim. Acta 411, 1841–1848 (2010).
Sintubin, L., Verstraete, W. & Boon, N. Biologically produced nanosilver: current state and future perspectives. Biotechnol. Bioeng. 109, 2422–2436 (2012).
Seltenrich, N. Nanosilver: weighing the risks and benefits. Environ. Health Perspect. 121, A220–A225 (2013).
Maurer-Jones, M. A., Gunsolus, I. L., Murphy, C. J. & Haynes, C. L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 85, 3036–3049 (2013).
Wijnhoven, S. W. P. et al. Nano-silver - a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3, 109–138 (2009).
Massarsky, A., Trudeau, V. L. & Moon, T. W. Predicting the environmental impact of nanosilver. Environ. Toxicol. Pharmacol. 38, 861–873 (2014).
Li, W. R. et al. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 85, 1115–1122 (2010).
Rahman, M. F. et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 187, 15–21 (2009).
Asharani, P. V., Hande, M. P. & Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 10, 65 (2009).
Roh, J. Y. et al. Ecotoxicity of Silver Nanoparticles on the Soil Nematode Caenorhabditis elegans Using Functional Ecotoxicogenomics. Environ.Sci. Technol. 43, 3933–3940 (2009).
Park, S. Y. et al. Ecotoxicity of bare and coated silver nanoparticles in the aquatic midge, Chironomus riparius. Environ. Toxicol. Chem. 34, 2023–2032 (2015).
Hund-Rinke, K. et al. Regulatory Ecotoxicity Testing of Nanomaterials - Proposed Modifications of OECD Test Guidelines Based on Laboratory Experience with Silver and Titanium Dioxide nanoparticles. Nanotoxicology. 1–16 (2016).
Werlin, R. et al. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat. Nanotechnol. 6, 65–71 (2011).
Holbrook, R. D., Murphy, K. E., Morrow, J. B. & Cole, K. D. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat. Nanotechnol. 3, 352–355 (2008).
Judy, J. D., Unrine, J. M. & Bertsch, P. M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ. Sci. Technol. 45, 776–781 (2011).
McTeer, J., Dean, A. P., White, K. N. & Pittman, J. K. Bioaccumulation of silver nanoparticles into Daphnia magna from a freshwater algal diet and the impact of phosphate availability. Nanotoxicology 8, 305–316 (2014).
Chen, J., Li, H., Han, X. & Wei, X. Transmission and Accumulation of Nano-TiO2 in a 2-Step Food Chain (Scenedesmus obliquus to Daphnia magna). Bull Environ. Contam. Toxicol. 95, 145–149 (2015).
Chae, Y. & An, Y. J. Toxicity and transfer of polyvinylpyrrolidone-coated silver nanowires in an aquatic food chain consisting of algae, water fleas, and zebrafish. Aquat. Toxicol. 173, 94–104 (2016).
Kim, S. W., Kwak, J. I. & An, Y. J. Multigenerational study of gold nanoparticles in Caenorhabditis elegans: transgenerational effect of maternal exposure. Environ. Sci. Technol. 47, 5393–5399 (2013).
Guo, X. et al. Synergistic effects induced by a low dose of diesel particulate extract and ultraviolet-A in Caenorhabditis elegans: DNA damage-triggered germ cell apoptosis. Chem. Res. Toxicol. 27, 990–1001 (2014).
Du, H. et al. Endosulfan isomers and sulfate metabolite induced reproductive toxicity in Caenorhabditis elegans involves genotoxic response genes. Environ. Sci. Technol. 49, 2460–2468 (2015).
Xiu, Z. M., Zhang, Q. B., Puppala, H. L., Colvin, V. L. & Alvarez, P. J. J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 12, 4271–4275 (2012).
Chernousova, S. & Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Edit 52, 1636–1653 (2013).
Li, Y., Zhang, W., Niu, J. & Chen, Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 47, 10293–10301 (2013).
Chamakura, K., Perez-Ballestero, R., Luo, Z., Bashir, S. & Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 84, 88–96 (2011).
Yuan, Z. et al. Interaction of silver nanoparticles with pure nitrifying bacteria. Chemosphere 90, 1404–1411 (2013).
Hou, W. C., Westerhoff, P. & Posner, J. D. Biological accumulation of engineered nanomaterials: a review of current knowledge. Environ. Sci. Process Impacts 15, 103–122 (2013).
Mohan, N., Chen, C. S., Hsieh, H. H., Wu, Y. C. & Chang, H. C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 10, 3692–3699 (2010).
Qu, Y. et al. Full assessment of fate and physiological behavior of quantum dots utilizing Caenorhabditis elegans as a model organism. Nano Lett. 11, 3174–3183 (2011).
Yoo-iam, M., Chaichana, R. & Satapanajaru, T. Toxicity, bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem. Spec. Bioavailab. 26, 257–265 (2014).
Harvey, S. C., Shorto, A. & Viney, M. E. Quantitative genetic analysis of life-history traits of Caenorhabditis elegans in stressful environments. BMC Evol. Biol. 8, 15 (2008).
Liu, W. et al. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4, 319–330 (2010).
Wang, X. et al. Use of Coated Silver Nanoparticles to Understand the Relationship of Particle Dissolution and Bioavailability to Cell and Lung Toxicological Potential. Small 10, 385–398 (2014).
Gliga, A. R., Skoglund, S., Wallinder, I. O., Fadeel, B. & Karlsson, H. L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 11, 11 (2014).
Ahamed, M. et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 233, 404–410 (2008).
Austin, C. A. et al. Distribution of silver nanoparticles in pregnant mice and developing embryos. Nanotoxicology 6, 912–922 (2012).
Contreras, E. Q., Puppala, H. L., Escalera, G., Zhong, W. & Colvin, V. L. Size-dependent impacts of silver nanoparticles on the lifespan, fertility, growth, and locomotion of Caenorhabditis elegans. Environ. Toxicol. Chem. 33, 2716–2723 (2014).
Yang, X. Y. et al. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis elegans. Environ. Sci. Technol. 46, 1119–1127 (2012).
Kelly, K. O., Dernburg, A. F., Stanfield, G. M. & Villeneuve, A. M. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156, 617–630 (2000).
Craig, A. L., Moser, S. C., Bailly, A. P. & Gartner, A. Methods for studying the DNA damage response in the Caenorhabdatis elegans germ line. Methods Cell Biol. 107, 321–352 (2012).
Badireddy, A. R., Wiesner, M. R. & Liu, J. Detection, characterization, and abundance of engineered nanoparticles in complex waters by hyperspectral imagery with enhanced Darkfield microscopy. Environ. Sci. Technol. 46, 10081–10088 (2012).
Yang, X. et al. Silver nanoparticle behavior, uptake, and toxicity in Caenorhabditis elegans: effects of natural organic matter. Environ. Sci. Technol. 48, 3486–3495 (2014).
Uchiyama, T. & Watanabe, K. Substrate-induced gene expression (SIGEX) screening of metagenome libraries. Nat Protoc. 3, 1202–1212 (2008).
Kubitschek, H. E. Cell volume increase in Escherichia coli after shifts to richer media. J. Bacteriol. 172, 94–101 (1990).
McCulloch, D. & Gems, D. Body size, insulin/IGF signaling and aging in the nematode Caenorhabditis elegans. Exp. Gerontol. 38, 129–136 (2003).
Kubitschek, H. E., Baldwin, W. W., Schroeter, S. J. & Graetzer, R. Independence of buoyant cell density and growth rate in Escherichia coli. J. Bacteriol. 158, 296–299 (1984).
Acknowledgements
This work was supported by the National Basic Research 973 Program (2014CB932002), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14030502), and the National Natural Science Foundation of China (81301182).
Author information
Authors and Affiliations
Contributions
X.L., S.M.X. and L.J.W. conceived and designed the experiments. X.L. executed the trophic transfer and toxicity experiments, and Y.N.Y. performed imaging and the ICP-AES analysis. L.Z.L. and S.P.C. performed flow cytometer analysis. A.X. commented on the project. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Luo, X., Xu, S., Yang, Y. et al. Insights into the Ecotoxicity of Silver Nanoparticles Transferred from Escherichia coli to Caenorhabditis elegans. Sci Rep 6, 36465 (2016). https://doi.org/10.1038/srep36465
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36465
This article is cited by
-
Impact of co-exposure to titanium dioxide nanoparticles (TiO2 NPs) and lead (Pb) on African catfish Clarias gariepinus (Burchell, 1922) fed contaminated copepods (Eucyclop sp.)
Environmental Science and Pollution Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.