Abstract
Conjunctival goblet cells synthesize and secrete mucins which play an important role in protecting the ocular surface. Pro-resolution mediators, such as lipoxin A4 (LXA4), are produced during inflammation returning the tissue to homeostasis and are also produced in non-inflamed tissues. The purpose of this study was to determine the actions of LXA4 on cultured human conjunctival goblet cell mucin secretion and increase in intracellular [Ca2+] ([Ca2+]i) and on histamine-stimulated responses. LXA4 increased mucin secretion and [Ca2+]i, and activated ERK1/2 in human goblet cells. Addition of LXA4 before resolvin D1 (RvD1) decreased RvD1 responses though RvD1 did not block LXA4 responses. LXA4 inhibited histamine-stimulated increases in mucin secretion, [Ca2+]i, and ERK1/2 activation through activation of β-adrenergic receptor kinase 1. We conclude that conjunctival goblet cells respond to LXA4 through the ALX/FPR2 receptor to maintain homeostasis of the ocular surface and regulate histamine responses and could provide a new therapeutic approach for allergic conjunctivitis and dry eye diseases.
Similar content being viewed by others
Introduction
Conjunctival goblet cells are specialized cells that span the thickness of the conjunctiva from the ocular surface to the stroma. These cells synthesize and secrete mucins which include the gel forming mucin MUC5AC in humans and in rats1,2. These mucins are responsible for maintenance of ocular surface hydration, lubrication, and prevention of destructive interaction of foreign bodies and pathogens with the conjunctiva. Goblet cells also play a role in the innate immune response of the conjunctiva and can be activated by cytokines produced during inflammation3,4.
In the context of the ocular surface, the types of inflammation observed include seasonal allergic conjunctivitis, and dry eye syndrome5,6. Allergic conjunctivitis alone affects 15–25% of Americans6. Dry eye disease is a chronic, multifactorial disease and can be a result of graft vs host disease, autoimmune diseases, normal aging or refractive and cataract surgeries7,8,9,10,11. It has been estimated that the overall cost of dry eye disease treatment in the United States is more than $3.8 billion though that number is likely underestimated12. Uncontrolled inflammation is a hallmark of these ocular surface diseases causing redness, itching, and discomfort and creating a significant impact on quality of life. There are few effective treatments for these diseases and most are only palliative.
During the allergic response, mast cells are recruited to the ocular surface, degranulate, and release histamine and leukotrienes (LT)13,14. We previously showed that goblet cells of the conjunctiva play an active role in the response of the ocular surface to histamine and leukotriene challenge15,16,17. All four receptors (H1-H4) for histamine as well as cysteinyl leukotriene receptors, CysLT1 and CysLT2, are expressed in goblet cells17. Activation of each of the these receptor subtypes caused an increase in intracellular [Ca2+] ([Ca2+]i) and high molecular weight glycoconjugate secretion including MUC5AC15,17.
Termination of inflammation occurs with the biosynthesis of the specialized pro-resolution mediators (SPMs) resolvins (Rvs), lipoxins (LX), maresins, and protectins from omega-3 and -6 essential fatty acids18. These resolution-phase mediators alter the magnitude and the duration of the inflammatory response through mechanisms involving counter regulation of inflammatory mediators as well as phagocytosis of microbes and cell debris18. Recent evidence suggests that LXs and Rvs also play a role under normal, physiological conditions19,20,21,22,23. In conjunctival goblet cells, RvD1 and its epimer aspirin-triggered RvD1 (AT-RvD1), and RvE1 appear to have two functions (1) alone these compounds increase [Ca2+]i, activate extracellular regulated kinase (ERK) 1/2, and stimulate mucin secretion and (2) block LTD4- and histamine-stimulated increase in [Ca2+]i and mucin secretion19,20.
LXA4 and lipoxin B4 (LXB4) are both biosynthesized from arachidonic acid. LXA4 binds to the ALX/FPR2 receptor causing a conformational change leading to stimulation of pro-resolution pathways24. Similar to RvD1 and AT-RvD1, LXA4, LXB4, and several stable analogs of LXA4 alone increased [Ca2+]i in conjunctival goblet cells from rats. LXA4 also increased mucin secretion utilizing the signaling pathways of phospholipase C, -D, and A219. The increase in [Ca2+]i stimulated by LXA4 was directly linked to mucin secretion as chelation of intracellular Ca2+ blocked secretion19. In the present study, we investigated the actions of LXA4 with cultured human conjunctival goblet cells, as well as the impact of LXA4 on histamine-stimulated increase in [Ca2+]i, mucin secretion, and ERK 1/2 activation in rat and human goblet cells. In human goblet cells, LXA4 binds to the ALX/FPR2 and GPR32 receptors while RvD1 binds to GPR32 receptors. In rat, LXA4 and RvD1 preferentially bind to the ALX/FPR2 receptor. In addition, we report that LXA4 utilizes β-adrenergic receptor kinase (βARK) 1 to counter-regulate the H1 histamine receptor.
Results
Action of LXA4 on [Ca2+]i and Protein Secretion in Human Goblet Cells
We previously demonstrated that LXA4 stimulated an increase in [Ca2+]i and mucin secretion in rat goblet cells19. To investigate the actions of LXA4 on goblet cells grown from human conjunctiva, cells were stimulated with LXA4 from 10−11 M–10−9 M and the increase in [Ca2+]i was measured. Pseudo colored images of goblet cells treated with increasing concentrations of LXA4 are shown in Fig. 1A. LXA4 increased [Ca2+]i in a concentration dependent manner with increases in peak [Ca2+]i of 97.6 ± 31.2, 762.0 ± 259.4, and 50.0 ± 14.4 nM at 10−11, 10−10, and 10−9 M, respectively (Fig. 1B,D). The increase at 10−10 M LXA4 was increased above basal (p = 0.03).
Lipoxin A4 (LXA4) Increases Intracellular [Ca2+] ([Ca2+]i) in Human Conjunctival Goblet Cells.
Pseudo-color images of goblet cells stimulated with increasing concentrations of LXA4 (10−11–10−9 M) is shown in (A). Effect of LXA4 (black line, LXA4 10−11 M; red line, LXA4 10−10 M; green line, LXA4 10−9 M) on [Ca2+]i over time is shown in (B) Goblet cells were preincubated with BOC-2 (10−4 M) for 30 min prior to addition of LXA4. and [Ca2+]i was measured. Effect over time with LXA4 10−10 M is shown in (C) Change in peak [Ca2+]i was determined (closed triangles) and is shown in (D). Change in peak [Ca2+]i was calculated and is shown in (D). Data are either the mean of the response over time from 3 individuals (B) or individual values or mean ± SD from the same 3 individuals (D).
LXA4 has been shown to bind to the ALX/FPR2 receptor in goblet cells isolated from rat conjunctiva19. To determine whether LXA4 acts via ALX/FPR2 receptors in human goblet cells, goblet cells were pretreated with the ALX/FPR2 antagonist BOC-2 (10−4 M) for 30 min, prior to addition of LXA4 (10−10 M). Similar to rat goblet cells, in cultured human cells BOC-2 inhibited LXA4-stimulated increase in [Ca2+]i at 10−10 M by 98.5 ± 1.1% to 13.3 ± 11.7 nM (p=0.05) (Fig. 1C,D).
Next, the actions of LXA4 on glycoprotein secretion from cultured human goblet cells was determined. Goblet cells were serum-starved for 2 h and LXA4 was added (10−10–10−8 M) for 2 h and glycoconjugate secretion measured. LXA4 (10−9 M) increased secretion 2.6 ± 0.1 fold above basal (p = 0.01, Fig. 2). In cells from the same individuals, histamine, as a positive control, increased glycoconjugate secretion 2.5 ± 0.3 fold above basal (p = 0.005, data not shown). These data show that in human goblet cells, similar to rat goblet cells, LXA4 activates the ALX/FPR2 receptor to increase [Ca2+]i and stimulate glycoconjugate secretion.
Presence and Localization of ALX/FPR2 Receptors in Human Conjunctival Goblet Cells
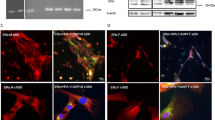
As LXA4 stimulates an increase in [Ca2+]i and glycoconjugate secretion and the ALX/FPR2 receptor inhibitor, BOC-2, blocks LXA4-stimulated increase in [Ca2+]i, we confirmed that ALX/FPR2 is expressed in human goblet cells. RT-PCR, using primers specific for this receptor, was performed. As shown in Fig. 3A, one band at the expected size was detected. The receptor is also expressed at the protein level as detected by western blot analysis from cells grown from 3 individuals (Fig. 3B). The ALX/FPR2 receptor is known to be glycosylated which could account for the multiple bands observed24. In cultured human goblet cells, ALX/FPR2 (shown in red) was present throughout the cytosol of the cells (Fig. 3C). UEA-1, shown in green was used to confirm the identity of cultured goblet cells (Fig. 3C). There was substantial overlap in the localization of ALX/FPR2 and UEA-1. These data confirm that ALX/FPR2 is present in human goblet cells.
ALX/FPR2 Receptor is Present in Human Conjunctival Goblet Cells.
RNA was isolated from cultured goblet cells, and RT-PCR performed with primers to human ALX/FPR2 receptor and is shown in (A). ALX/FPR2 was also detected by Western blot analysis in human conjunctival goblet cells (B). Each lane in B represents a different individual. ALX/FPR2 was also detected by immunofluorescent techniques (C). ALX/FPR2 is shown in red (left micrograph) while UEA-1, which binds to L-fucose containing high moleculuar weight glycoproteins including mucins in goblet cells, is shown in green (middle micrograph). Overlay of ALX/FPR2 and UEA-1 is shown in right micrograph. Micrographs are representative of results from 3 individuals.
Interaction of LXA4 and RvD1 via ALX/FPR2 and GPR32
The ALX/FPR2 receptor has multiple agonists which can bind to it. These agonists include RvD1, the protein annexin A1, as well as LXA425. In addition to ALX/FPR2, RvD1 and LXA4 also bind to the receptor GPR3226, which we previously demonstrated to be present in cultured human goblet cells20. We explored the interaction between LXA4 and RvD1 with ALX/FPR2 and GPR32 in human goblet cells. In a first set of experiments, we determined the extent to which RvD1 binds to ALX/FPR2 in human goblet cells. Goblet cells were preincubated with ALX/FPR2 inhibitor BOC-2 (10−4 M) prior to stimulation with RvD1 (10−8 M) and [Ca2+]i was measured. In the absence of inhibitor, RvD1 increased [Ca2+]i by 242.8 ± 70.8 nM (Fig. 4A). Preincubation with BOC-2 had no effect on the RvD1 response (p = 0.14).
Receptor Interaction of Resolvin D1 (RvD1) and Lipoxin A4 (LXA4) in Human Conjunctival Goblet Cells.
Human goblet cells were preincubated with BOC-2 (10−4 M) or vehicle for 30 min prior to addition of RvD1 and [Ca2+]i was measured. Change in peak [Ca2+]i was calculated and shown in (A). RvD1 was added alone or 5 min after addition of RvD1 or LXA4 (B,C) or LXA4 was added alone or 5 min after addition of LXA4 or RvD1 (B,D) and [Ca2+]i measured. Panel (B) is mean of [Ca2+]i over time while (C,D) are mean ±SD in the change in peak [Ca2+]i from 3 individuals. Data from (B–D) are from the same individuals. Arrows indicate addition of either RvD1 or LXA4. *indicates significance difference from basal; #indicates significance difference from RvD1 or LXA4.
In a second set of experiments, the following experimental paradigm was used: addition of first agonist was followed 5 minutes later by addition of second agonist and the increase in peak [Ca2+]i. was measured approximately 30 seconds after addition of agonist. In cultured human goblet cells, the addition of RvD1 (10−8 M) first caused a peak increase in [Ca2+]i of 256.2 ± 62.5 nM (Fig. 4B,C). A second addition of RvD1, 5 minutes after the first, resulted in a peak increase of [Ca2+]i of 17.9 ± 13.8 nM (Fig. 4B,C). This was a decrease from the response obtained when RvD1 was added first (p = 0.04). If LXA4 is added first and RvD1 is added 5 min later, the RvD1 response was 11.3 ± 11.3 nM. This is also a decrease from the response obtained if RvD1 is added first (p = 0.03).
If LXA4 (10−9 M) is added first, the initial response obtained was a change in peak [Ca2+]i of 576.5 ± 149.8 nM (Fig. 4B,D). A second addition of LXA4 resulted in a decrease from the first peak and was 20.4 ± 13.8 nM (p = 0.04). If RvD1 is given first, the addition of LXA4 resulted in change in peak [Ca2+]i of 391.7 ± 53.7 nM. This was not different from the result obtained with LXA4 added first (p = 0.4, Fig. 4B,D) though it is increased from basal (p = 0.002). The data shown in Fig. 4C,D are from the same individuals.
These data indicate that in human goblet cells, RvD1 preferentially binds to GPR32 which desensitizes the receptor to a second addition of RvD1 while the LXA4 response is not altered. In contrast, LXA4 binds to both the ALX/FPR2 and GPR32 receptor and a second addition of LXA4 desensitizes both receptors to a subsequent addition of either LXA4 or RvD1.
LXA4 Inhibits Histamine-stimulated Increase in Glycoconjugate Secretion, [Ca2+]i, and ERK Activation
To examine the effects of LXA4 on histamine-stimulated glycoconjugate mucin secretion, rat goblet cells were pretreated with LXA4 (10−10–10−9 M) for 30 min and stimulated with histamine (10−5 M) for 2 h. In untreated rat cells, histamine increased mucin secretion 1.9 ± 0.1 fold increase above basal (p=0.002). LXA4 blocked histamine-stimulated secretion by 75.8 ± 8.8 and 90.8 ± 4.0% at 3 × 10−10 and 10−9 M, respectively (p = 0.001 and 0.00002, Fig. 5A). In human goblet cells, histamine increased mucin secretion 2.5 ± 0.3 fold increase above basal (p=0.005). Preincubation with LXA4 10−10 and 10−9 M blocked histamine-stimulated secretion by 85.5 ± 14.5 and 87.3 ± 8.4%, respectively (p = 0.004 and 0.0003, Fig. 5B).
LXA4 Blocks Histamine-stimulated Glycoconjugate Secretion from Rat and Human Goblet Cells.
Goblet cells from rat (A) or human (B) were preincubated with LXA4 (10−10–10−8 M) for 30 min prior to addition of histamine (His, 10−5 M) for 2 h and glycoconjugate secretion measured by ELLA. Data are mean ± SD from 3 rats or 3 individuals. *indicates significance difference from histamine alone.
We previously established that histamine increases [Ca2+]i in a concentration-dependent manner which was blocked by RvD1 and AT-RvD120. To determine if LXA4 also blocks histamine responses, cultured goblet cells were preincubated with LXA4 prior to stimulation with histamine. In goblet cells cultured from rat, histamine (10−5 M) increased [Ca2+]i. with a peak of 587.1 ± 92.3 nM (p = 0.0002, Fig. 6A). Preincubation with LXA4 (10−10–10−8 M) decreased histamine-stimulated increase in [Ca2+]i at 10−9 and 10−8 M with maximum inhibition occurring at 10−9 M LXA4 which decreased the histamine response by 64.1 ± 14.1% to 185.6 ± 52.1 nM (p = 0.01, Fig. 6A,B).
LXA4 Uses ALX/FPR2 to Block Histamine-stimulated Increase in [Ca2+]i in Rat and Human Goblet Cells.
Goblet cells from rat (A,B) or human (C,D) were preincubated with LXA4 (10−10–10−8 M) for 30 min prior to addition of histamine (His, 10−5 M). Panels A and C are mean of [Ca2+]i over time. Arrows indicate addition of histamine. Panels B,D are mean ± SD in the change in peak [Ca2+]i from 8 rats or 4 humans. Rat goblet cells were stimulated with either histamine (His, 10−5 M); LXA4 (10−9 M) for 30 min prior to addition of histamine; or preincubated with ALX/FPR2 inhibitor BOC-2 (10−4 M) 15 min prior to addition of LXA4 (10−9 M) which was added 30 min before histamine. The change in peak [Ca2+]i was measured and is shown in (E). Data are mean ± SD from 4 rats. *Indicates significance difference from histamine alone. #Indicates significance difference from histamine.
Similar results were obtained with cultured human goblet cells. Histamine (10−5 M) stimulated an increase in peak [Ca2+]i 1152.5 ± 173.9 nM (p=0.006, Fig. 6C,D). Preincubation with LXA4 (10−10–10−8 M) decreased histamine-stimulated increase in [Ca2+]i at all concentrations with maximum inhibition occurring at 10−10 M LXA4 which decreased the histamine response by 66.1 ± 7.3% to 378.6 ± 92.6 nM (p = 0.005, Fig. 6C,D).
To ensure that the actions of LXA4 on histamine response are mediated by the ALX/FPR2 receptor, rat goblet cells were pretreated with BOC-2 (10−4 M) for 15 min prior to addition of LXA4 (10−9 M) for 30 min. The [Ca2+]i was then measured in response to histamine (10−5 M). In the absence of BOC-2 and LXA4, the change in peak [Ca2+]i in response to histamine was 586.8 ± 173.1 nM (p = 0.01, Fig. 6E). LXA4 added first reduced the histamine response by 90.3 ± 1.9% to 55.5 ± 8.5 nM (p = 0.03). Preincubation with BOC-2 reversed the inhibition by LXA4 on the histamine response and increased [Ca2+]i by 495.0 ± 76.6 nM (Fig. 6E).
Histamine activates ERK 1/2 to stimulate glycoconjugate secretion which was also blocked by RvD1 and AT-RvD115,20. To determine if LXA4 also blocks histamine-stimulated ERK 1/2 activity, rat goblet cells were preincubated with LXA4 (10−10–10−8 M) for 30 min prior to incubation with histamine (10−6 M) for 5 min and ERK 1/2 activity was measured. Histamine increased ERK 1/2 activity 1.3 ± 0.1 fold increase above basal (p=0.04, Fig. 7A,B). LXA4 decreased this response at all concentrations (Fig. 7A). When four independent experiments were analyzed, inhibition with LXA4 10−8 M decreased histamine response by 64.4 ± 19.4% to 1.1 ± 0.1 fold increase above basal (p = 0.002, Fig. 7B).
LXA4 Blocks Histamine-stimulated ERK 1/2 Activation in Rat Goblet Cells.
Rat goblet cells were preincubated with LXA4 (10−10–10−8 M) for 30 min prior to addition of histamine (His, 10−6 M) for 5 min and amount of activated (phosphorylated) and total ERK determined by Western blot analysis. Representative blot is shown in (A). Upper blot has been rearranged for ease of comparison. Mean ± SD from 4 rats are shown in (B) *indicates significant difference from histamine alone.
ALX/FPR2 Uses βARK1, but Not Protein Kinase C, to Block the H1 Histamine Receptor Simulated Increase in [Ca2+]i
Examination of the H1 histamine receptor for phosphorylation sites using Scan Site (http://scansite.mit.edu/), showed that this receptor has consensus sequences for β-adrenergic receptor kinase 1 (βARK1), also known as G-protein coupled receptor kinase (GRK)-2 and protein kinase C (PKC). We previously demonstrated that RvD1 binding to GPR32 activates both these kinases to counter-regulate the H1 histamine receptor to block the increase in [Ca2+]i20. To determine if ALX/FPR2 and LXA4 also use βARK1 and/or PKC to counter regulate histamine H1 receptor, rat goblet cells were pretreated with either LXA4 or LXA4 plus inhibitors to βARK1 and PKC. The increase in [Ca2+]i in response to the specific H1 receptor agonist, histamine dimaleate was measured in cultured rat goblet cells. Pretreatment with LXA4 decreased the histamine dimaleate stimulated increase in [Ca2+]i from 823.7 ± 154.1 nM above basal in the absence of LXA4 to 140.9 ± 68.7 nM (p = 0.02, Fig. 8A,B). βARK1 inhibitor peptide (10−6 M) alone did not have an effect of the histamine dimaleate (p = 0.16, Fig. 8A,B). When cells were pretreated with βARK1 inhibitor peptide followed by LXA4, blockage of the histamine dimaleate response by LXA4 was completely reversed (Fig. 8A,B).
LXA4 Uses β-Adrenergic Receptor Kinase 1 to Block H1 Histamine Receptor.
Rat goblet cells from rat were stimulated with either histamine dimaleate (His Dim, 10−6 M); preincubated with LXA4 (10−9 M) for 30 min prior to addition of the His Dim; or preincubated with β-adrenergic receptor kinase 1 inhibitor peptide (βARK 1 Inh peptide, 10−6 M, (A,B) or with the PKC inhibitor Ro317549 (10−7 M, (C,D) added 30 min before His Dim; or preincubated with β-adrenergic receptor kinase 1 inhibitor peptide (βARK 1 Inh peptide, 10−6 M, (A,B) or with the PKC inhibitor Ro317549 (10−7 M, (C,D) for 15 min prior to addition of LXA4 that was added 30 min before His Dim. Change in [Ca2]i over time is shown in (A,C) and change in peak [Ca2+]i is shown in (B,D). Data are mean ± SD from 3 (A,B) or 4 (C,D) individuals. *indicates significance difference from basal; #indicates significance difference from His Dim alone.
To investigate the role of PKC in counter-regulation of H1 receptor by LXA4, rat goblet cells were preincubated with the PKC inhibitor Ro317549 (10−7 M). In these experiments, histamine dimaleate increased [Ca2+]i by 751.7 (p=0.01, ± 216.1 nM (Fig. 8C,D). This response was decreased by LXA4 and was 193.3 ± 11.3 nM (p = 0.04, Fig. 8C,D). Ro317549 alone had no effect on histamine dimaleate response (Fig. 8C,D). Addition of the PKC inhibitor prior to LXA4 had no effect on the LXA4 blockage of histamine dimaleate (Fig. 8C,D).
These data indicate that the activation of the ALX/FPR2 uses βARK1 but not PKC to counter regulate the H1 histamine receptor in rat goblet cells. This is in contrast to RvD1 that uses both βARK1 and PKC to counter regulate the H1 receptor.
Discussion
Our results demonstrate that LXA4 plays a role in goblet cell function in both normal non-inflamed conditions and acute inflammatory conditions. Our hypothesis is that the ALX/FPR2 receptor is present in human conjunctival goblet cells and activation of the receptor by LXA4 stimulates an increase in [Ca2+]i and mucin secretion, which in rat goblet cells is protective in the eye and involves activation of phospholipase (PL) C, PLD, and PLA2 signaling pathways (Fig. 9A)19. In circumstances such as inflammation or pharmacological addition, LXA4 inhibits histamine-stimulated increase in [Ca2+]i, ERK 1/2 activation, and mucin secretion through the counter-regulation of histamine receptor by βARK1 (Fig. 9B). This may be relevant in controlling excessive histamine release into the conjunctiva.
Schematic Diagram of Pathways Activated by LXA4 in Rat and Human Conjunctival Goblet Cells to Stimulate Mucin Secretion and Inhibit Histamine-stimulated Mucin Secretion.
In rat goblet cells, histamine via the H1 histamine receptor subtype activates phospholipase (PL) –C to stimulate extracellular-regulated kinase 1/2 (ERK 1/2) which leads to mucin secretion. In addition, inositol trisphosphate (IP3) is produced which also leads to release of Ca2+i and activation of Ca2+ channels leading to mucin secretion Also in rat goblet cells activation of the ALX/FPR2 receptor stimulates PLC, -D, and A2. These phospholipases activate ERK 1/2 through phosphorylation (pERK 1/2), and protein kinase C (PKC). IP3 is produced which leads to release of Ca2+i and activation of Ca2+ channels leading to mucin secretion. (A) Activation of ALX/FPR2 by either LXA4 or RvD1 activates β-adrenergic receptor kinase 1 (βARK1) to counter-regulate the H1 histamine receptor to prevent histamine-stimulated mucin secretion (B). In human goblet cells, RvD1 binds to GPR32 receptor and regulates goblet cells (A,B). The rat homolog of the human GPR32 if present remains to be identified.
It is currently not known if any cells in the conjunctiva, including goblet cells, produce and secrete LXA4. Along these lines, Gronert et al. have demonstrated that the epithelial cells of the cornea endogenously express LXA4 and the amount is increased upon wounding22. This LXA4 could then diffuse via the tears to the goblet cells to stimulate mucin secretion.
While LXA4 is an appreciated pro-resolution mediator, the results from several studies indicate that LXA4 and other pro-resolution mediators can also play a role within other organs in, physiological conditions that maybe organ specific. For example, LXA4 is endogenously produced in the cornea and lacrimal gland under non-inflamed conditions17. RvD1 and AT-RvD1, similar to LXA4, alone stimulate conjunctival goblet cell functions20. These results imply that these mediators could assist in the maintenance of the normal homeostasis of the ocular surface by regulating goblet cell mucin secretion that is linked to ocular surface health.
Allergic conjunctivitis is the most common type of inflammation of the ocular surface. In this condition, histamine interacts with H1-H4 histamine receptors, all of which are expressed in rat and human conjunctival goblet cells15. Histamine also increases [Ca2+]i and mucin secretion in a concentration dependent manner15. Pre-incubation with LXA4 blocked histamine-stimulated increase in [Ca2+]i, mucin secretion and ERK 1/2. Thus, LXA4 likely acts as a pro-resolution mediator acting on goblet cells of the conjunctiva to return mucin levels to normal. LXA4 is likely to have similar effects on histamine-stimulated responses in other tissues. For example, LXA4 inhibits histamine release from human lung mast cells27 and histamine-stimulated paw edema in mice28.
This study examined the actions of LXA4 on conjunctival goblet cells only. The ocular surface consists of multiple cell types and is covered by tears, which are a complex film that overspreads the ocular surface29. The actions of LXA4 have not been tested on other types of cells on the ocular surface nor in the presence of tears.
Cultured human goblet cells often react similarly to LXA4 as cultured rat goblet cells. In goblet cells from both species, LXA4 stimulated an increase [Ca2+]i, and mucin secretion to the same extent (current study and20). Mucin secretion stimulated by cysteinyl leukotrienes in human goblet cells was also similar to that obtained with rat goblet cells17. There does appear to be several differences between rat and human goblet cells. In human goblet cells, the concentration LXA4 required to maximally inhibit histamine-stimulated increase in [Ca2+]i was 10 fold less that than that required in rat goblet cells. An additional difference was demonstrated by experiments involving interactions of LXA4 and RvD1 with their receptors. In rat goblet cells, initial addition of either LXA4 or RvD1 blocked the increase in [Ca2+]i stimulated by a second addition of either LXA4 or RvD1 indicating that these two SPMs bind to the same receptor19. However in human goblet cells, while an initial addition of LXA4 blocks the RvD1 response, an initial addition of RvD1 does not block the LXA4 response. In addition, BOC-2 does not alter RvD1-stimulated increase in [Ca2+]i. These results support the notion that in human cells RvD1 preferentially activates GPR32 while LXA4 activates both receptors (Fig. 9). LXA4 is an established agonist of ALX/FPR2 and has been shown to bind to GPR32 in human phagocytes26. It is also known that RvD1 binds to both ALX/FPR2 and GPR3226. At this point it is not known if a rat homolog of GPR32 is present and functional in rat goblet cells. Since GPR32 has not yet been identified in rat, it is possible that RvD1 only binds to ALX/FPR2 in these rat cells. There are many other situations in which rat differs from human including regulatory T cell phenotypes30, wound healing in skin31, and glomerulonephritis32.
We previously showed the mechanism by which RvD1 prevents the actions of histamine in rat goblet cells20. We found that RvD1 counter-regulates the H1 histamine receptor by activation of both βARK1 and PKC to prevent the H1 specific agonist-stimulated increase in [Ca2+]i20. In contrast to RvD1, only an inhibitor of βARK1 reversed the LXA4 inhibition of H1 histamine receptor. Cooray et al. have demonstrated that ALX/FPR2 receptor can form hetero- and homodimers depending on the agonist bound24. Thus RvD1 and LXA4 could form different dimer formations in rat goblet cells.
The signaling pathways activated by LXA4 after binding to ALX/FPR2 are dependent on the cell type (Table 1). LXA4 acting through ALX/FPR2 stimulated an increase in [Ca2+]i, chemotaxis and adherence in human monocytes33,34. In human neutrophils, ALX/FPR2 activation leads to lipid remodeling, arachidonic acid release, and activation of phospholipase D (PLD) via PKC with a small increase in [Ca2+]i35. LXA4 had no effect on [Ca2+]i36 in human astrocytoma cells while it increased [Ca2+]i, in human bronchial epithelia as well as increased the number of tight junctions and Cl− secretion and decreased Na+ absorption37,38,39. Thus LXA4 can have variable effects on [Ca2+]i and other cell functions and cellular responses to LXA4 need to be determined for each cell type.
In conclusion, we demonstrate that ALX/FPR2 receptors are present on cultured human goblet cells, and that LXA4 alone increases [Ca2+]i, mucin secretion and ERK 1/2 activation. In addition, LXA4 counter-regulates the H1 histamine receptor to block its activation thereby returning the ocular surface to homeostasis. LXA4 thus plays a critical role in ocular surface health and maintenance in physiological conditions. In addition, LXA4 protects the ocular surface from challenges of the external environment that induce ocular surface inflammatory and allergic diseases. Thus LXA4 and this receptor axis may provide the basis for new therapeutic treatments for these diseases.
Materials and Methods
Synthetic LXA4 was purchased from EMD Millipore (Billerica, MA) and RvD1 was purchased from Cayman Chemical, Ann Arbor, MI). Both compounds were dissolved in ethanol as supplied by the manufacturer and were stored at −80 °C with minimal exposure to light. Immediately prior to use, the SPMs were diluted in with Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES, 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]) to the desired concentrations and added to the cells. The cells were then incubated at 37 °C in the dark. Daily working stock dilutions were discarded following each experiment. N-BOC-Phe-Leu-Phe-Leu-Phe (BOC-2) was purchased from Genescript (Piscataway, NJ).
Human Tissue
Human conjunctiva was obtained from Eversight (Ann Arbor, MI). Tissue was placed in Optisol media within 18 h after death.
Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing between 125 and 150 g were anesthetized with CO2 for 1 min, decapitated, and the bulbar and forniceal conjunctival membranes removed from both eyes. All experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee and carried out in accordance to the protocols approved by this committee.
Cell Culture
Goblet cells from human and rat conjunctiva were grown in organ culture as described and extensively characterized previously4,16,17,40,41,42. The tissue plug was removed after nodules of cells were observed. First passage goblet cells were used in all experiments. The identity of cultured cells was periodically checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin Ulex europaeus agglutinin (UEA)-1 (detects goblet cell secretory product) to ensure that goblet cells predominated.
Measurement of [Ca2+]i
Goblet cells were incubated for 1 h at 37 °C with KRB-HEPES with 0.5% BSA containing 0.5 μM fura-2/AM (Invitrogen, Grand Island, NY), 8 μM pluronic acid F127, and 250 μM sulfinpyrazone followed by washing in KRB-HEPES containing sulfinpyrazone. Inhibitors were added for the last 30 min of the fura-2 incubation. Calcium measurements were made with a ratio imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. At least 10 cells were selected in each experimental condition. Data were collected in real time and are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]i was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data are not shown, the plateau [Ca2+]i was affected similarly to the peak [Ca2+]i.
Measurement of Glycoconjugate Secretion
Cultured goblet cells were serum starved for 2 h before use and then stimulated with either LXA4 or histamine in serum-free RPMI 1640 supplemented with 0.5% BSA for 2 h. Inhibitors were added 30 min prior to stimulation. Goblet cell secretion was measured using an enzyme-linked lectin assay (ELLA) with the lectin UEA-I. UEA-1 detects high molecular weight glycoconjugates containing L-fucose including mucin MUC5AC produced by goblet cells43. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion as described earlier17. Glycoconjugate secretion was expressed as fold increase over basal that was set to 1.
Reverse Transcriptase (RT)-PCR
Cultured human goblet cells were homogenized in TRIzol and total RNA was isolated. One microgram of purified total RNA was used for complementary DNA (cDNA) synthesis using the Superscript First-Strand Synthesis system for RT-PCR (Invitrogen, Carlsbad,CA). The cDNA was amplified by the polymerase chain reaction (PCR) using primers specific to human ALX/FPR2 receptor using the Jumpstart REDTaq Readymix Reaction Mix (Sigma-Aldrich, St. Louis, MO) in a thermal cycler (Master Cycler, Eppendorf, Hauppauge, NY). The primers were from published sequences44. The forward primer sequence was GGA TTT GCA CCC ACT GCA TTT and reverse primer was ATC CAA GGT CCG AGA TCA C. These primers generated a product of 528 base pairs. β−Actin served as the positive control. The primers were from published sequences45. The conditions were as follows: 5 min at 95 °C followed by 35 cycles of 1 min at 94 °C, 3 s at annealing temperature for 1 min at 72 °C with a final hold at 72 °C for 10 min. Samples with no cDNA served as the negative control. Amplification products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining.
Western blotting analyses
Cultured goblet cells were homogenized in RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The lysate was centrifuged at 2000 g for 30 min at 4 °C. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and processed for western blotting. The antibody against the ALX/FPR2 receptor (Novus Biologics, Littleton, CO) was diluted 1:1000. To measure activation of ERK 1/2, LXA4 was added 30 min prior to histamine (10−6 M) for 5 min. The antibody against phosphorylated (active) ERK 1/2 was used diluted 1:200 and total ERK 1/2 (Santa Cruz Biotechnologies, Santa Cruz, CA) was diluted 1:500. Immunoreactive bands were visualized by the enhanced chemiluminescence method. The films were analyzed with Image J software (http://rsbweb.nih.gov/ij/). Values for phosphorylated ERK 1/2 were normalized to total ERK 1/2. Control value was set as 1.
Immunofluorescence Microscopy
First passage cells were grown on glass cover slips and were fixed in 4% formaldehyde diluted in phosphate buffered saline (PBS, 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 (pH 7.2)) for 4 hours at 4 °C. The coverslips were rinsed for 5 minutes in PBS, and nonspecific sites were blocked by incubation with 1% bovine serum albumin, and 0.2% Triton X-100 in PBS for 45 minutes at room temperature. ALX/FPR2 receptor antibody (Novus Biologics) was used at 1:100 dilution overnight at 4 °C. UEA-1 directly conjugated to FITC (Sigma-Aldrich, St. Louis, MO) was used at a dilution of 1:300 to identify goblet cells. Secondary antibodies were conjugated to Cy 3 (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at a dilution of 1:150 for 1 h at room temperature. Negative control experiments included incubation with the isotype control antibody. The cells were viewed by fluorescence microscopy (Eclipse E80i; Nikon, Tokyo, Japan) and micrographs were taken with a digital camera (Spot; Diagnostic Instruments, Inc, Sterling Heights, MI).
Statistical analysis
Results were expressed as the fold-increase above basal. Results are presented as mean ± SD. Data were analyzed by ANOVA followed by post-hoc Tukey or Student’s t-test. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Hodges, R. R. et al. Lipoxin A4 Counter-regulates Histamine-stimulated Glycoconjugate Secretion in Conjunctival Goblet Cells. Sci. Rep. 6, 36124; doi: 10.1038/srep36124 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Inatomi, T., Tisdale, A. S., Zhan, Q., Spurr-Michaud, S. & Gipson, I. K. Cloning of rat Muc5AC mucin gene: comparison of its structure and tissue distribution to that of human and mouse homologues. Biochem Biophys Res Commun 236, 789–797, doi: 10.1006/bbrc.1997.7051 (1997).
Jumblatt, M. M., McKenzie, R. W. & Jumblatt, J. E. MUC5AC mucin is a component of the human precorneal tear film. Invest Ophthalmol Vis Sci 40, 43–49 (1999).
Garcia-Posadas, L. et al. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol 9, 206–217, doi: 10.1038/mi.2015.53 (2016).
McGilligan, V. E. et al. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS One 8, e74010, doi: 10.1371/journal.pone.0074010 (2013).
Leonardi, A., Motterle, L. & Bortolotti, M. Allergy and the eye. Clin Exp Immunol 153 Suppl 1, 17–21, doi: 10.1111/j.1365-2249.2008.03716.x (2008).
Gomes, P. J. Trends in prevalence and treatment of ocular allergy. Current opinion in allergy and clinical immunology 14, 451–456, doi: 10.1097/ACI.0000000000000100 (2014).
Kasetsuwan, N., Satitpitakul, V., Changul, T. & Jariyakosol, S. Incidence and pattern of dry eye after cataract surgery. PLoS One 8, e78657, doi: 10.1371/journal.pone.0078657 (2013).
Levinson, B. A. et al. Referrals to the Wills Eye Institute Cornea Service after laser in situ keratomileusis: reasons for patient dissatisfaction. Journal of cataract and refractive surgery 34, 32–39, doi: 10.1016/j.jcrs.2007.08.028 (2008).
Jabbur, N. S., Sakatani, K. & O’Brien, T. P. Survey of complications and recommendations for management in dissatisfied patients seeking a consultation after refractive surgery. Journal of cataract and refractive surgery 30, 1867–1874, doi: 10.1016/j.jcrs.2004.01.020 (2004).
Lemp, M. A. et al.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. The ocular surface 5, 75–92 (2007).
Gipson, I. K. et al.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. The ocular surface 5, 179–193 (2007).
McDonald, M., Patel, D. A., Keith, M. S. & Snedecor, S. J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. The ocular surface 14, 144–167, doi: 10.1016/j.jtos.2015.11.002 (2016).
Fukagawa, K. et al. Histamine and tryptase levels in allergic conjunctivitis and vernal keratoconjunctivitis. Cornea 13, 345–348 (1994).
Miller, S., Cook, E., Graziano, F., Spellman, J. & Yanni, J. Human conjunctival mast cell responses in vitro to various secretagogues. Ocul Immunol Inflamm 4, 39–50, doi: 10.3109/09273949609069126 (1996).
Hayashi, D. et al. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Vis Sci 53, 2993–3003, doi: 10.1167/iovs.11-8748 (2012).
Li, D., Carozza, R. B., Shatos, M. A., Hodges, R. R. & Dartt, D. A. Effect of histamine on Ca(2+)-dependent signaling pathways in rat conjunctival goblet cells. Invest Ophthalmol Vis Sci 53, 6928–6938, doi: 10.1167/iovs.12-10163 (2012).
Dartt, D. A. et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol 186, 4455–4466, doi: 10.4049/jimmunol.1000833 (2011).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101, doi: 10.1038/nature13479 (2014).
Hodges, R. R. et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol In Press (2016).
Li, D. et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol 6, 1119–1130, doi: 10.1038/mi.2013.7 (2013).
Gao, Y. et al. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. Journal of immunology 195, 3086–3099, doi: 10.4049/jimmunol.1500610 (2015).
Gronert, K. Lipoxins in the eye and their role in wound healing. Prostaglandins Leukot Essent Fatty Acids 73, 221–229, doi: 10.1016/j.plefa.2005.05.009 (2005).
Canny, G. O. & Lessey, B. A. The role of lipoxin A4 in endometrial biology and endometriosis. Mucosal immunology 6, 439–450, doi: 10.1038/mi.2013.9 (2013).
Cooray, S. N. et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proceedings of the National Academy of Sciences of the United States of America 110, 18232–18237, doi: 10.1073/pnas.1308253110 (2013).
Chiang, N. et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological reviews 58, 463–487, doi: 10.1124/pr.58.3.4 (2006).
Krishnamoorthy, S. et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA 107, 1660–1665, doi: 10.1073/pnas.0907342107 (2010).
Martin, N. et al. Primary human airway epithelial cell-dependent inhibition of human lung mast cell degranulation. PLoS One 7, e43545, doi: 10.1371/journal.pone.0043545 (2012).
Menezes-de-Lima, O. Jr., Kassuya, C. A., Nascimento, A. F., Henriques, M. & Calixto, J. B. Lipoxin A4 inhibits acute edema in mice: implications for the anti-edematogenic mechanism induced by aspirin. Prostaglandins & other lipid mediators 80, 123–135, doi: 10.1016/j.prostaglandins.2006.05.016 (2006).
Gipson, I. K. Age-related changes and diseases of the ocular surface and cornea. Invest Ophthalmol Vis Sci 54, ORSF48–ORSF53, doi: 10.1167/iovs.13-12840 (2013).
Rodriguez-Perea, A. L., Arcia, E. D., Rueda, C. M. & Velilla, P. A. Phenotypic characterization of regulatory T cells in humans and rodents. Clin Exp Immunol, doi: 10.1111/cei.12804 (2016).
Rittie, L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal 10, 103–120, doi: 10.1007/s12079-016-0330-1 10.1007/s12079-016-0330-1 [pii] (2016).
Muhammad, S. Nephrotoxic nephritis and glomerulonephritis: animal model versus human disease. Br J Biomed Sci 71, 168–171 (2014).
Romano, M., Maddox, J. F. & Serhan, C. N. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. Journal of immunology 157, 2149–2154 (1996).
Maddox, J. F. et al. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. The Journal of biological chemistry 272, 6972–6978 (1997).
Fiore, S., Romano, M., Reardon, E. M. & Serhan, C. N. Induction of functional lipoxin A4 receptors in HL-60 cells. Blood 81, 3395–3403 (1993).
Decker, Y., McBean, G. & Godson, C. Lipoxin A4 inhibits IL-1beta-induced IL-8 and ICAM-1 expression in 1321N1 human astrocytoma cells. American journal of physiology. Cell physiology 296, C1420–C1427, doi: 10.1152/ajpcell.00380.2008 (2009).
Grumbach, Y., Quynh, N. V., Chiron, R. & Urbach, V. LXA4 stimulates ZO-1 expression and transepithelial electrical resistance in human airway epithelial (16HBE14o-) cells. American journal of physiology. Lung cellular and molecular physiology 296, L101–L108, doi: 10.1152/ajplung.00018.2008 (2009).
Bonnans, C., Mainprice, B., Chanez, P., Bousquet, J. & Urbach, V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. The Journal of biological chemistry 278, 10879–10884, doi: 10.1074/jbc.M210294200 (2003).
Al-Alawi, M. et al. Physiological levels of lipoxin A4 inhibit ENaC and restore airway surface liquid height in cystic fibrosis bronchial epithelium. Physiological reports 2, doi: 10.14814/phy2.12093 (2014).
Shatos, M. A. et al. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Investigative ophthalmology & visual science 42, 1455–1464 (2001).
Shatos, M. A. et al. Isolation and characterization of cultured human conjunctival goblet cells. Investigative ophthalmology & visual science 44, 2477–2486 (2003).
Shatos, M. A., Gu, J., Hodges, R. R., Lashkari, K. & Dartt, D. A. ERK/p44p42 mitogen-activated protein kinase mediates EGF-stimulated proliferation of conjunctival goblet cells in culture. Investigative ophthalmology & visual science 49, 3351–3359, doi: 10.1167/iovs.08-1677 (2008).
Hodges, R. R. et al. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res 103, 99–113, doi: 10.1016/j.exer.2012.08.010 S0014-4835(12)00268-0 [pii] (2012).
Cattaneo, F., Parisi, M. & Ammendola, R. WKYMVm-induced cross-talk between FPR2 and HGF receptor in human prostate epithelial cell line PNT1A. FEBS letters 587, 1536–1542, doi: 10.1016/j.febslet.2013.03.036 (2013).
Iwamoto, S., Mihara, K., Downing, J. R., Pui, C. H. & Campana, D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117, 1049–1057, doi: 10.1172/JCI30235 (2007).
El Kebir, D. et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. Journal of immunology 179, 616–622 (2007).
Acknowledgements
This work was supported by NIH R0EY019470 to DAD and RO1GM38765 to CNS and P30 EY003790 to SERI/MEE.
Author information
Authors and Affiliations
Contributions
R.R.H. conducted experiments, designed experiments, acquired data, analyzed data, wrote manuscript D.L. conducted experiments M.A.S. conducted experiments C.N.S. provided reagents, designed experiments, wrote manuscript D.A.D. designed experiments, wrote manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hodges, R., Li, D., Shatos, M. et al. Lipoxin A4 Counter-regulates Histamine-stimulated Glycoconjugate Secretion in Conjunctival Goblet Cells. Sci Rep 6, 36124 (2016). https://doi.org/10.1038/srep36124
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36124
This article is cited by
-
Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model
Mucosal Immunology (2019)
-
Context-Dependent Regulation of Conjunctival Goblet Cell Function by Allergic Mediators
Scientific Reports (2018)
-
AT-RvD1 Promotes Resolution of Inflammation in NOD/ShiLtJ mice
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.