Abstract
Chronic kidney disease (CKD) is a significant risk factor for contrast induced acute kidney injury (CI-AKI) after percutaneous coronary intervention (PCI). This study included 1592 CKD patients extracted from a prospective multicenter, all comer-based registry of patients undergoing PCI. In multivariate logistic analysis for CI-AKI development, a significant linear trend was observed between the quartiles of HDL-C (quartile 1 vs. 2: odds ratio [OR], 0.716; 95% confidence interval [CI], 0.421–1.219; quartile 1 vs. 3: OR, 0.534; 95% CI, 0.301–0.947; quartile 1 vs. 4: OR, 0.173; 95% CI, 0.079–0.377; P for trend < 0.001). HDL-C quartiles were also negatively correlated with the incidence of CI-AKI; 19.0%, 12.1%, 8.7%, and 3.7% for quartile 1(Q1) (<34 mg/dL), Q2 (34–40 mg/dL), Q3 (40–48 mg/dL), and Q4 (>48 mg/dL) respectively (P < 0.001 overall and for the trend). Multivariate Cox regression analysis for the long term mortality, the highest HDL-C quartile was associated with decreased mortality compared with the lowest HDL-C quartile (hazard ratio [HR] 0.516, 95% CI, 0.320–0.832, P = 0.007). Our study suggests more intensive strategies should be considered for preventing CI-AKI in CKD patients with low serum HDL-C level who is planned for PCI.
Similar content being viewed by others
Introduction
As both the number of elderly people and the prevalence of diabetes mellitus (DM) continue to rise, the incidence and prevalence of chronic kidney disease (CKD) increase rapidly1,2. These CKD patients are the population at risk of cardiovascular disease such as ischemic heart disease (IHD) and cerebrovascular accident (CVA)3. Endovascular procedure based on angiography using contrast media has been increasingly employed in cardiovascular patients because it is less invasive than open surgery and minimizes the procedure-related risk that may be related to procedure itself4,5. However, contrast media are nephrotoxic and sometimes lead to irreversible renal damage.
Contrast-induced acute kidney injury (CI-AKI) after primary percutaneous coronary intervention (PCI) is known to be associated with the increase in mortality during admission and the development of major cardiac adverse events even after discharge6,7. Older age, anemia, contrast volume, peri-procedural hemodynamic instabilities such as the use of intra-aortic balloon pump, initial lower left ventricular ejection fraction (LVEF), DM and acute hyperglycemia have been also reported as the risk factors for CI-AKI but the degree of the underlying CKD is the most important determinant for CI-AKI development8,9,10,11. Only limited data are available in terms of risk factors of CI-AKI in CKD patients. We conducted this observational cohort study to find the risk factors for CI-AKI in the CKD patients undergoing PCI.
Results
Baseline demographic, clinical and laboratory profiles
Among the 1592 patients in this study, 193 (12.1%) patients developed CI-AKI. As shown in Table 1, The CI-AKI group was older and had a higher prevalence of DM and CVA. BMI, LVEF, hemoglobin, albumin and pre-procedural Modification of Diet in Renal Disease estimated glomerular filtration rate (MDRD eGFR) levels were lower, while the level of pre-procedural plasma glucose, uric acid and phosphorus were higher in the CI-AKI group. In the lipid profile, only high density lipoprotein cholesterol (HDL-C, median: 35.0 mg/dL, interquartile range [IQR]: 29.0 to 41.0 mg/dL vs. median: 41.0 mg/dL, IQR: 34.0 to 49.0 mg/dL, P < 0.001) level were lower in the CI-AKI group.
Predictors of the development of CI-AKI and long term mortality
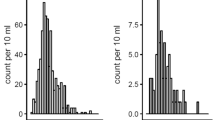
Univariate and multivariate logistic regression analyses were performed to assess the effects of variables on CI-AKI development. Potential confounders (features that differed between the two groups) and variables with P < 0.1 in univariate analyses were included in the multivariate model. In multivariate analysis including HDL-C as quartiles rather than continuous variables, a significant linear trend was observed between the quartiles of HDL-C (quartile 1 vs. 2: odds ratio [OR], 0.716; 95% confidence interval [CI], 0.421–1.219; quartile 1 vs. 3: OR, 0.534; 95% CI, 0.301–0.947; quartile 1 vs. 4: OR, 0.173; 95% CI, 0.079–0.377; P for trend < 0.001) In addition, history of CVA (OR 2.023, 95% CI 1.142–3.587; P = 0.016), LVEF (OR 0.960, 95% CI 0.945–0.975; P < 0.001) and non-HDL-C (OR 1.006, 95% CI 1.001–1.010; P = 0.013) were independent variables for the development of CI-AKI (Table 2). HDL-C levels as quartiles were also negatively correlated with the incidence of CI-AKI whereas non-HDL-C levels as quartiles were not positively correlated with the incidence of CI-AKI (Fig. 1).
(A) Relationship between the pre-procedural HDL cholesterol level and CI-AKI. The association between HDL cholesterol level and the percentage of patients with CI-AKI was significant (P < 0.001 overall and for the trend). (B) Relationship between the pre-procedural non-HDL cholesterol level and CI-AKI. The association between pre-procedural non-HDL cholesterol level and the percentage of patients with CI-AKI was not significant (P = 0.149 overall and for the trend).
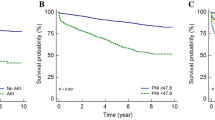
In multivariate Cox regression analysis of the long term mortality, the hazard ratio (HR) of HDL-C quartile 4, using the HDL-C quartile 1 as the reference category, was 0.516 (95% CI, 0.320–0.832, P = 0.007). In addition, age (HR 1.063, 95% CI 1.043–1.084; P < 0.001), LVEF (HR 0.963, 95% CI 0.952–0.975; P < 0.001), albumin (HR 0.516, 95% CI 0.320–0.832; P < 0.001) and CI-AKI (HR 1.063, 95% CI 1.043–1.084; P < 0.001) (Table 3).
Figure 2 shows the Kaplan-Meier plot of patient survival by HDL-C quartiles. As shown, the survival was lower in the patients with the lowest HDL-C quartile compared with those with the highest HDL-C quartile (P = 0.001).
Discussion
Our study showed a higher HDL-C level was associated with the decreased risk of CI-AKI and the better long term survival in CKD patients undergoing PCI.
The CKD population is known to be at higher risk of cardiovascular diseases, because their CKD state itself is associated with increased inflammation and oxidative stress, all of which are well known etiologies of atherosclerosis12,13. Percutaneous interventional procedures performed in this population are at higher risk of CI-AKI4. The contrast medium during the procedure is known to constrict renal arteries and decrease renal blood flow which contributes to ischemic insults in the kidneys. Concentrated stasis of iodinated contrast in renal tubules is also known to cause direct renal tubular toxicities and injuries. The resultant reactive oxygen species-mediated oxidative stress by these two mechanisms induces an inflammatory response in the kidneys, which may manifest as CI-AKI14,15,16. Hypotension or dehydration aggravates these ischemic renal insults, whereas N-acetylcysteine, which has been used for prevention of CI-AKI, is known to detoxify reactive oxygen species by acting as a free radical scavenger. CI-AKI may develop in patients with IHD undergoing coronary angiography and is known to be associated with poor clinical outcomes although the causes of CI-AKI can be multifactorial.
Dyslipidemia in CKD patients is characterized by dysregulation of the synthesis and activity of HDL-C and of the metabolism of triglyceride rich apolipoprotein B containing lipoproteins, which lead to the elevated plasma triglyceride and depressed HDL-C levels17. The combined oxidative stress, inflammation and dyslipidemia in CKD can accelerate atherosclerosis, the basic pathophysiology of all kinds of vascular disease. There is also a point of view that dyslipidemia makes significant contribution to CKD development suggesting that glomerulosclerosis and progressive glomerular and tubulointerstitial diseases are part of a spectrum of inter-related clinical disorders, including atherosclerosis, dyslipidemia, and oxidative and inflammatory stresses18,19. Several epidemiology studies have also documented an association between dyslipidemia and the CKD progression; the population with normal renal function who had low HDL-C and high non-HDL-C levels were at increased risk of CKD development20 whereas increased HDL-C levels were associated with a decreased risk of CKD progression21. Hypertriglyceridemia is also reported as an independent risk factor for developing CKD including proteinuria22,23.
It has not yet been established whether dyslipidemia per se is a risk factors for CI-AKI, despite the fact that several clinical studies have shown a benefit of pre-procedural statin in reducing CI-AKI24,25,26,27,28. The role of HDL-C in the development of CI-AKI is currently unknown. Only one study showed that a low HDL-C level was one of the significant risk factor of CI-AKI after PCI29. HDL-C, in addition to its well-known reverse cholesterol transport effect that lowers the total cholesterol level, has anti-oxidant, anti-inflammatory, and anti-apoptotic effects against the development and progression of atherosclerosis30,31. The functional integrity of HDL-C is as important as HDL-C level itself for its antiatherogenic effect. Some trials attempted to increase the HDL level by using torcetrapib, a cholesteryl ester transfer protein inhibitor, in which increased HDL levels were paradoxically linked to increased cardiovascular morbidities and mortalities30,32. Increasing these functional properties of HDL cannot be achieved by simply increasing its levels. Furthermore, recent studies suggest that systemic oxidative stress and inflammation in CKD substantially reduce the antioxidant and anti-inflammatory capabilities of HDL-C and even can convert HDL-C into a prooxidant and proinflammatory agent17,33.
Our study has limitations. First, the data were retrospectively analyzed, so unadjusted confounding factors may exist. Second, we showed that HDL cholesterol levels were inversely correlated with CI-AKI development without performing any functional assessment, despite the fact that recent studies have dealt with the quality of HDL cholesterol rather than its quantity.
In conclusion, low HDL-C level is associated with the increased risk of CI-AKI and long term mortality in the CKD patients undergoing PCI. Our study suggests that more intensive strategies including proper hydration, N-acetylcysteine use, exposure to less volume of contrast media, and careful monitoring of urine output and renal function, should be considered for preventing CI-AKI in CKD patients with low serum HDL-C level who is planned for PCI.
Methods
Study population
The study population was extracted from from the Catholic Medical Center percutaneous coronary intervention (COACT) registry, a prospective multicenter, all comer-based registry of patients undergoing PCI with drug eluting stents (DES) in 8 hospitals affiliated with the Catholic Medical Center in South Korea between January 1, 2004 to December 31, 2013. Among the 8334 patients with follow up laboratory data of more than 2 weeks duration after the index PCI, patients with end stage renal disease, those on hemodialysis or peritoneal dialysis (n = 426) and renal transplant recipients (n = 125) were excluded.
A total of 7783 patients were subsequently identified. In the patients with acute coronary syndrome (ACS), emergency coronary angiography(CAG) with PCI (within 2 hours) was performed in ST segment elevation MI (STEMI). In Non-STEMI (NSTEMI) and unstable angina, emergency CAG with PCI was also performed when refractory or recurrent angina, signs/symptoms of heart failure, new/worsening mitral regurgitation, hemodynamic instability, sustained ventricular tachycardia, or ventricular fibrillation was present. Otherwise, early (within 24 hours) or delayed invasive (within 25–72 hours) PCI was performed based on the discretion of the interventional cardiologist. Therefore the entire patient cohort can be divided into two groups consisting of emergency PCI (n = 2950) and non-emergency PCI (n = 4833). The non-emergency group was comprised of patients with ACS treated by early or delayed invasive PCI and patients diagnosed with stable angina who were electively treated by PCI. From this non-emergency PCI group, we extracted 1592 CKD patients who were diagnosed with stable angina and electively treated by elective PCI. CKD was defined as MDRD eGFR less than 60 mL/min/1.73 m2, the presence of albuminuria (urinary albumin-to-creatinine ratio greater or equal than 30 mg/g) or proteinuria (a spot urine protein-to-creatinine ratio [UPCR] greater or equal than 300 mg/g) for >3 months, which is the accepted cutoff value of CKD by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines34. All patients received pre-procedure intravenous hydration with half normal saline at the rate of 1 mL/kg body weight per hour. After exposure to contrast medium, patients again received half normal saline intravenously at a rate of 1 mL/kg/h for 12 hours. This hydration rate was reduced in patients with decreased LVEF or overt heart failure. The contrast agent used for primary PCI was iodixanol, an isosmolar nonionic dimer (Visipaque; GE Healthcare, Princeton NJ, USA).The registry was approved by the Institutional Review Board of the Catholic Medical Center of Korea (IRB No.XC11RIMI0107K).
Definition
CI-AKI was defined as an absolute increase in serum creatinine (SCr) by >0.3 mg/dL or a relative increase of SCr by >50%, in accordance with the Acute Kidney Injury Network criteria. CI-AKI was diagnosed based on pre- and post-PCI SCr measurements. The pre-PCI SCr level was defined as the level measured just before the procedure. The post-PCI SCr level was defined as the highest level measured within 7 days of PCI.
The MDRD eGFR (mL/min/1.73 m2) was calculated using the equation: eGFR = 175 × SCr (mg/dL)−1.154 × (age)−0.203 × (0.742 if female).
Collection of clinical and laboratory data
Variables included in the analysis were: demographics (age, gender, height, and body weight [BW]), medical history (presence of DM, hypertension and CVA), smoking status, LVEF measured by echocardiography, laboratory findings; hemoglobin, albumin, phosphorus, uric acid, pre-procedural plasma glucose, UPCR, lipid profile including total cholesterol (TC), HDL-C, non-HDL-C and measured low density lipoprotein cholesterol (LDL-C) levels except triglyceride level because triglyceride level should be measured only in the fasting state, measured before PCI, and SCr levels before and after PCI measured during hospitalization, and contrast volume (CV) used during PCI. After hospital discharge, assessment of the survival status and validation of clinical outcomes were performed by collecting records from the outpatient clinic or by follow-up telephone interviews.
Statistical analyses
Continuous variables are presented as mean ± SD and were compared using t-test for independent samples. Variables not normally distributed are presented as median and IQR and were compared with the Mann–Whitney U test. Categorical variables were compared using a chi-square test or Fisher’s exact test, as appropriate. Binary logistic regression for estimating the OR and 95% CI was used to identify the predictive factors for the development of CI-AKI. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional-hazard regression models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for the long term mortality. Variables with a variance inflation factor greater than 10 were considered to have a multicollinearity problem. All p values were two-tailed, and P values < 0.05 were considered significant. Statistical analyses were performed using SPSS version 15.0 for Windows (SPSS, Chicago, IL, USA).
Additional Information
How to cite this article: Park, H. S. et al. HDL Cholesterol Level Is Associated with Contrast Induced Acute Kidney Injury in Chronic Kidney Disease Patients Undergoing PCI. Sci. Rep. 6, 35774; doi: 10.1038/srep35774 (2016).
References
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351, 1296–1305, 10.1056/NEJMoa041031 (2004).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538, 10.1046/j.1523-1755.2002.00600.x (2002).
McCullough, P. A. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J. Am. Coll. Cardiol. 41, 725–728 (2003).
Davidson, C. et al. Contrast medium use. Am. J. Cardiol. 98, 42–58, 10.1016/j.amjcard.2006.01.023 (2006).
McCullough, P. A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 51, 1419–1428, 10.1016/j.jacc.2007.12.035 (2008).
Solomon, R. J. et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clinical journal of the American Society of Nephrology : CJASN 4, 1162–1169, 10.2215/cjn.00550109 (2009).
Mehran, R. et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J. Am. Coll. Cardiol. 44, 1393–1399, 10.1016/j.jacc.2004.06.068 (2004).
Calvin, A. D., Misra, S. & Pflueger, A. Contrast-induced acute kidney injury and diabetic nephropathy. Nature reviews. Nephrology 6, 679–688, 10.1038/nrneph.2010.116 (2010).
Marenzi, G. et al. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am. Heart J. 160, 1170–1177, 10.1016/j.ahj.2010.09.022 (2010).
Marenzi, G. et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 44, 1780–1785, 10.1016/j.jacc.2004.07.043 (2004).
Stolker, J. M. et al. Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography. J. Am. Coll. Cardiol. 55, 1433–1440, 10.1016/j.jacc.2009.09.072 (2010).
Kovesdy, C. P., Trivedi, B. K. & Anderson, J. E. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Advances in chronic kidney disease 13, 183–188, 10.1053/j.ackd.2006.01.005 (2006).
Vaziri, N. D. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. American journal of physiology. Renal physiology 290, F262–F272, 10.1152/ajprenal.00099.2005 (2006).
Toprak, O. et al. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant 23, 853–859, 10.1093/ndt/gfm691 (2008).
Goodman, A. I. et al. Heme oxygenase-1 protects against radiocontrast-induced acute kidney injury by regulating anti-apoptotic proteins. Kidney Int. 72, 945–953, 10.1038/sj.ki.5002447 (2007).
Efrati, S. et al. Differential effects of N-acetylcysteine, theophylline or bicarbonate on contrast-induced rat renal vasoconstriction. Am. J. Nephrol. 29, 181–191, 10.1159/000154471 (2009).
Vaziri, N. D., Navab, M. & Fogelman, A. M. HDL metabolism and activity in chronic kidney disease. Nature reviews. Nephrology 6, 287–296, 10.1038/nrneph.2010.36 (2010).
Gyebi, L., Soltani, Z. & Reisin, E. Lipid nephrotoxicity: new concept for an old disease. Current hypertension reports 14, 177–181, 10.1007/s11906-012-0250-2 (2012).
Ruan, X. Z., Varghese, Z. & Moorhead, J. F. An update on the lipid nephrotoxicity hypothesis. Nature reviews. Nephrology 5, 713–721, 10.1038/nrneph.2009.184 (2009).
Schaeffner, E. S. et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 14, 2084–2091 (2003).
Muntner, P., Coresh, J., Smith, J. C., Eckfeldt, J. & Klag, M. J. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 58, 293–301, 10.1046/j.1523-1755.2000.00165.x (2000).
Massy, Z. A. et al. Dyslipidaemia and the progression of renal disease in chronic renal failure patients. Nephrol Dial Transplant 14, 2392–2397 (1999).
Tozawa, M. et al. Triglyceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int. 62, 1743–1749, 10.1046/j.1523-1755.2002.00626.x (2002).
Leoncini, M. et al. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J. Am. Coll. Cardiol. 63, 71–79, 10.1016/j.jacc.2013.04.105 (2014).
Roberts, A. Prevention: Rosuvastatin can prevent contrast-induced AKI. Nature reviews. Cardiology 10, 679, 10.1038/nrcardio.2013.166 (2013).
Han, Y. et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J. Am. Coll. Cardiol. 63, 62–70, 10.1016/j.jacc.2013.09.017 (2014).
Patti, G. et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty–contrast-induced nephropathy] trial. Am. J. Cardiol. 108, 1–7, 10.1016/j.amjcard.2011.03.001 (2011).
Ball, T. & McCullough, P. A. Statins for the prevention of contrast-induced acute kidney injury. Nephron. Clinical practice 127, 165–171, 10.1159/000363202 (2014).
Fu, N. K. et al. Risk factors and scoring system in the prediction of contrast induced nephropathy in patients undergoing percutaneous coronary intervention. Zhonghua yi xue za zhi 92, 551–554 (2012).
Eren, E., Yilmaz, N. & Aydin, O. High Density Lipoprotein and it’s Dysfunction. The open biochemistry journal 6, 78–93, 10.2174/1874091x01206010078 (2012).
Cannon, C. P. High-density lipoprotein cholesterol and residual cardiometabolic risk in metabolic syndrome. Clinical cornerstone 8 (suppl 6), S14–S23 (2007).
Podrez, E. A. Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin. Exp. Pharmacol. Physiol. 37, 719–725, 10.1111/j.1440-1681.2010.05380.x (2010).
Moradi, H., Pahl, M. V., Elahimehr, R. & Vaziri, N. D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Translational research: the journal of laboratory and clinical medicine 153, 77–85, 10.1016/j.trsl.2008.11.007 (2009).
Summary of Recommendation Statements. Kidney international supplements 3, 5–14, 10.1038/kisup.2012.77 (2013).
Author information
Authors and Affiliations
Contributions
H.S.P., C.J.K., K.C., D.C.J. and K.-B.S. researched idea and study design. C.J.K., B.-H.H., T.-H.K., C.-S.P., J.M.L., H.-Y.K., D.S.J. and K.-D.Y. performed data collection. H.S.P., C.J.K., Y.S.K., H.-J.P., S.-H.H., S.W.J., P.-J.K., K.C., D.C.J. and K.-B.S. analyzed and interpreted data. H.S.P., C.J.K., K.C., D.C.J. and K.-B.S. performed statistical analysis. All authors read and agreed to the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Park, H., Kim, C., Hwang, BH. et al. HDL Cholesterol Level Is Associated with Contrast Induced Acute Kidney Injury in Chronic Kidney Disease Patients Undergoing PCI. Sci Rep 6, 35774 (2016). https://doi.org/10.1038/srep35774
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35774
This article is cited by
-
Preprocedural Lp(a) level and ApoB/ApoA-Ι ratio and the risk for contrast-induced acute kidney injury in patients undergoing emergency PCI
Lipids in Health and Disease (2021)
-
Effect of iodinated contrast media on renal perfusion: A randomized comparison study in pigs using quantitative contrast-enhanced ultrasound (CEUS)
Scientific Reports (2017)
-
Efficacy of alprostadil for preventing of contrast-induced nephropathy: A meta-analysis
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.