Abstract
Association between allergic conditions and prostate cancer risk has been investigated for many years. However, the results from available evidence for the association are inconsistent. We conducted a meta-analysis to evaluate the relationship between allergic conditions (asthma, atopy, hay fever and “any allergy”) and risk of prostate cancer. The PubMed and Embase databases were searched to screen observational studies meeting our meta-analysis criteria. Study selection and data extraction from included studies were independently performed by two authors. Twenty studies were considered eligible involving 5 case-control studies and 15 cohort studies. The summary relative risk (RR) for developing prostate cancer risk was 1.04 (95%CI: 0.92–1.17) for asthma, and 1.25 (95%CI: 0.74–2.10) for atopy, 1.04 (95%CI: 0.99–1.09) for hay fever, 0.96 (95%CI: 0.86–1.06) for any allergy. In the Subgroup and sensitivity analysis, similar results were produced. Little evidence of publication bias was observed. The present meta-analysis of observational studies indicates that no indication of an association between allergic conditions and risk of prostate cancer was found, and the meta-analysis does not support neither the original hypothesis of an overall cancer protective effect of allergic conditions, nor that of an opposite effect in the development of prostate cancer.
Similar content being viewed by others
Introduction
Prostate cancer is one of most frequently diagnosed cancer in Western countries and is the second most common cancer in men, following lung cancer, worldwide1,2. In 2015, up to 220,800 men were diagnosed with prostate cancer, and 27,540 men will die of it in the United States1. The incidence and mortality rates of prostate cancer vary markedly among different ethnic groups, with the lowest rates found in China and other parts of Asia and the highest rates detected in Western populations3. The etiology of prostate cancer comprises multiple factors. Established risk factors for prostate cancer included obesity, old age, ethnicity, androgen, and environmental factor, androgen, and diet4,5,6,7. There are some studies addressing systematic inflammation conditions and immune response that contribute to prostate tumorigenesis8,9.
Allergy-related carcinogenesis is a topic of interest but has generated considerable controversy. Considering the impact of the prostate cancer risk potentially resulting from allergic diaseases, a number of studies have explored the association between allergic diseases and prostate cancer risk10,11,12,13,14,15,16,17,18,19. However, individual studies have yielded inconsistent or conflicting findings, possibly caused by limitation associated with an individual study. In a previous meta-analysis of studies (only included nine studies), asthma, hay fever and allergy were not associated with prostate cancer risk, but not for atopy20. Two additional studies were not included in the meta-analysis21,22. Subsequent publishing studies have also found inconsistent results of associations between allergic disease and prostate cancer risk, with positive association23,24,25,26, inverse association27, and no association28,29,30,31. To shed light on these contradictory results and to more precisely evaluate the relationship among allergic diseases and prostate cancer, we performed an up-dated meta-analysis of published studies. Nevertheless, clarifying a relationship might emphasize the importance of considering additional preventative methods for prostate cancer. The study was reported in accordance with the Preferred Reporting Items for Systematic Reviews32.
Results
Literature search
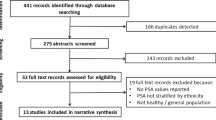
Following the development of our search strategy, 1,890 records were initially retrieved. After excluding the duplicates and articles that did not meet the inclusion criteria, we reviewed 34 possible relevant studies in full-text. A total of 14 studies were excluded for the following reasons: Two articles were covered the same population27,31; six articles were narrative reviews33,34,35,36,37,38; one article reported the association allergic disorders and pancreatic cancer39; and six articles were not related to outcome of interest40,41,42,43,44,45. Finally, 20 studies that met inclusive criteria were included in the meta-analysis (Fig. 1).
Study characteristics
The Tables 1 and 2 shows the descriptive data for all included studies. A total of 20 studies, comprising 5 case-control studies with 2,924 incident cases and 7,175 controls and 15 cohort studies including 16,526 cases and 1,681,562 pariticipants, contributed to the meta-analysis. These studies were published from 1985 to 2015. The number of prostate cancer patients ranged from 1 to 6,294 in the case-control studies and from 10 to 1,936 in the cohort studies. Ten studies were conducted in Europe10,12,13,14,17,23,24,28,29,30, six in the North America11,16,18,19,22,31, two in Australia15,25 and two in Asia21,26. Eighteen studies reported findings for prostate cancer incidence10,11,12,13,14,16,17,18,19,21,22,23,24,25,26,28,29,31, whereas only two studies reported results for prostate cancer mortality16,30. We included a total of 5,757 prostate cancer deaths, 16,526 prostate cancer cases in the meta-analysis. The maximum numbers (1,102,247) of participants were from the Canada prospective study and minimum number (1,522) were from Australia prospective study in the cohort studies, while the maximum incident patients (1,936) were from the Canada Montreal PROtEuS study and minimum incident patients (10) were from Japan study in the case-control studies. The exposure categories that were measured were: 1) asthma; 2) hay fever (rhinitis); 3) atopy; and 4) any allergy (atopy and/or asthma, hay fever, or other allergic disease). For the assessment of allergic conditions, allergen-specific IgE measurement, skin prick testing, self-reported questionnaire, interviews by medical staff, and hospital discharge register were employed. For the assessment of prostate cancer, fourteen studies reported that cancers were histologically confirmed or were identified from national/regional cancer registeries, which we assumed verified the cancer pathologically. The remaining studies based their cancer diagnoses on different criteria; admission/discharge diagnoses; an automated general practice database; a linked national death register; review of hospital and nursing home recorder and death certificates. Six studies were designed to evaluate OR10,17,18,19,22,31, five evaluated HR15,25,26,29,30, three evaluated RR11,16,21,28, while five studies compared observed cancer incidence rates in cohort of patients with asthma or hay fever/allergic rhinitis against expected numbers estimated from population-based cancer registries and their effect estimates were standardized incidence ratio (SIR)13,14,23,24 or standardized mortality ratio(SMR)12.
Adjusted effect estimates could be determined for most cohort and case-control studies. Most risk estimates were adjusted for age (n = 12)10,11,15,17,18,19,22,25,26,28,30,31, smoking (n = 10)10,11,15,16,17,18,19,25,28,29 and body mass index (n = 8)10,15,16,17,25,28,29,30. Some studies were also controlled for alcohol consumption (n = 5)10,16,17,25,29 and race (n = 3)16,19,28, but few studies were adjusted for family history of prostate cancer (n = 2), total energy intake (n = 1)25, and intake of vegetable and red meat (n = 1)16. None of the studies were adjusted for exposure to heavy metals and androgen.
The methodological quality of the included studies was generally good. The NOS scores ranged from five to seven (Table 3). The median NOS score was 6.0.
Asthma and risk of prostate cancer
The association of asthma with prostate cancer was investigated in 17 studies10,11,12,13,14,15,16,17,18,21,22,24,25,26,28,30,31. Thirteen studies reported HR/RR/OR10,11,15,16,17,18,21,22,25,26,28,30,31, while four studies reported the SIR/SMR12,13,14,24. The combined relative risk was 1.04 (95%CI: 0.92–1.17), with significant heterogeneity (Pfor heterogeneity = 0.000; I2 = 73.2%), while the pooled SIR was 1.00 (95%CI: 0.68–1.47), with significant heterogeneity (Pfor heterogeneity = 0.000; I2 = 97.7%) (Fig. 2). In subgroup and sensitivity analysis, the results showed basically consistent with the overall analysis (Table 4). When we stratified the analysis by geographic region, the pooled RR was 0.95 (95%CI: 0.81–1.12) for studies conducted in North America, 0.90 (0.81–1.00) for studies conducted in Europe, and 4.55 (0.23–89.94) for studies conducted in Asia. In the subgroup analysis stratified by NOS quality, the combined RR was 1.90 (95%CI: 0.29–12.61) for low quality studies and 1.02 (95%CI: 0.93–1.13) for high quality studies. We restricted each analysis to study design, the combined RR was 1.02 (95%CI: 0.91–1.15) among case-control studies and 1.20 (94%CI: 0.69–2.09) among cohort studies, respectively. Nevertheless, when we stratified the analysis by adjusted for age, race, BMI, cigarette smoking and alcohol drinking, asthma was also not associated with risk of prostate cancer. In a sensitivity analysis, similar results were observed, which ranged from 1.01 (95%CI: 0.88–1.16) with significant heterogeneity (Pfor heterogeneity = 0.000, I2 = 73.7%) (excluding the study by Severi G et al.25) to 1.07 (95%CI: 0.93–1.23) with significant heterogeneity (Pfor heterogeneity = 0.000, I2 = 69.2%) (excluding the study by Platz et al.28). Only four of studies on asthma specially investigated advanced prostate cancer risk (advanced prostate cancer was defined as T3-4 and PSA > 50 ng/ml or Gleason grade ≥ 8) and asthma25,28, the summary RR was 0.86 (95%CI: 0.54–1.37), with significant heterogeneity (Pfor heterogeneity = 0.079; I2 = 67.6%). Egger funnel plot asymmetry test (P = 0.865) and Begg rank correlation test (P = 0.502) were performed to assess publication bias and the funnel plot symmetry (Fig. 3) was examined. Finally, no proof of publication bias was obtained. Because of limited number of studies, we fail to conduct subgroup and sensitivity, publication analysis for studies reported the SIR or SMR.
Hay fever and risk of prostate cancer
The association of hay fever with prostate cancer was examined in 7 studies10,11,16,18,23,28,31. The six studies reported RR/OR10,11,16,18,28,31 (Fig. 4), while SIR was reported in only one study, which reported a positive association between hay fever/allergic rhinitis and prostate cancer risk23. The combined relative risk was 1.04 (95%CI: 0.99–1.09), with low heterogeneity (Pfor heterogeneity = 0.428; I2 = 0.0%). Subgroup and sensitivity analysis produced similar results (Table 4). In subgroup analysis stratified by geographic region, asthma was not associated with risk of prostate cancer (RR: 0.97; 95% CI: 0.88–1.06) in the four studies in North Ameria, but not in the two studies in Europe (RR:1.07; 95%CI: 1.01–1.13). Compared with a low NOS score (RR = 1.02, 95%CI: 0.94–1.11), the association was higher among studies with high NOS score (RR = 1.15, 95% CI: 0.78–1.70). When we restricted each analysis to study design, the combined RR was 1.03 (95%CI: 0.93–1.14) among case-control studies and 1.02 (95%CI: 0.76–1.36) cohort studies, respectively. When the analysis was restricted to studies adjusted for race, BMI and cigarette smoking, we found no association between asthma and prostate cancer, but not for studies adjusted for age and alcohol drinking. In a sensitivity analysis, similar results were observed, which ranged from 0.97 (95%CI: 0.88–1.06) with low heterogeneity (Pfor heterogeneit = 0.360, I2 = 9.0%) (excluding the study by Platz et al.28) to 1.07 (95%CI: 1.01–1.13) with significant heterogeneity (Pfor heterogeneit = 0.025, I2 = 58.4%) (excluding the study by Turne et al. which reported the association between asthma and mortality of prostate cancer16). Both the Begg rank correlation test (P = 0.707) and the Egger linear regression test (P = 0.775) in the meta-analysis indicated no significant publication bias. Because number of included studies less than 10, we did not perform the funnel plot.
Atopy and risk of prostate cancer
The association of atopy with prostate cancer was investigated in 3 studies10,15,29. The combined relative risk was 1.25 (95%CI: 0.74–2.10), with notable heterogeneity (Pfor heterogeneity = 0.024; I2 = 73.2%) (Fig. 5). In subgroup and sensitivity analysis, the results showed basically consistent with the overall analysis (Table 4). The include studies achieved six or more stars and considered to be of high quality, so the result was consistent with overall analysis. When the analysis was restricted to studies adjusted for age, race, BMI, alcohol drinking and cigarette smoking, we found no association between asthma and prostate cancer. In a sensitivity analysis, similar results were observed, which ranged from 1.05 (95%CI: 0.62–1.77) with low heterogeneity (Pfor heterogeneity = 0.036, I2 = 77.6%) (excluding the study by Talbot-Smith et al.15) to 1.59 (95%CI: 0.93–2.71) with significant heterogeneity (Pfor heterogeneity = 0.193, I2 = 41.0%) (excluding the study by Skaaby et al.29). Both Egger’s test (P = 1.000) and Bgger’s test (P = 0.761) showed no publication bias. Because number of included studies less than 10, we did not perform the funnel plot.
Any allergy and risk of prostate cancer
The association of asthma with prostate cancer was investigated in 6 studies10,11,16,19,22,31. The combined relative risk was 0.96 (0.86–1.06), with low heterogeneity (Pfor heterogeneity = 0.287; I2 = 19.3%). Subgroup and sensitivity analysis yielded similar result (Fig. 6). In subgroup and sensitivity analysis, the results showed basically consistent with the overall analysis (Table 4). When we stratified the analysis by geographic region, the pooled RR was 0.96 (95%CI: 0.84–1.10) for 5 studies conducted in North America, 0.98 (95%CI: 0.66–1.45) for 1 studies conducted in Europe. Stratifying by study design, the combined RR was 1.06 (95%CI: 0.84–1.33) among case-control studies and 0.88 (95%CI: 0.75–1.04) among cohort studies, respectively. Compared with a low NOS score (SMR = 2.08, 95%CI: 0.73–5.91), the association was significant among studies with high NOS score (OR = 1.51, 95%CI: 1.14–1.98). In subgroup analysis adjusted for risk factors, including age, race, smoking and alcohol drinking, the results was consistent with overall analysis. In a sensitivity analysis, similar results were observed, which ranged from 0.93 (95%CI: 0.86–1.00) with low heterogeneity (Pfor heterogeneity = 0.612, I2 = 0.0%) (excluding the study by Mill et al.11) to 0.99 (95%CI: 0.82–1.19) with significant heterogeneity (Pfor heterogeneity = 0.194, I2 = 34.1%) (excluding the study by Turner et al.16). The Begg rank correlation test (P = 0.133) and Egger linear regression test (P = 0.489) also indicated no evidence of publication bias. Because number of included studies less than 10, we did not perform the funnel plot.
Discussion
Allergic diseases (immune mediated conditions), encompassing hay fever, allergic asthma and atopy, are caused by inappropriate immunological response to antigens that do not elicit response in most individuals. Allergy-related carcinogenesis is a topic of interest but has generated considerable controversy. There has been a long-standing interest in determining whether individuals with allergic diseases have an altered risk of developing cancer. Several studies investigated the association between allergic diseases and specific cancers. Asthma, hay fever, and atopy have been associated with the risk of several specific cancers, such as pancreatic cancer, lymphomas, brain tumors, breast cancer and leukemia, although inconsistently10,14,15,46,47. Allergic diseases could theoretically both prevent and induce the development of several specific cancers. Two hypotheses that attempt to explain the possible mechanism between allergic diseases and cancer are immune surveillance and the antigenic stimulation theory48. Allergy might enhance the human immune system to recognize and eliminate cancer cells. In contrast, the antigenic stimulation hypothesis proposes that hyperactive immune conditions trigger chronic cellular inflammation, resulting in DNA mutation in dividing cells and inevitably leading to cancer development49. The determination of whether asthma, hay fever, atopy and any allergy are associated with prostate cancer has been evaluated in a small number of studies. The small number of prior studies evaluating allergic disease and prostate cancer has not produced consistent results, inverse12,16,18, null13,14,15,17,28,29,30,31, and positive associations10,11,19,21,22,23,24,25,26,27 have been reported. The inconsistent results of previous studies may be due to insufficient study sample size, publication bias, selection bias, lack of adjustment for confounding factors, and the use of different definitions of allergic diseases. A recent meta-analysis of the few studies suggested that asthma (N = 8, pooled RR: 0.93; 95%CI: 0.76–1.15), hay fever (N = 5, pooled RR: 0.96; 95%CI: 0.87–1.05), and any allergy (N = 4, pooled RR: 1.01; 95%CI: 0.87–1.17), but not for atopy (N = 3, pooled RR: 1.43; 95%CI: 1.08–1.91)20. However, studies published subsequent to this report have reported inconsistent results23,24,25,26,27,28,29,30,31. In addition, the relation between allergic diseases and cancer risk remain unclear, and appear to be site-specific, we conducted an up-dated meta-analysis to summarize the current proof to evaluate the association between allergic conditions and prostate cancer risk. The meta-analysis suggested that there is little observational support for the two theories in the development of prostate cancer. The effect of hyperreactive state and/or immune surveillance theories maybe mutually offset in the body.
The present meta-analysis exhibited several strengths, compared to the previous published meta-analysis. The first research highlight of this meta-analysis is its large sample size. The large number of total cases provided high statistical power to quantitatively evaluate the association between allergic conditions and prostate cancer risk. In addition, we expand the meta-analysis and the association between different geographic regions and study design, adjusted for covariates was also explored. Second, publication bias is a potential concern in any meta-analysis because small studies with null results do not get published. However, in our meta-analysis, we found little evidence of publication bias.
Nevertheless, there are some several limitations in the present meta-analysis. First, case-control studies have intrinsic limitations, such as selective bias and recall or memory bias. This limitation can partly explained the different results between case-control and cohort studies in the stratified analysis. Second, we cannot exclude the possibility that the observed null relationship between allergic disorders and prostate cancer risk is attributed to confounding factors. Majority of the studies were adjusted for potential confounding factors, but not all potential confounders were adjusted in every study. Although the allergic conditions and prostate cancer share common some potential confounders, such as cigarette smoking, alcohol drinking, old age, BMI and race, in analysis stratified by adjusting the smoking status, age, alcohol drinking BMI and race, similar results were obtained. Third, measurement error in assessment of allergic conditions are known to bias effect estimates, however, none of included studies in the meta-analysis made any corrections for measurement errors. An accurate assessment of allergic conditions remains a challenge, because these measures are based on different assessment methods, such as allergen-specific IgE measurement, skin prick testing, self-reported questionnaire, interviewed by medical staff, and hospital discharge register. Diagnosis of allergic conditions was largely based on a self-reported. Only a few studied were based on measurements that may be condisered to be more objective: serum LgE or skin prick tests. The increasing errors in measurements become inevitable. The imprecise measurement of allergic disorders might have attenuated the true associations. Fourth, potential sources of between-study heterogeneity, which is common in meta-analysis, should be explored although the low heterogeneity was found between hay fever or atopy and prostate cancer risk. Results from subgroup and sensitivity analysis indicated that geographic region, study design, quality of NOS may be potential sources of heterogeneity. Thirds, in subgroup analysis, there was a marginally positive association between hay fever and prostate cancer risk in studies that adjusted for age and alcohol drinking and conducted in the Europe, but a null association among studies adjusted for cigarette smoking and race. Due to the numerous comparisons and few included studies, this positive finding may have been a chance finding. It indicated that more relevant articles are needed to further explore this association. Fifth, analysis of the length of the induction period allows for characterising an exposure–outcome relationship and for falsifying the pathway assumed. The longer the supposed time sequence between the exposure and the occurrence of the outcome, the more crucial to analyze the empirical induction period. The average follow-up ranged from 5.05 years to 43 years among included cohort studies. Patients were followed up over five years in majority of the studies (92.8%). Therefore, the observation period in the included cohort studies covered a reasonable induction period. Overall, these limitations may affect our final conclusions.
In the meta-analysis, we found no indication of an association between allergic conditions (asthma, atopy, hay fever, or any allergy) and risk of prostate cancer, and there is little observational support for the immune surveillace theory or antigenic stimulation theory in the development of prostate cancer risk. However, these results should be carefully interpreted because of the significant heterogeneity among studies and potential confounders. Additional large-scale and high-quality prospective studies are needed to confirm the association between allergic conditions and risk of prostate cancer.
Methods
Literature search
A literature search was performed in March 15, 2016 without restriction to regions, publication types, or languages. The primary sources were the electronic databases of Pubmed and Embase databases. To identify eligible studies, the main search employed various combinations of Medical Subject Headings (MeSH) and non-MeSH terms “prostate carcinoma” OR “prostatic cancer” OR “prostate cancer” OR “prostatic carcinoma” combined with “asthma” OR “asthma*” OR “allergy” OR “allerg*” OR “atopy”. The main search was completed independently by two investigators. Any discrepancy was solved by consultation of an investigator, not involved in the initial procedure. Moreover, the reference lists of all the studies and published systematic reviews, meta-analysis were also screened to identify other potentially eligible studies.
Study selection
To minimize the differences between studies, we imposed the following methodological restrictions for the inclusion criteria: 1) study design of interest was either cohort or case control study 2) the exposure of interest was allergic conditions (asthma, atopy, hay fever and “any allergy”); 3) the outcome was prostate cancer; 4) the study reported enough information to extract effect estimates and the corresponding 95% confidence intervals. In case of multiple publications, only the most recent or comprehensive one was considered eligible. Two authors (JKS and XHY) independently evaluated the eligibility of all retrieved studies and disagreements were resolved through discussion or consultation with a third author (LHG).
Data extraction
Data from the included studies were extracted and summarized independently by two of the authors using a pre-standardized data extraction form. Any disagreement was resolved by the senior author (ZWD). The following data were extracted from each study: first author, publication year, study design, country, sex, total number of cases and subjects for cohort studies, total number of cases and controls for case-control studies, assessment methods for allergic diseases, quantitative effect estimates (expressed as an odds ratio, hazard risk, relative risk, standardized mortality ratio, or standardized incidence ration) and 95%CI and variables adjusted for in the meta-analysis. The OR in one study18 was not extracted, thus, we computed the crude risk estimates and corresponding CI. Two review authors (SJK and ZJG) independently extracted data from the selected studies. Any disagreements were resolved through discussion and consensus.
Quality assessment
Two review authors (SJK and ZJG) independently assessed the risk of bias using the Newcastle-Ottawa Scale (NOS), which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study50,51. The studies achieving six or more stars were considered to be of high quality.
Statistical analysis
Because the incidence of prostate cancer risk was low, the OR/HR was considered as approximation of RR, SMR was also considered as equivalent of SIR52. We computed the combined RR and SIR and corresponding 95%CI from the estimated reported in each study. The aggregated results and 95%CIs for effect sizes were calculated using inverse-variance weighted random-effects meta-analysis, which incorporated both within-study and between-study variability53. I2 was used to assess heterogeneity across studies, with I2 values of 0%, 25%, 50% and 75% representing no, low, moderate and high heterogeneity, respectively. Subgroup analysis was stratified by geographic region, study design, quality of NOS scale, body mass index (BMI) and whether the study adjusted for risk factors, including age, race, alcohol drinking and smoking. The sensitivity analysis was also conducted by removing one study at a time and the rest analyzed to determine whether an individual study affected the aggregate result or not. Small study bias, consistent with publication bias, was evaluated by statistical tests (Begg rank correlation test54 and Egger’s linear regression test55), the visual examination of funnel plot when the number of included studies ≥10, and the results were considered to indicate publication bias when P < 0.10. All statistical analyses were conducted using Stata version 13.1 (Stata Corp., College Station, TX, USA).
Additional Information
How to cite this article: Zhu, J. et al. Association between allergic conditions and risk of prostate cancer: A Prisma-Compliant Systematic Review and Meta-Analysis. Sci. Rep. 6, 35682; doi: 10.1038/srep35682 (2016).
References
Siegel, R. L. et al. Cancer statistics for Hispanics/Latinos. CA: a cancer journal for clinicians 65, 457–480, doi: 10.3322/caac.21314 (2015).
Jemal, A. et al. Global cancer statistics. CA: a cancer journal for clinicians 61, 69–90, doi: 10.3322/caac.20107 (2011).
Zhu, Y., Wang, H. K., Qu, Y. Y. & Ye, D. W. Prostate cancer in East Asia: evolving trend over the last decade. Asian journal of andrology 17, 48–57, doi: 10.4103/1008-682X.132780 (2015).
Allott, E. H., Masko, E. M. & Freedland, S. J. Obesity and prostate cancer: weighing the evidence. Eur Urol 63, 800–809, doi: 10.1016/j.eururo.2012.11.013 (2013).
Venkateswaran, V. & Klotz, L. H. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nature reviews. Urology 7, 442–453, doi: 10.1038/nrurol.2010.102 (2010).
Bostwick, D. G. et al. Human prostate cancer risk factors. Cancer 101, 2371–2490, doi: 10.1002/cncr.20408 (2004).
Hsing, A. W. & Chokkalingam, A. P. Prostate cancer epidemiology. Frontiers in bioscience: a journal and virtual library 11, 1388–1413 (2006).
Gurel, B. et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23, 847–856, doi: 10.1158/1055-9965.EPI-13-1126 (2014).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nature reviews. Cancer 7, 256–269, doi: 10.1038/nrc2090 (2007).
Wang, H. et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. International journal of cancer. Journal international du cancer 119, 695–701, doi: 10.1002/ijc.21883 (2006).
Mills, P. K., Beeson, W. L., Fraser, G. E. & Phillips, R. L. Allergy and cancer: organ site-specific results from the Adventist Health Study. American journal of epidemiology 136, 287–295 (1992).
Kallen, B., Gunnarskog, J. & Conradson, T. B. Cancer risk in asthmatic subjects selected from hospital discharge registry. The European respiratory journal 6, 694–697 (1993).
Vesterinen, E., Pukkala, E., Timonen, T. & Aromaa, A. Cancer incidence among 78,000 asthmatic patients. International journal of epidemiology 22, 976–982 (1993).
Eriksson, N. E., Holmen, A., Hogstedt, B., Mikoczy, Z. & Hagmar, L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy 50, 718–722 (1995).
Talbot-Smith, A., Fritschi, L., Divitini, M. L., Mallon, D. F. & Knuiman, M. W. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. American journal of epidemiology 157, 606–612 (2003).
Turner, M. C. et al. Cancer mortality among US men and women with asthma and hay fever. American journal of epidemiology 162, 212–221, doi: 10.1093/aje/kwi193 (2005).
Gonzalez-Perez, A., Fernandez-Vidaurre, C., Rueda, A., Rivero, E. & Garcia Rodriguez, L. A. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol Drug Saf 15, 131–138, doi: 10.1002/pds.1163 (2006).
Vena, J. E. et al. Allergy-related diseases and cancer: an inverse association. American journal of epidemiology 122, 66–74 (1985).
McWhorter, W. P. Allergy and risk of cancer. A prospective study using NHANESI followup data. Cancer 62, 451–455 (1988).
Vojtechova, P. & Martin, R. M. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer causes & control: CCC 20, 1091–1105, doi: 10.1007/s10552-009-9334-y (2009).
Ohrui, T. et al. Risk of prostate cancer in older Japanese asthmatics. Journal of the American Geriatrics Society 50, 202 (2002).
Lightfoot, N. et al. Medical history, sexual, and maturational factors and prostate cancer risk. Ann Epidemiol 14, 655–662, doi: 10.1016/j.annepidem.2003.11.006 (2004).
Hemminki, K. et al. Risk of cancer in patients with medically diagnosed hay fever or allergic rhinitis. International journal of cancer. Journal international du cancer 135, 2397–2403, doi: 10.1002/ijc.28873 (2014).
Ji, J. et al. Cancer risk in hospitalised asthma patients. British journal of cancer 100, 829–833, doi: 10.1038/sj.bjc.6604890 (2009).
Severi, G. et al. Asthma, asthma medications, and prostate cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 19, 2318–2324, doi: 10.1158/1055-9965.EPI-10-0381 (2010).
Su, Y. L., Chou, C. L., Rau, K. M. & Lee, C. T. Asthma and Risk of Prostate Cancer: A Population-Based Case-Cohort Study in Taiwan. Medicine 94, e1371, doi: 10.1097/MD.0000000000001371 (2015).
El-Zein, M. et al. History of asthma or eczema and cancer risk among men: a population-based case-control study in Montreal, Quebec, Canada. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology 104, 378–384, doi: 10.1016/j.anai.2010.03.003 (2010).
Platz, E. A. et al. Asthma and risk of lethal prostate cancer in the Health Professionals Follow-Up Study. International journal of cancer. Journal international du cancer 137, 949–958, doi: 10.1002/ijc.29463 (2015).
Skaaby, T., Nystrup Husemoen, L. L., Roswall, N., Thuesen, B. H. & Linneberg, A. Atopy and development of cancer: a population-based prospective study. The journal of allergy and clinical immunology. In practice 2, 779–785, doi: 10.1016/j.jaip.2014.06.010 (2014).
Taghizadeh, N. et al. Objective allergy markers and risk of cancer mortality and hospitalization in a large population-based cohort. Cancer causes & control: CCC 26, 99–109, doi: 10.1007/s10552-014-0489-9 (2015).
Weiss, D. et al. Asthma, allergy and the risk of prostate cancer: results from the Montreal PROtEuS study. Cancer epidemiology 38, 695–699, doi: 10.1016/j.canep.2014.10.003 (2014).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339, b2535, doi: 10.1136/bmj.b2535 (2009).
Josephs, D. H., Spicer, J. F., Corrigan, C. J., Gould, H. J. & Karagiannis, S. N. Epidemiological associations of allergy, IgE and cancer. Clinical and Experimental Allergy 43, 1110–1123 (2013).
Turner, M. C., Chen, Y., Krewski, D. & Ghadirian, P. An overview of the association between allergy and cancer. International journal of cancer. Journal international du cancer 118, 3124–3132, doi: 10.1002/ijc.21752 (2006).
Wang, H. & Diepgen, T. L. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy 60, 1098–1111, doi: 10.1111/j.1398-9995.2005.00813.x (2005).
Merrill, R. M., Isakson, R. T. & Beck, R. E. The association between allergies and cancer: what is currently known? Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology 99, 102–116; quiz 117-109, 150, doi: 10.1016/S1081-1206(10)60632-1 (2007).
Josephs, D. H., Spicer, J. F., Corrigan, C. J., Gould, H. J. & Karagiannis, S. N. Epidemiological associations of allergy, IgE and cancer. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 43, 1110–1123, doi: 10.1111/cea.12178 (2013).
Sherman, P. W., Holland, E. & Sherman, J. S. Allergies: their role in cancer prevention. The Quarterly review of biology 83, 339–362 (2008).
Olson, S. H. et al. Serum immunoglobulin e and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23, 1414–1420, doi: 10.1158/1055-9965.EPI-13-1359 (2014).
Sumino, K. et al. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. The Journal of asthma: official journal of the Association for the Care of Asthma 51, 306–314, doi: 10.3109/02770903.2013.879881 (2014).
Alderson, M. Mortality from malignant disease in patients with asthma. Lancet (London, England) 2, 1475–1477 (1974).
Vandentorren, S. et al. Long-term mortality among adults with or without asthma in the PAARC study. The European respiratory journal 21, 462–467 (2003).
Lindelof, B., Granath, F., Tengvall-Linder, M. & Ekbom, A. Allergy and cancer. Allergy 60, 1116–1120, doi: 10.1111/j.1398-9995.2005.00808.x (2005).
Markowe, H. L. et al. Prognosis in adult asthma: a national study. Br Med J (Clin Res Ed) 295, 949–952 (1987).
Petroianu, A., Chaves, D. N. & De Oliveira, O. Jr. Comparative incidence of allergy in the presence or absence of cancer. The Journal of international medical research 23, 358–363 (1995).
Petridou, E. T. et al. Breast cancer risk in relation to most prevalent IgE specific antibodies: a case control study in Greece. Anticancer research 27, 1709–1713 (2007).
Zhou, M. H. & Yang, Q. M. Association of asthma with the risk of acute leukemia and non-Hodgkin lymphoma. Molecular and clinical oncology 3, 859–864, doi: 10.3892/mco.2015.561 (2015).
Pardoll, D. Does the immune system see tumors as foreign or self? Annual review of immunology 21, 807–839, doi: 10.1146/annurev.immunol.21.120601.141135 (2003).
Lu, H., Ouyang, W. & Huang, C. Inflammation, a key event in cancer development. Molecular cancer research: MCR 4, 221–233, doi: 10.1158/1541-7786.MCR-05-0261 (2006).
Hartling, L. et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. Journal of clinical epidemiology 66, 982–993, doi: 10.1016/j.jclinepi.2013.03.003 (2013).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605, doi: 10.1007/s10654-010-9491-z (2010).
Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiologic reviews 9, 1–30 (1987).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Controlled clinical trials 7, 177–188 (1986).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997).
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81170699, 81360119), Projects of Guangdong Key Laboratory of Clinical Molecular Medicine and Diagnostics, Guangzhou Municipal Science and Technology Key Project (2014J4100072), Outstanding Youth Science and Technology Talent Cultivating Object of Guizhou Province in 2013 (2013–18), International Scientific and Technological Cooperation Projects of Guizhou Province (20137031), China Postdoctoral Science Foundation (2014M562157), Zhejiang provincial medical and health science and technology project(2016KYB265), Guizhou Province Science and Technology Agency - Guizhou Provincial People’s Hospital Joint Fund SY[2010]3124.
Author information
Authors and Affiliations
Contributions
J.S., W.Z., J.Z., Z.L. and J.H. wrote the main manuscript text; J.S., H.L., Y.L., D.Y. and Z.J. prepared Figures 1–6, J.S., N.J., C.W. and Z.S. contributed data analysis; All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhu, J., Song, J., Liu, Z. et al. Association between allergic conditions and risk of prostate cancer: A Prisma-Compliant Systematic Review and Meta-Analysis. Sci Rep 6, 35682 (2016). https://doi.org/10.1038/srep35682
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35682
This article is cited by
-
Atopic allergic conditions and prostate cancer risk and survival in the Multiethnic Cohort study
British Journal of Cancer (2023)
-
Allergy, asthma, and the risk of breast and prostate cancer: a Mendelian randomization study
Cancer Causes & Control (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.