Abstract
Alcoholic hepatitis (AH) is the most severe form of alcoholic liver disease for which there are no effective therapies. Patients with AH show impaired hepatocyte proliferation, expansion of inefficient ductular cells and high lipopolysaccharide (LPS) levels. It is unknown whether LPS mediates ductular cell expansion. We performed transcriptome studies and identified keratin 23 (KRT23) as a new ductular cell marker. KRT23 expression correlated with mortality and LPS serum levels. LPS-TLR4 pathway role in ductular cell expansion was assessed in human and mouse progenitor cells, liver slices and liver injured TLR4 KO mice. In AH patients, ductular cell expansion correlated with portal hypertension and collagen expression. Functional studies in ductular cells showed that KRT23 regulates collagen expression. These results support a role for LPS-TLR4 pathway in promoting ductular reaction in AH. Maneuvers aimed at decreasing LPS serum levels in AH patients could have beneficial effects by preventing ductular reaction development.
Similar content being viewed by others
Introduction
Alcoholic liver disease (ALD) is the main cause of cirrhosis worldwide1 and the main driver of health expenditure in hospitalized patients with liver disease in the US2. In clear contrast with the recent advances in viral hepatitis, there are no targeted therapies for patients with ALD. In particular, there are no effective therapies for alcoholic hepatitis (AH), a frequent and severe presentation of ALD patients that bears a high short-term mortality rate3. Mortality associated with AH is due to profound liver failure and portal hypertension, leading to complications such as variceal bleeding, renal failure and sepsis4,5. Patients with severe AH are particularly prone to bacterial infections, reflecting intense derangement of immune function6. The available therapy (i.e. prednisolone) does not improve survival beyond one month, and targeted therapies are urgently needed7,8,9. Identifying molecular and cellular drivers of AH is a prerequisite to develop such therapies. In fact, there is a current effort by public agencies in the US (i.e. NIH-sponsored international consortia on translational research in AH) to identify novel targets for therapy.
The pathogenesis of AH is largely unknown. Translational studies using human samples have identified several potential molecular targets including the CXC chemokine family, tumor necrosis factor receptor superfamily member 12A, osteopontin, chemokine (C-C motif) ligand 20, members of the inflammasome, interleukin-22, the Hedgehog signaling pathway and macrophage migration inhibitory factor10,11,12,13,14,15,16. Moreover, gut-derived bacterial products such as lipopolysaccharide (LPS) are believed to play a major role by inducing liver inflammation and fibrogenesis through toll-like receptor 4 (TLR4) expressed in both parenchymal and non-parenchymal cells17. Strategies aimed at modifying bacterial dysbiosis and ameliorating intestinal barrier dysfunction and the resulting translocation of endotoxin into the portal circulation may be beneficial in patients with AH18,19. We recently found that LPS serum levels predict mortality in patients with AH and are associated with a poor response to corticosteroids20. The mechanisms by which increased LPS levels are associated with a poor outcome are largely unknown. Human studies suggest that LPS could mediate immune paralysis in these patients and favor infections21,22. We hypothesize that LPS could also play a role in the impaired hepatocellular regeneration in these patients. Recent studies strongly suggest that an inefficient ductular reaction (mostly composed by liver progenitor cells -LPC-) could play a role in AH23,24. Furthermore, markers of hepatic ductular reaction at admission correlate with liver injury and closely predict short-term mortality in AH23 and patients non-responding to therapy show a massive expansion of ductular cells in the liver explants24. Little is known on the factors that regulate the growth and fate of ductular cells in the setting of AH. Investigating the biological properties of these cells could favor the development of novel targeted therapies for AH.
LPS is known to regulate the proliferation and fate in bone marrow, endothelial and dental progenitor cells through TLR4 signaling25,26,27,28,29. It is plausible that increased LPS levels also play a role in the expansion of inefficient ductular cells in AH. To test this hypothesis, we conducted a systems biology approach including a comparative transcriptome analysis of liver from patients with AH and non-alcoholic steatohepatitis (NASH) to find novel markers of ductular cells. The “structural molecule activity” pathway was found to be the most dysregulated pathway, and keratin 23 (KRT23) was the most upregulated gene in this family. Importantly, this keratin was expressed in the ductular reaction in humans and mice. Based on these recent data, we hypothesized that the LPS-TLR4 pathway may stimulate the expansion of ductular reaction and regulates the biological properties of ductular cells in AH.
Results
Identification of KRT23 as a Marker of Ductular Cells in AH
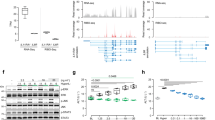
Comparative gene expression profile analysis was performed in patients with severe AH (n = 15, Table 1), NASH (n = 8, Supplementary Table 1) and normal controls (n = 7) that underwent microarray analysis in our lab11,30 in order to identify genes specifically dysregulated in this severe form of ALD. Unsupervised clustering analysis revealed a unique gene expression signature of livers from patients with AH compared to NASH (Fig. 1a). To visualize the main differences in global transcriptional patterns between the three study groups we performed multidimensional scaling analysis of the 30 samples that underwent array profiling (Fig. 1b). Importantly, marked differences in gene expression were noted between livers from patients with AH, patients with NASH and normal controls (P < 0.001). A Venn diagram illustrates different groups of genes differentially regulated in both diseases (Fig. 1c). We found the majority of genes were differentially dysregulated between AH and NASH (1,200 upregulated and 854 downregulated in AH vs. NASH, respectively). Additionally, 296 genes were similarly dysregulated in AH and NASH compared to normal livers, while a smaller group of genes were specifically dysregulated in NASH compared to AH (148 upregulated and 102 downregulated, respectively). Functional analysis of the transcriptome data (GOstats) revealed key pathways differentially regulated in patients with AH when compared to NASH and control livers. Among these pathways, the “structural molecule activity” pathway was found to be the most significantly upregulated in AH (P = 7.5 × 10−9) (Supplementary Table 2). The most upregulated genes that were differentially regulated in this and other significant pathways between patients with AH and NASH are shown in Table 2.
Comparative Functional Analysis of the Transcriptome in AH and NASH.
(a) Heatmap display of the genes with the most significantly different expression between patients with AH, patients with NASH and healthy controls. Rows represent genes, and columns represent samples. The intensity of each color denotes the standardized ratio between each value and the average expression of each gene across all samples. Red pixels correspond to an increased abundance of mRNA in the indicated liver biopsy sample, whereas green pixels indicate decreased mRNA levels. (b) Multidimensional scaling analysis representing the 30 samples that underwent array profiling. The different samples are placed in the three-dimensional space according to their mRNA expression. AH samples are represented in magenta, NASH in light blue and normal livers in red. (c) Venn diagram showing the overlapping genes that were significantly upregulated (red) or downregulated (green). Venn diagram shows genes specifically upregulated (1200 genes) or downregulated (854 genes) in AH as well as genes specifically upregulated (148 genes) or downregulated (102 genes) in NASH. (d) Heatmap display of keratins with the most significantly different expression between AH and NASH and control livers. The intensity of each color denotes the standardized ratio between each value and the average expression of each gene across all samples. Red pixels correspond to an increased abundance of mRNA in the indicated liver biopsy sample, whereas green pixels indicate decreased mRNA levels.
Next, we sought to identify novel specific biomarkers exclusively overexpressed in patients with AH. We focused on the “structural molecule activity” pathway, the most dysregulated pathway in our functional analysis. Among the genes most upregulated in this pathway, we found several keratins (KRT23, keratin 19 -KRT19-, and keratin 7 -KRT7-) as well as genes encoding procollagens I-V (Table 2). Because keratins are novel biomarkers in liver disease that also regulate cell fate, we further analyzed this subgroup of genes. Figure 1c shows the expression of genes encoding keratins, which were markedly upregulated in AH. Among them, we found KRT23 to be the most upregulated gene in AH compared to NASH and normal livers (45.8 and 126-fold increase, respectively, P < 0.001 for both).
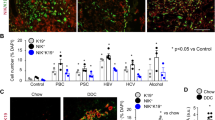
Based on previous studies from our lab showing that most upregulated genes in AH are ductular reaction markers23, we hypothesized that KRT23 is predominantly expressed in these cells. Immunohistochemistry studies in patients with AH, including double staining studies using two ductular reaction markers (KRT7, epithelial cell adhesion molecule -EPCAM-) clearly demonstrated that KRT23 is preferentially expressed in the ductular reaction cells in AH (Fig. 2a,b). KRT23 showed a strong overlap with KRT7 and co-localized in the same cell as EPCAM. KRT23 is also weakly expressed in some hepatoblasts in the vicinity of the ductular reaction. These results strongly suggest that KRT23 is mainly expressed in the ductular reaction (likely LPCs) in patients with AH.
Identification of KRT23 as a Marker of Ductular Cells in AH.
(a) Representative micrographs of KRT23 protein expression in paraffin sections of liver biopsies from normal livers and from patients with AH, stained with anti-KRT23 antibody (x100 magnification). (b) Immunofluorescence staining shows co-localization of KRT23 and LPCs markers (KRT7 and EPCAM) in paraffin sections of liver biopsies from patients with AH (x100 magnification). (c) Krt23 hepatic gene expression measured in a mouse model of progenitor cell expansion (DDC diet for 4 weeks, n = 6 compared to uninjured mouse liver (control, n = 6). (d) Representative images of liver KRT23 staining in mice treated for 4 weeks with a DDC diet and in uninjured mice (x200 magnification). (e) Krt23 and LPCs markers (Epcam, Krt7, Krt19) gene expression was significantly enriched in LCP cells (n = 3) vs. a non-LPC population (n = 3).
Expression of KRT23 in Animal Models of Ductular Reaction
The current experimental models of ALD do not reproduce the main histological features of severe AH (i.e. advanced fibrosis, bilirrubinostasis and ductular reaction) that are associated with a poor outcome23,31,32 but rather mimic moderate alcoholic liver disease. To overcome this difficulty, we used different animal models of liver injury which reproduce unique features of AH. To explore the expression of KRT23 in the ductular reaction we used a well-characterized experimental model which causes advanced fibrosis, cholestasis and progenitor cell expansion (i.e. DDC diet). We found that KRT23 gene and protein expression were dramatically induced in DDC-treated mice. Interestingly, KRT23 protein was detected within the ductular reaction (Fig. 2c,d). To further confirm that KRT23 is expressed in LPCs, we analyzed hepatic gene expression of primary LPCs isolated from DDC-treated mice33. Expression of KRT23 as well as specific cell markers of LPCs were significantly enriched in LPC cells vs. non-LPC population (Fig. 2e). Overall, these results indicate that KRT23 is expressed in the ductular reaction and LPCs in livers with cholestasis and progenitor cell expansion.
Expression of KRT23-positive Ductular Cells in AH: Correlation with Disease Outcome
To confirm the microarray results, we analyzed KRT23 hepatic gene expression in a large cohort of patients with AH and other diseased controls by real time PCR. The results confirmed a dramatic upregulation of KRT23 in patients with AH (n = 51) compared with normal livers (n = 7) and other liver diseases including compensated cirrhosis (n = 10), hepatitis C (n = 10, HCV) and NASH (n = 14), (P < 0.001) (Fig. 3a). KRT23 expression was also upregulated, but to a lesser extent, in patients with HCV (P < 0.005) and compensated cirrhosis (P < 0.001) compared to the control liver samples. Elevated circulating levels of soluble keratin 18 have been detected in several liver diseases and may serve as a biomarker for identifying steatohepatitis34,35. We then assessed serum KRT23 concentration and plasma free and microparticle-bound proteins by ELISA as a potential biomarker of liver disease. KRT23 serum concentration was higher in patients with AH as compared to patients with compensated cirrhosis (Fig. 3b). Since other keratins (i.e. keratin 18) are typically bound to microparticles, we next explored whether KRT23 is also linked to these small molecules. We found that the vast majority of KRT23 in plasma was not bound to microparticles (Supplementary Fig. 1a,b).
KRT23 expression correlates with disease severity and key features of AH.
(a) KRT23 hepatic gene expression measured by qPCR in patients with AH (n = 51), HCV (n = 10), compensated cirrhosis (n = 10) and NASH (n = 14), compared to normal livers (n = 7). (b) KRT23 peripheral serum levels are elevated in patients with AH (n = 34) when compared to patients with cirrhosis (n = 14). (c) Correlation between KRT23 hepatic gene expression (fold change vs. normal livers) and clinical features in patients with AH (n = 51). Both MELD score as well as ABIC score correlated with KRT23 hepatic gene expression. (d) Kaplan-Meier’s curve analysis illustrates the relationship between KRT23 hepatic gene expression with 90-day mortality in patients with AH. A cut-off value of 250-fold expression (fold change vs. normal livers) defined patients with low and high KRT23 gene expression with the best sensitivity and specificity. Portal hypertension (hepatic venous pressure gradient – HVPG – mmHg) was higher in patients with higher KRT23 gene expression levels (>250-fold).
To gain insight on the pathogenic role of KRT23-positive ductular cells in AH, we next explored whether its expression correlated with disease severity in the cohort of patients with AH (n = 51). KRT23 hepatic gene expression (expressed as fold change vs. normal livers) positively correlated with the main prognostic scores in patients with AH. Hepatic KRT23 correlated with Model for End-stage Liver Disease (MELD; P = 0.01) and Age/Bilirubin/International normalized ratio/Creatinine (ABIC; P = 0.01) scores (Fig. 3c). We also observed increased hepatic KRT23 mRNA levels in patients who died within 90 days after admission compared with those who survived (522-fold vs. 245-fold induction, respectively; P = 0.01). In addition, to evaluate if KRT23 can serve as a predictor of short-term mortality, we performed a Kaplan-Meier analysis. A value of 250-fold expression (fold expression vs. normal livers) was identified as the cut-off value with better sensitivity and specificity to define patients with low and high KRT23 gene expression (area under the receiver characteristic curve - AUROC = 0.72 [95% CI 0.51–0.91]). KRT23 hepatic gene expression predicted short-term mortality in patients with AH (Fig. 3d). Finally, we analyzed if KRT23 gene expression correlated with the degree of portal hypertension, a major pathophysiological event in these patients. We observed a higher degree of portal hypertension in patients with higher levels of hepatic KRT23 expression compared to those with lower levels of hepatic KRT23 expression (hepatic venous pressure gradient 14 ± 6 mmHg vs. 21 ± 5 mmHg, respectively; P < 0.001) (Fig. 3d). Overall, these results suggest that KRT23-positive ductular cells may play a role in the pathophysiology of AH and that KRT23 might be used as a biomarker to predict short-term outcomes in patients with AH.
Role of LPS-TLR4 Pathway on Expression of KRT23-positive Ductular Cells
LPS plays a major role in several liver diseases36,37,38,39. Because LPS is a major driver in AH and correlates with patient outcome20, we next explored if LPS stimulates the expression of ductular cell makers using different experimental approaches. First, we showed that LPS serum levels strongly correlated with hepatic expression of KRT23 in patients with AH (P < 0.001, Fig. 4a). To uncover the mechanisms driving the increase of KRT23-positive ductular cells in AH, we used different animal models of liver injury which are representative of some of the key events that occur in AH, such as ethanol consumption, fibrosis and endotoxaemia. First, we showed that LPS administration markedly induced Krt23 expression in Balb/c mice (Fig. 4b). Then, we explored if LPS further increases the expression of Krt23 in livers with advanced fibrosis. To test this hypothesis we induced advanced fibrosis with chronic carbon tetrachloride (CCl4). As shown in Fig. 4c LPS administration further increased the expression for Krt23 in fibrotic livers. These results were confirmed in precision-cut liver slices (Supplementary Fig. 2a–h). Finally, we used a protocol to generate LPCs from pluripotent stem cells. Differentiation of these stem cells into LPCs resulted in the upregulation of keratins including KRT23 and KRT7 as well as EPCAM, a ductular reaction marker. Notably, expression of these markers was increased in cells incubated with LPS (Fig. 4d and Supplementary Fig. 3a–c). These results, together with the finding that LPS serum levels correlated with KRT23 expression in AH, strongly suggest that LPS could drive expression of KRT23-positive ductular cells.
KRT23 hepatic expression in animal models of liver injury and in LPCs.
(a) Relationship between KRT23 hepatic gene expression and LPS serum levels in patients with AH (n = 39). Correlation between LPS serum levels and ABIC score in patients with AH. (b) Krt23 hepatic gene expression in a mouse model of acute liver injury. Krt23 gene expression in mice treated with LPS (n = 6) compared to control mice (n = 6). (c) Krt23 hepatic gene expression in a mouse model of advanced fibrosis. Krt23 in mice treated CCl4 plus LPS (n = 8) compared to mice treated with CCl4 alone (n = 6). (d) Gene expression of KRT23 and LPCs markers (KRT7 and EPCAM) in a model of liver progenitor cell differentiation from 3 independent experiments.
Subsequently, we investigated whether TLR4 mediates the effect of LPS on the development of KRT23-positive ductular reaction. To test this hypothesis, we first found that TLR4 small interfering RNA (Tlr4 siRNA) blocked LPS-induced Krt23 in precision-cut liver slices (Supplementary Fig. 2a,b). Next, we performed in vivo loss-of-function studies using Tlr4 deficient mice. We first caused advanced fibrosis by inducing chronic cholestasis through prolonged bile duct ligation (BDL), which is known to increase serum LPS levels40. Mice with BDL showed a marked upregulation of KRT23 (both at the gene and protein levels), KRT7, and EPCAM, all of which were heavily blunted in mice lacking TLR4 (Fig. 5a,b). To a lesser extent, these results were also observed in TLR4-KO mice chronically exposed to alcohol (Tsukamoto-French model) (Fig. 5c,d). Overall, these results suggest that the LPS-TLR4 pathway mediates the development of ductular reaction and KRT23 expression in chronic liver injury.
LPS-TLR4 induced liver damage is mediated by ductular cells in animal models of liver injury.
(a) Gene expression of Krt23 and LPCs markers (Krt7 and Epcam) in WT and Tlr4-KO mice subjected to sham operation (n = 3) or BDL. BDL mice were sacrificed 5 (n = 4) or 21 days (n = 8) after operation. (b) Representative micrographs of KRT23 protein expression in WT and Tlr4-KO mice subjected to sham operation or BDL. (c) Gene expression of Krt23 and LPCs markers in WT and Tlr4-KO mice subjected to Tsukamoto-French model of ethanol damage (4 weeks, n = 6) compared to uninjured control mice (n = 4). (d) Representative micrographs of Krt23 protein expression in WT and Tlr4-KO mice subjected to Tsukamoto-French model and uninjured control mice. Bars, 100 μm.
Regulation of KRT23 Expression by Histone Acetylation in LPCs
Because ALD is characterized by profound epigenetic changes41 and KRT23 is sensitive to histone deacetylases (HDAC) inhibition42, we next investigated whether KRT23 expression in ductular cells is governed by histone acetylation in LPCs. First, we showed that an exogenous inhibitor of HDACs (sodium butyrate -NaB-) considerably increased Krt23 in bipotential mouse adult liver cells (BAML) isolated from mice (Fig. 6a). We recently demonstrated that AH is characterized by a distinct decrease of sirtuin 1 (SIRT1), a powerful HDAC43. To test if SIRT1 also plays a role in KRT23 induction, we first investigated if NaB treatment on BAML cells is associated with a decrease in SIRT1 levels. As shown in Fig. 6b, SIRT1 levels are downregulated in this model. SIRT1 levels are also downregulated in the model of human LPC differentiation in culture (Supplementary Fig. 6a,b). The role of SIRT1 in inducing KRT23 expression was demonstrated in mice deficient for Sirt1 exposed to chronic ethanol (Supplementary Fig. 5a,b). The absence of Sirt1 in mice exposed to alcohol was associated with increased Krt23 expression. These results suggest that histone acetylation regulates KRT23 expression in LPCs.
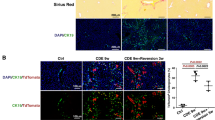
KRT23 regulation by HDACs in mice liver progenitor cells and KRT23 effects on collagen synthesis.
(a) Effect of HDAC inhibition by NaB administration on Krt23 gene expression in BAML cells (3 independent experiments). (b) Gene expression of Sirt1, an HDAC, in a BAML cells treated with NaB (3 independent experiments). (c) Correlation between KRT23 hepatic gene expression and COL1A1 hepatic gene expression in patients with AH (n = 39). (d) Effect of HDACs inhibition by NaB administration on Col1a1 gene expression in BAML cells. (e) Silencing of Krt23 by means of siRNA inhibited NaB induced Col1a1 gene expression in BAML cells (3 independent experiments).
Fibrogenic Roles of KRT23 in Ductular Cells
Because severe fibrosis is a hallmark find in AH that predicts survival in these patients31, and levels of KRT23 correlated with the degree of portal hypertension in patients with AH, we finally explored the potential fibrogenic role of KRT23 in ductular cells. We found that the expression of KRT23 closely correlated with the expression of collagen type I alpha 1 (COL1A1) (Fig. 6c). Since ductular cells express COL1A1, we next investigated if KRT23 stimulates profibrogenic actions in mouse BAML cells. BAML cells treated with NaB also showed Col1a1 over-expression in a Krt23-dependent manner (Fig. 6d), since this profibrogenic response was blunted in cells transfected with a Krt23 siRNA (Fig. 6e). Taken together, these results suggest that KRT23 may be promoting pro-fibrogenic actions in ductular cells.
Discussion
The current paradigm is that AH is due to a flare of hepatic inflammation caused by neutrophils in a previously damaged liver. However, we recently found that neutrophil infiltrate is associated with a better prognosis in AH31 and that severe AH is characterized by poor hepatocellular function and an inefficient accumulation of ductular cells23,24. Therefore, our efforts are currently focused in finding the molecular drivers causing this massive accumulation of ductular cells. To identify new markers of ductular cells, we used a highly translational approach. We first compared the transcriptome of patients with AH and NASH, and found that the “structural molecule activity” pathway was the most specifically dysregulated in patients with AH. A detailed analysis of this family of genes allowed us to identify several keratins that were profoundly upregulated in the livers with AH. Among them, we found that KRT23 was by far the most upregulated gene in this family. Interestingly, KRT23 has been previously reported as a biomarker for steatohepatitis44. In the Starmann et al. study, patients with steatohepatitis presented an advance degree of fibrosis. Moreover, 5 out of 8 patients with steatohepatitis included in their microarray cohort presented ALD. These are common traits between Starmann et al. and the AH cohort of the present study. Thus, KRT23 may be expressed in cases of steatohepatitis with cirrhosis.
Because many of the most overexpressed genes in AH are mainly expressed in ductular cell/LPCs, we hypothesized that KRT23 could mainly be expressed in these cells. Our further histological studies in human samples and experimental approaches in animal models of ductular reaction strongly suggest that this keratin is mainly expressed in the ductular reaction/LPCs population. Our results are in accordance with a recent study from Guldiken et al. showing that KRT23 is a maker of mouse and human ductular reaction45. Thus, we used KRT23 together with other known markers of LPCs (KRT7 and EPCAM) to study the mechanisms leading to the massive accumulation of ductular cells in livers with severe AH.
It is well known that proliferative bile ductular reaction not only occurs in AH but also in various liver diseases including acute-on-chronic liver failure and advanced ALD46. Whether the ductular reaction contributes to liver regeneration is still unclear as experimental studies show contradictory results47,48. Using genetic lineage-tracing, we previously showed that the biliary compartment probably does not play a role during normal liver homeostasis. However, following profound liver injury, biliary cells give rise to an expanding LPC population that have the potential to generate hepatocytes33. Yet, the contribution of the ductular reaction in the regeneration of the failing liver in AH is probably minimal. This assertion is based on two previous observations from our research groups. First, the accumulation of ductular/LPCs in patients with AH, assessed as the expression of specific cells markers, closely predicts patient mortality23. In other words, the more ductular cells that accumulate in the liver, the worse the patient’s short-term prognosis is. Second, explants from patients that did not respond to corticosteroids showed a massive accumulation of ductular cells together with poor hepatocellular function and proliferation24. Importantly, in these patients with burn-out AH, bipotent LPCs are mainly engaged to differentiate into a biliary phenotype, rather than into hepatocytes. Altogether, these results strongly suggest that the ductular reaction does not contribute to the regeneration of dying hepatocytes. On the contrary, it is plausible that accumulation of these cells produces deleterious effects such as lobular fibrosis. Our results show that LPCs express collagen and support this hypothesis. Interestingly, our immunohistochemistry study in patients with AH revealed that some hepatoblasts in the vicinity of the ductular reaction weakly express KRT23. KRT23 expression in hepatoblasts is also suggested in Guldiken et al.45. This finding suggests that cells derived from LPCs progressively lose this marker. However, it is also possible that in the context of AH, stressed hepatocytes undergo reprogramming and de novo express keratin including KRT23. Further studies should test this intriguing hypothesis and should evaluate the gene expression profile and biological actions of LPCs within the ductular reaction in non-alcoholic forms of acute-on-chronic liver failure.
An important finding of our study is that ductular reaction and the consequent KRT23 expression are driven by LPS/TLR4. This finding suggests that increased LPS in patients with severe forms of AH could promote ductular reaction and keratin expression. The fact that LPS levels correlate with KRT23 gene expression and that mice lacking Tlr4 have attenuated ductular reaction and keratin expression (Krt23 and Krt7) support this hypothesis. Moreover, IL-1ß induces Krt23 gene expression in hepatocellular and bile duct cells lines suggesting an association with inflammatory reaction45. There is mounting evidence that LPS plays multiple pathogenic roles in AH and is a major driver in this condition21. First, LPS serum levels measured at admission predict multiple organ failure, mortality and corticosteroids response in patients with AH20. Second, LPS serum levels correlate with neutrophil dysfunction and survival in AH, suggesting that the LPS-TLRs pathway plays a major role in immune paralysis in this population49. Third, TLRs mediate the release of inflammatory and fibrogenic mediators from immune and non-parenchymal cells caused by gut-derived endotoxin50. All together, these studies indicate that targeting LPS translocation from the gut represents an appealing strategy to treat patients with AH.
We also explored other mechanisms regulating KRT23 expression. KRT23 expression is known to be sensitive to histone deacetylase inhibition42. We demonstrated that KRT23 gene expression is strongly induced in LPCs exposed to exogenous inhibitors of HDACs. To explore if endogenous HDACs also play a role on KRT23 expression, we focused on sirtuin 1, which we recently showed to be downregulated in patients with AH43. Interestingly, KRT23 induction correlated with decreased SIRT1 in a cell model of differentiation of LPCs from stem cells, and Krt23 protein expression was increased in Sirt1-KO mice exposed to chronic ethanol. Collectively, our results suggest that histone acetylation regulates KRT23 expression in ductular cells. Further studies are needed to investigate the regulation of ductular KRT23-positive cells through histone acetylation in AH.
Finally, we explored whether KRT23 regulates the biological functions of ductular cells/LPCs. As stated above, in patients with AH, cells within the ductular reaction have a highly proliferative phenotype that is probably linked to poor differentiation into mature hepatocytes24. Moreover, AH is characterized by pericellular and sinusoidal fibrosis, and we previously demonstrated that the stage of fibrosis is significantly associated with 90-day survival in these patients31. Therefore, we explored the potential role of LPS-TLR4-KRT23 on collagen expression of LPCs. We found that KRT23 regulates the expression of COL1A1 in ductular cells. In fact, several studies indicate that the inhibition of the LPC response to liver injury is associated with a reduction in the severity of hepatic fibrosis51,52. The role of collagen production by ductular reaction cells in AH needs to be further explored, as myofibrobalsts are probably the main fibrogenic cell type in liver diseases regardless of the origin. Further studies should investigate whether targeting these actions in LPCs has beneficial effects in AH.
Our study has potential limitations. First, the exact contribution of ductular cells to liver regeneration in the setting of AH is largely unknown. Second, there are no well-established animal models of AH that mimic the main findings in humans (i.e. severe fibrosis and cholestasis), since available models only show signs of mild to moderate steatohepatitis. To overcome these limitations, we are currently optimizing the isolation procedure of single cells from biopsies form patients with AH. Single-cells studies will allow us to further characterize the origin and biological properties of ductular cells.
In summary, the present study provides evidence that KRT23, one of the most upregulated genes in AH, is a novel marker of ductular cells and correlates with disease severity. Moreover, we found that the LPS-TLR4 pathway drives accumulation KRT23-positive ductular cells, and that KRT23 which regulates their pro-fibrogenic functions in vitro. Further preclinical studies in models of true AH are required to determine if targeting KRT23-positive cells is an effective and safe therapeutic strategy to reinstate differentiation of these cells into mature hepatocytes and improve liver function.
Methods
Patients
Patients admitted to the Liver Unit of the Hospital Clínic, Barcelona, between January 2007 and December 2009 with clinical, analytical and histological features of AH were included in a prospective fashion. Experiments were performed with approval and under the accordance of the relevant guidelines established by The Ethics Committee of the Hospital Clinic of Barcelona. All patients included gave written informed consent. The inclusion criteria have been previously described10,53,54. Microarray data were deposited in NCBI’s Gene Expression Omnibus (GEO; accession number GSE28619). Patients with malignancies or any other potential cause of liver disease were excluded from the study. Liver biopsies were obtained using a transjugular approach. A fragment of liver tissue, serum and plasma were obtained at the time biopsy. A total of 51 patients with AH were included (Table 1). As diseased controls, we included liver tissue from treatment-naïve chronic hepatitis C virus (HCV) infection (genotype 1) (n = 10), compensated alcoholic cirrhosis patients without active alcohol intake for at least 6 months (n = 10) as well as patients with morbid obesity and NASH (n = 14) according to Kleiner’s criteria55. Only patients with NASH without evidence of excessive alcohol intake (>20 gr/d) or other potential cause of liver disease were included (Supplementary Table 1). Additionally, as normal controls we included fragments of normal livers obtained from optimal cadaveric liver donors (n = 3) or resection of liver metastases (n = 4). Criteria for selected normal livers have been described in detail previously11.
Comparative Microarray Studies
Comparative analysis of microarray data from patients with AH and NASH was performed. Whole transcriptome analysis from these two types of patients was previously performed by our group11,30. All microarray studies were performed at the same time using Human Genome U133A plus 2.0 GeneChip (Affymetrix Hgu133plus, Santa Clara, CA). Differential expression was assessed by using linear models and empirical Bayes moderated t-statistics. Linear Models for Microarray Analysis (LIMMA) R-package software was used for the analysis of gene expression microarray data56. Group comparisons and determinations of false discovery rates (FDR computation using Benjamini-Hochberg procedure)57 were performed. Functional analysis of gene expression data was conducted using the R/Bioconductor package GOstats and the GO database (http://www.geneontology.org). Only genes that could be associated with a unique EntrezGene ID were used. Among them those were selected representing a change of 1.5-fold or greater and moderated p-value < 0.05. The hypergeometric distribution was used to evaluate the probability of randomly observing the enrichment for each GO term58.
Experimental Models of Liver Injury and Progenitor Cell Expansion in Mice
The current experimental models of ALD do not reproduce the main histological features of severe AH but rather mimic moderate alcoholic liver disease32. Through the study we used different animal models to better mimic particular features of AH. All mice used were male.
To investigate the effects of the LPS-TLR4 pathway in KRT23 induction we used a model of acute liver injury (LPS) and advanced fibrosis (CCl4 followed by a single dose of LPS)11. Male 8-week-old Balb/c mice were purchased from Charles River (Charles River, l’Arbresle, France). For the acute liver injury model mice were injected LPS (Sigma-Aldrich) intravenously at a dose of 10 mg/kg body weight (n = 6) or saline as control (n = 6) and were sacrificed 4 hours after the injection. For the advanced fibrosis model, mice were injected carbon tetrachloride (CCl4) intraperitoneally (diluted 1:4 in corn oil; Sigma-Aldrich, St Louis, MO) at a dose of 0.5 ml/kg body weight twice per week for a total of 5 injections. Control mice (n = 6) were given vehicle (corn oil, Sigma-Aldrich). CCl4 treated mice were injected intravenously either LPS at a dose of 10 mg/kg body weight (n = 8) or saline as control (n = 6) and were sacrificed 4 hours after the injection.
A model of ductular reaction expansion was induced by administering a 0.1% 3,5-diethoxycarbonyl-1,4-dihydro-collidin (DDC) diet for 3 weeks (n = 6). LPCs and non-LPCs cells were isolated by liver perfusion method from Hnf1βCreER/R26RYfp uninjured and from mice treated with a DDC containing diet (Sigma-Aldrich) for 3 weeks as previously described33.
Pathogen-free 8-week-old male TLR4-KO mice (originally generated by Dr. Shizuo Akira, Osaka University, Japan), and wild-type (WT) littermates were subjected to a model of ductular reaction (BDL) or to a model of alcoholic steatohepatitis (Tsukamoto-French)59. Briefly, the murine TLR4 genomic clone was screened from the 129/SvJ mouse genomic library (Stratagene, La Jolla, CA). A targeting vector was designed and electroporated into E14.1 embryonic stem cells. Two embryonic stem cell clones were selected for microinjection into blastocysts. These clones gave germline transmission and were interbred to generate homologous gene targeted mice. TLR4 knowckout mice were backcrossed at least 10 generations onto the C57BL/6 background. Bile duct ligation (BDL) was performed as described previously50,60. Bile duct ligated and sham-operated mice (n = 3) were sacrificed 5 (n = 4) or 21 (n = 8) days after surgery. Tsukamoto-French model of continuous ethanol infusion in mice was performed in as described previously50,61. Ethanol infusion was increased until it reached 29.4 g/kg/day at 4 weeks (n = 6; control mice n = 4).
A model of chronic plus single binge ethanol in Sirt1-KO mice was used to explore the role of HDACs as modulators of KRT23 expression. Liver-specific sirtuin KO mice (Sirt1-KO) (n = 6) mice and their age-matched WT littermates (n = 6) were subjected to a model of chronic plus single binge ethanol consumption as previously described (NIAAA model)43. Sirt1 allele with floxed exon 4 was backcrossed 5 times into the C57BL/6 background. It was then bred with mice expressing the Cre recombinase driven by the albumin promoter to generate liver specific Sirt1 knockout mice (Sirt1 LKO) in over 98% C57BL/6 background. SIRT1 LKO mice and their age-matched littermate Lox controls (Cre−/−, Sirt1flox/flox) older than 6-week of age were used in the present study. All animal procedures were approved by The University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell cultures and in vitro assays
To investigate the effect of LPS on ductular cells, the NIH-approved human pluripotent stem cells line H1 (WA01, WiCell Research Institute, Wisconsin, MA) underwent differentiation following The National Institutes of Health Guidelines on Human Stem Cell Research, as described previously62. Briefly, differentiation of H1 PSCs cells was induced by culturing the cells in priming medium (media formulation described in Supplementary methods). After 72 hours, differentiation medium was added for 5 days. At day 8, cells were cultured in maturation and maintenance medium for 8 days. Cells were collected for RNA and protein extraction as well as immunofluorescense analysis after 5 days in differentiation medium and after 8 days in maturation medium. Thirty-six hours before harvest, cells were treated with LPS (1 μg/mL, Sigma-Aldrich) or phosphate buffered saline (PBS, Sigma-Aldrich).
BAML cells were isolated from control mice as previously described63 and were maintained on type-I collagen-coated plates in MCDB201/DMEM medium. BAML cells were treated with the HDAC inhibitor sodium butyrate (NaB, 4 mM, Sigma-Aldrich) for 24 h in fresh culture medium. Transfection with either scrambled or KRT23 small interfering RNA (siRNA; Life Technologies) was performed for 24 h and followed by treatment with 4 mM NaB for 24 h in fresh culture medium. Lipofectamine® 3000 reagent (Life Technologies) was used according to the manufacturer’s instructions. Transduction experiments with adenovirus vectors containing either a specific human KRT23 sequence (Ad-KRT23) or a GFP sequence (Ad-GFP)(SignaGen Laboratories, Rockville, MD) incubated in cell culture media for 12 h were performed on human HepaRG cells (Life Technologies).
Analysis of Serum KRT23 levels
Samples from patients with AH (n = 37) and compensated alcoholic cirrhosis (n = 14) were analyzed. To detect KRT23 serum levels, serum samples were collected from patients and stored at −80 °C. Serum samples were analyzed using commercially available immunoassays for KRT23 detection (CSB-EL012539HU ELISA kit; Cusabio, Wuhan, China). LPS serum levels were determined using the limulus amoebocyte lysate QCL-1000 test (Lonza Walkersville Inc, Walkersville, MA).
Statistics
Continuous variables were described as means (±standard error) or medians (interquartile range). Comparisons between groups were performed using the Student’s t test or the Mann-Whitney U test, depending on their normality test. Differences between categorical variables were assessed by Fisher’s exact test or the chi-square test with Yates correction for continuity, when necessary. Correlations between variables were evaluated using Spearman’s rho or Pearson’s r, when appropriate. The area under the receiver characteristic curve (AUROC) analysis was used to determine the best cut-off value and the accuracy (sensitivity and specificity) of continuous variables associated with 90-day mortality. A comparative risk analysis was performed using the Kaplan-Meier method. Comparisons were performed by the log-rank test. All statistical analyses were performed using SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL).
Additional Information
How to cite this article: Odena, G. et al. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci. Rep. 6, 35610; doi: 10.1038/srep35610 (2016).
References
Global Status Report on Alcohol and Health–2014 ed., 390 (World Health Organization, Geneva, Switzerland, 2014).
Peery, A. F. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187 e1171–1173, 10.1053/j.gastro.2012.08.002 (2012).
Lucey, M. R., Mathurin, P. & Morgan, T. R. Alcoholic hepatitis. The New England journal of medicine 360, 2758–2769, 10.1056/NEJMra0805786 (2009).
Rincon, D. et al. Prognostic value of hepatic venous pressure gradient for in-hospital mortality of patients with severe acute alcoholic hepatitis. Alimentary pharmacology & therapeutics 25, 841–848, 10.1111/j.1365-2036.2007.03258.x (2007).
Altamirano, J. et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 10, 65–71 e63, 10.1016/j.cgh.2011.09.011 (2012).
Louvet, A. et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 137, 541–548, 10.1053/j.gastro.2009.04.062 (2009).
Altamirano, J. & Bataller, R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nature reviews. Gastroenterology & hepatology 8, 491–501, 10.1038/nrgastro.2011.134 (2011).
Thursz, M. R. et al. Prednisolone or pentoxifylline for alcoholic hepatitis. The New England journal of medicine 372, 1619–1628, 10.1056/NEJMoa1412278 (2015).
Singh, S. et al. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology, 10.1053/j.gastro.2015.06.006 (2015).
Dominguez, M. et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 136, 1639–1650, 10.1053/j.gastro.2009.01.056 (2009).
Affo, S. et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62, 452–460, 10.1136/gutjnl-2011-301146 (2013).
Morales-Ibanez, O. et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology 58, 1742–1756, 10.1002/hep.26521 (2013).
Affo, S. et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 63, 1782–1792, 10.1136/gutjnl-2013-306098 (2014).
Ki, S. H. et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52, 1291–1300, 10.1002/hep.23837 (2010).
Jung, Y. et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134, 1532–1543, 10.1053/j.gastro.2008.02.022 (2008).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. The Journal of clinical investigation 122, 3476–3489, 10.1172/jci60777 (2012).
Gao, B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. Journal of gastroenterology and hepatology 27 Suppl 2, 89–93, 10.1111/j.1440-1746.2011.07003.x (2012).
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585, 10.1053/j.gastro.2011.09.002 (2011).
Schnabl, B. & Brenner, D. A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524, 10.1053/j.gastro.2014.01.020 (2014).
Michelena, J. et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology (In Press), 10.1002/hep.27779 (2015).
Bataller, R. & Mandrekar, P. Identifying molecular targets to improve immune function in alcoholic hepatitis. Gastroenterology 148, 498–501, 10.1053/j.gastro.2015.01.013 (2015).
Stadlbauer, V. et al. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. American journal of physiology. Gastrointestinal and liver physiology 296, G15–G22, 10.1152/ajpgi.90512.2008 (2009).
Sancho-Bru, P. et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 55, 1931–1941, 10.1002/hep.25614 (2012).
Dubuquoy, L. et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut (In Press), 10.1136/gutjnl-2014-308410 (2015).
Li, C. et al. Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll-like receptor 4 mediated nuclear factor kappaB pathway. Stem cell research & therapy 5, 67, 10.1186/scrt456 (2014).
He, J. et al. The expression of functional Toll-like receptor 4 is associated with proliferation and maintenance of stem cell phenotype in endothelial progenitor cells (EPCs). Journal of cellular biochemistry 111, 179–186, 10.1002/jcb.22686 (2010).
He, W. et al. LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. Journal of cellular physiology 230, 554–561, 10.1002/jcp.24732 (2015).
Giuliani, M. et al. TLR ligands stimulation protects MSC from NK killing. Stem cells (Dayton, Ohio) 32, 290–300, 10.1002/stem.1563 (2014).
Liotta, F. et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem cells (Dayton, Ohio) 26, 279–289, 10.1634/stemcells.2007-0454 (2008).
Lopez-Vicario, C. et al. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 63, 344–355, 10.1136/gutjnl-2012-303179 (2014).
Altamirano, J. et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 146, 1231–1239 e1231–e1236, 10.1053/j.gastro.2014.01.018 (2014).
Mandrekar, P., Bataller, R., Tsukamoto, H. & Gao, B. Alcoholic hepatitis: Translational approaches to develop targeted therapies. Hepatology, 10.1002/hep.28530 (2016).
Rodrigo-Torres, D. et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology 60, 1367–1377, 10.1002/hep.27078 (2014).
Musso, G., Gambino, R., Cassader, M. & Pagano, G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Annals of medicine 43, 617–649, 10.3109/07853890.2010.518623 (2011).
Seidel, N. et al. The extent of liver steatosis in chronic hepatitis C virus infection is mirrored by caspase activity in serum. Hepatology 42, 113–120, 10.1002/hep.20747 (2005).
Choi, Y. et al. The role of the gut barrier function in the pathophysiology of viral liver cirrhosis. Hepato-gastroenterology 58, 1244–1247, 10.5754/hge10338 (2011).
Feld, J. J., Meddings, J. & Heathcote, E. J. Abnormal intestinal permeability in primary biliary cirrhosis. Digestive diseases and sciences 51, 1607–1613, 10.1007/s10620-006-9544-z (2006).
Farhadi, A. et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver international: official journal of the International Association for the Study of the Liver 28, 1026–1033, 10.1111/j.1478-3231.2008.01723.x (2008).
Jain, L. et al. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 44, 1027–1031, 10.1016/j.dld.2012.07.002 (2012).
Giacometti, A. et al. Administration of protegrin peptide IB-367 to prevent endotoxin induced mortality in bile duct ligated rats. Gut 52, 874–878 (2003).
Mandrekar, P. Epigenetic regulation in alcoholic liver disease. World journal of gastroenterology: WJG 17, 2456–2464, 10.3748/wjg.v17.i20.2456 (2011).
Zhang, J. S., Wang, L., Huang, H., Nelson, M. & Smith, D. I. Keratin 23 (K23), a novel acidic keratin, is highly induced by histone deacetylase inhibitors during differentiation of pancreatic cancer cells. Genes, chromosomes & cancer 30, 123–135 (2001).
Yin, H. et al. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology 146, 801–811, 10.1053/j.gastro.2013.11.008 (2014).
Starmann, J. et al. Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PloS one 7, e46584, 10.1371/journal.pone.0046584 (2012).
Guldiken, N. et al. Keratin 23 is a general stress-inducible marker of mouse and human ductular reaction in liver disease. Journal of hepatology, 10.1016/j.jhep.2016.04.024 (2016).
Spee, B. et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 59, 247–257, 10.1136/gut.2009.188367 (2010).
Yanger, K. et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell stem cell 15, 340–349, 10.1016/j.stem.2014.06.003 (2014).
Michalopoulos, G. K., Barua, L. & Bowen, W. C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 41, 535–544, 10.1002/hep.20600 (2005).
Markwick, L. J. et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 148, 590–602 e510, 10.1053/j.gastro.2014.11.041 (2015).
Seki, E. et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature medicine 13, 1324–1332, 10.1038/nm1663 (2007).
Davies, R. A., Knight, B., Tian, Y. W., Yeoh, G. C. & Olynyk, J. K. Hepatic oval cell response to the choline-deficient, ethionine supplemented model of murine liver injury is attenuated by the administration of a cyclo-oxygenase 2 inhibitor. Carcinogenesis 27, 1607–1616, 10.1093/carcin/bgi365 (2006).
Knight, B., Tirnitz-Parker, J. E. & Olynyk, J. K. C-kit inhibition by imatinib mesylate attenuates progenitor cell expansion and inhibits liver tumor formation in mice. Gastroenterology 135, 969–979, 979.e961, 10.1053/j.gastro.2008.05.077 (2008).
Colmenero, J. et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology 132, 687–697, 10.1053/j.gastro.2006.12.036 (2007).
Dominguez, M. et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. The American journal of gastroenterology 103, 2747–2756, 10.1111/j.1572-0241.2008.02104.x (2008).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321, 10.1002/hep.20701 (2005).
Xia, X., McClelland, M. & Wang, Y. WebArray: an online platform for microarray data analysis. BMC bioinformatics 6, 306, 10.1186/1471-2105-6-306 (2005).
Smyth, G. K. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor Statistics for Biology and Health (eds Gentleman, Robert et al.) Ch. 23, 397–420 (Springer New York, 2005).
Falcon, S. & Gentleman, R. Using GOstats to test gene lists for GO term association. Bioinformatics (Oxford, England) 23, 257–258, 10.1093/bioinformatics/btl567 (2007).
Hoshino, K. et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. Journal of immunology (Baltimore, Md.: 1950) 162, 3749–3752 (1999).
Bataller, R. et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. Journal of Clinical Investigation 112, 1383–1394, 10.1172/jci200318212 (2003).
Tsukamoto, H. et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology 5, 224–232 (1985).
Hay, D. C. et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proceedings of the National Academy of Sciences of the United States of America 105, 12301–12306, 10.1073/pnas.0806522105 (2008).
Fougere-Deschatrette, C. et al. Plasticity of hepatic cell differentiation: bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivo. Stem cells (Dayton, Ohio) 24, 2098–2109, 10.1634/stemcells.2006-0009 (2006).
Acknowledgements
This work was performed in The Center for Gastrointestinal Biology and Disease (CGIBD) at UNC, Chapel Hill, NC. The authors are grateful to all the patients who took part in this study. They would also like to thank M. Humphries for technical help and E. Stein and V. Massey for critical reading of the manuscript. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAA)(1U01AA021908-01 and 1U01AA020821), and by grants from the Instituto de Salud Carlos III (ISCIII)(FIS PI041538, and FIS PS09/01164 to R. Bataller and J. Caballeria, respectively). P. Sancho-Bru is supported by ISCIII, Miguel Servet (CP11/00071) and by Fondo Europeo de Desarrollo Europeo (FEDER), Union Europea, “Una manera de hacer Europa”. E. Seki is supported by R01AA020172 and R01DK085252. M. You is supported by National Institute on Alcoholism and Alcohol Abuse (grants AA015951 and AA013623). G. Odena received a grant from the Asociacion Espanola para el Estudio del Higado and is partially funded by 1U01AA020821. J. Altamirano received a grant from Fundación Banco Bilbao Vizcaya Argentaria. D. Rodrigo-Torres received a grant from the Ministerio de Educacion, Cultura y Deporte, FPU program. S. Affo received a grant from IDIBAPS. O. Morales-Ibanez received a grant from the Generalitat de Catalunya for Research Stays Abroad, BE-DGR 2012.
Author information
Authors and Affiliations
Contributions
G.O. participated in the conception and design of this study, data analysis, interpretation, performance of the majority of the experiments and manuscript writing. J.J.L. analyzed data and interpreted the data of the microarray. J.A. performed the statistical analysis. J.C., D.R.-T., S.A., O.M.-I., H.M., J.Z. and R.D. performed important experiments. P.-E.R., J.C., P.G., V.A., F.C. and D.V. helped with the interpretation of the data and revision of the final version of the manuscript. M.Y., E.S. and P.S.-B. provided study materials, helped with the interpretation of the data and revision of the final version of the manuscript. R.B. was involved in the conception, design and supervision of this paper, obtaining funding, analysis and interpretation of the data and participated in drafting and critically revising the final version of the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Odena, G., Chen, J., Lozano, J. et al. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci Rep 6, 35610 (2016). https://doi.org/10.1038/srep35610
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35610
This article is cited by
-
Alterations in the hepatocyte epigenetic landscape in steatosis
Epigenetics & Chromatin (2023)
-
The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases
Journal of Gastrointestinal Surgery (2022)
-
Hepatic lipocalin 2 promotes liver fibrosis and portal hypertension
Scientific Reports (2020)
-
Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis
Nature Communications (2019)
-
Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis
Indian Journal of Gastroenterology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.