Abstract
The insect-transmitted protozoan parasite Trypanosoma cruzi is the causative agent of Chagas disease, and infects 5–8 million people in Latin America. Chagas disease is characterised by an acute phase, which is partially resolved by the immune system, but then develops as a chronic life-long infection. There is a consensus that the front-line drugs benznidazole and nifurtimox are more effective against the acute stage in both clinical and experimental settings. However, confirmative studies have been restricted by difficulties in demonstrating sterile parasitological cure. Here, we describe a systematic study of nitroheterocyclic drug efficacy using highly sensitive bioluminescence imaging of murine infections. Unexpectedly, we find both drugs are more effective at curing chronic infections, judged by treatment duration and therapeutic dose. This was not associated with factors that differentially influence plasma drug concentrations in the two disease stages. We also observed that fexinidazole and fexinidazole sulfone are more effective than benznidazole and nifurtimox as curative treatments, particularly for acute stage infections, most likely as a result of the higher and more prolonged exposure of the sulfone derivative. If these findings are translatable to human patients, they will have important implications for treatment strategies.
Similar content being viewed by others
Introduction
Chagas disease is caused by the insect-transmitted protozoan Trypanosoma cruzi and is the most important parasitic infection in Latin America, affecting 5–8 million people1. It is also becoming a global problem, with increasing numbers of symptomatic cases in non-endemic areas, including the USA and Europe2,3. The initial acute stage of Chagas disease is usually relatively mild, although in children it can be severe, and sometimes fatal. With the development of a cellular immune response, parasitemia is suppressed, but sterile immunity is not achieved. Initially, the chronic infection phase is asymptomatic, but ~30% of patients eventually develop pathology, often decades later. Cardiomyopathy occurs in the majority of these individuals, whilst a minority suffer digestive tract megasyndromes4,5.
The nitroheterocyclic compounds benznidazole and nifurtimox are the front-line Chagas disease drugs6,7. Unfortunately, they display a range of toxic side-effects, which can impact negatively on patient compliance. Furthermore, both require bioactivation by the same parasite nitroreductase, a potential source of cross-resistance8,9. Treatment failures are common and new drugs are urgently required. Recent clinical trials have had disappointing outcomes. Posaconazole, a potent ergosterol biosynthesis inhibitor, was found to display limited curative potential against chronic infections10, and benznidazole, although partially11 or highly10 effective at achieving parasitological cure, showed no significant benefit in patients who had already developed advanced chagasic cardiomyopathy11.
There is general consensus that nitroheterocylic drugs are more effective against T. cruzi infections during the acute stage than in the chronic stage. Although widely quoted12,13,14,15,16,17,18,19,20,21, there have been few systematic studies to support this assertion. One of the major problems has been the difficulty in unequivocally demonstrating sterile cure, both in human patients and animal models. To increase the accuracy and reproducibility of drug testing, we developed highly sensitive bioluminescence methodology based on the expression by trypanosomes of a red-shifted luciferase reporter22,23,24. This in vivo imaging procedure has a limit of detection of 100–1000 parasites, and facilitates the real-time tracking of parasite burden in individual mice during long-term experimental infections. With the T. cruzi CL Brener-BALB/c mouse-parasite combination, the parasite burden peaks 14 days post-infection, and then resolves to the chronic phase over the next 30–40 days23. Infections persist for at least a year in dynamic equilibrium, at levels 2–3 orders of magnitude below the acute stage. Parasites are pan-tropic in the acute stage, but in chronically infected mice, the large intestine and stomach are the primary reservoir sites, a feature that also occurs for other parasite-mouse genotype combinations. Transient bioluminescent foci can also be detected at peripheral sites during chronic infections, which fluctuate in a spatiotemporally dynamic manner, appearing and disappearing over a period of hours. This bioluminescence imaging system is more reliable than PCR-based approaches for tracking experimental T. cruzi infections and for confirming parasitological cure25.
Here, we describe the use of this predictive model to undertake a detailed comparison of the efficacy of the nitroheterocyclic agents benznidazole, nifurtimox, fexinidazole and fexindazole sulfone against acute and chronic T. cruzi infections.
Results
Nitroheterocyclic drugs cure chronic stage infections more effectively than acute stage infections
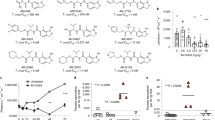
Using the T. cruzi CL Brener-BALB/c model, we found that chronic infections could be cured with 5 daily oral doses of 100 mg kg−1 benznidazole (ref. 25, Table 1, Fig. 1a). Drug efficacy was assessed by both in vivo and ex vivo imaging, with cyclophosphamide-induced immunosuppression to enhance the reactivation of any residual infection (Methods). When we applied this same treatment regimen to mice at the peak of the acute stage, none of the 30 mice tested were cured (Table 1, Fig. 1b). Although treatment initiated 14 days post-infection resulted in a rapid drop in the bioluminescence-inferred parasite burden, this was transient, with a rapid return to levels similar to those in non-treated mice. Treatment of acute stage infections had to be extended to 20 days to achieve curative outcomes comparable to 5 days treatment in the chronic stage (Table 1).
Treatment of chronic (a) and acute (b) stage Trypanosoma cruzi infections with nitroheterocyclic drugs assessed by in vivo imaging. BALB/c mice, infected with bioluminescent CL Brener parasites, were treated once daily by oral gavage for 5 days at 100 mg kg−1 with benznidazole (BZ), nifurtimox (NF), fexinidazole (FX), or fexinidazole sulfone (FXS). Treatment was initiated 114 days (chronic) or 14 days (acute) post-infection (see Table 1 for numbers of mice). Representative ventral images of 2 mice are shown for each drug, at various points post-infection. NT, non-treated (vehicle only). Mice with low/background levels of bioluminescence were immunosuppressed by cyclophosphamide (Methods), starting 135 or 31 days post-infection and are underlined with a red bar. All acute stage mice treated with BZ or NF were non-cured, and only immunosuppressed where necessary to confirm outcome. In acute stage infections, examples of both curative and non-curative outcomes with FX are shown. (c) Assessment of drug activity against acute stage infections by ex vivo imaging. Representative images of organs/tissues isolated 50 days post-infection (Methods) from NT (i), FX-treated (ii) and FXS-treated (iii) mice are shown. The locations of specific organs/tissues are illustrated in the left-hand image. The heat-map is on a log10 scale and indicates intensity of bioluminescence from low (blue) to high (red); the minimum and maximum radiances for the pseudocolour scale are shown.
Using the 5 day protocol, we also found nifurtimox to be highly effective at curing chronic infections, with 9/10 mice remaining bioluminescence negative after immunosuppression (Fig. 1a). In contrast, non-curative outcomes were observed in all cases when acute stage infections were treated with 100 mg kg−1 nifurtimox (Table 1, Fig. 1b). Therefore, with the 5 day treatment protocol, both of the front-line Chagas disease drugs are more effective against experimental infections in the chronic stage.
We next tested fexnidazole and its metabolite fexinidazole sulfone. Previous studies suggested that these nitroimidazoles might have greater in vivo activity than benznidazole26,27. When chronically infected mice were treated for 5 days with oral doses of 100 mg kg−1, there was a 100% cure rate in each case (Table 1, Fig. 1a). A similar outcome was observed when acute stage infections were treated with fexinidazole sulfone (Fig. 1b,c). Fexinidazole also showed significant efficacy during acute stage infections, curing 4/6 mice.
To further explore the 5 day treatment protocol for acute stage infections, we administered nitroheterocyclic drugs as two daily doses of 50 mg kg−1. With this schedule, benznidazole again failed to cure any mice (0/6) (Table 1, Fig. 2). Nifurtimox was more effective at reducing parasite burden, but only 1/6 mice was ultimately cured. In contrast, fexinidazole treatment was 100% successful (6/6) and fexinidazole sulfone curative in 5/6 cases. Fexinidazole sulfone was then investigated by administering single doses of 50 mg kg−1 for 5 days. With chronic stage infections, all 6 mice were cured. In contrast, none were cured when acute stage infections were treated, confirming the differential effectiveness of nitroheterocyclic drugs against the two disease stages (Table 1).
When chronically infected mice were treated daily with 30 mg kg−1 benznidazole for 20 days, there was a 100% cure rate (6/6) (Table 1, Fig. 3a). Against acute stage infections however, the 30 mg kg−1 dosing regimen was less effective, with only 2/6 mice cured (Fig. 3b). This stage-specific trend was also observed when treatment duration was reduced to 10 days; 4/6 chronically infected mice were cured, compared to 100% treatment failure (0/6) in the acute stage (Table 1, Fig. 3a,b). When 5 days treatment was assessed, we found that benznidazole could reduce bioluminescence to background levels in most chronically infected mice, but the effect was transient and the parasite burden rebounded, particularly after immunosuppression. In this model therefore, daily dosing with 30 mg kg−1 benznidazole is more effective against chronic than acute stage infections, with the duration of treatment being a major factor in determining outcome. Treatment of chronic infections for 20 days with doses as low as 10 mg kg−1 also resulted in a significant reduction in parasite burden, although in this case, only 1 out of 6 mice was ultimately cured (Table 1). Nifurtimox, fexinidazole and fexinidazole sulfone displayed significant activity at 30 mg kg−1 against chronic stage infections. For example, 10 days treatment of chronic infections with 30 mg kg−1 nifurtimox produced a higher cure rate (5/6) than 10 days treatment of acute stage infections with 100 mg kg−1 (1/6). As with benznidazole, restricting the treatment period to 5 days, reduced drug efficacy in all cases (Table 1).
Greater efficacy against chronic stage infections is not a model-specific phenomenon
We investigated if our observations were relevant to other experimental models of Chagas disease. First, we assessed whether fexinidazole sulfone was also more effective than benznidazole in BALB/c mice infected with the JR strain of T. cruzi. The CL Brener (lineage TcVI) and JR (lineage TcI) strains are highly divergent28, although both strains display similar infection profiles in a BALB/c background when monitored by bioluminescence imaging29. Treatment of acute stage infections with 100 mg kg−1 benznidazole for 5 days resulted in a rapid knockdown in bioluminescence (Fig. 4a, Table 1). However, as with CL Brener infections, this effect was transient and non-curative (Figs 1b and 4a). When JR-infected BALB/c mice were treated with fexinidazole sulfone using the same dosing regimen, all but one of the mice were successfully cured (Fig. 4a). Therefore, at similar doses, fexinidazole sulfone is more effective than benznidazole as a treatment for acute stage infections in two diverse T. cruzi strains.
We next examined the efficacy of both drugs in C3H mice. In this strain, the parasite burden is typically higher and more disseminated than in BALB/c mice29. When acute stage JR-infections were assessed following 5 days treatment (100 mg kg−1), the outcomes were similar to those in the BALB/c strain, with 100% treatment failure in the case of benznidazole (n = 5), but curative outcomes in all but one of the mice treated with fexinidazole sulfone (Fig. 4, Table 2). In C3H mice, infections with the JR strain display an extended acute phase, reaching chronic stage equilibrium later than in BALB/c mice29. For assessment of efficacy against chronic infections, treatment (5 days, 100 mg kg−1) was therefore delayed until day 161. In these circumstances, fexinidazole sulfone cured 5/5 mice, with 3/5 cured in the case of benznidazole (Fig. 4c). Thus, T. cruzi infections are easier to cure in the chronic stage in two distinct murine/parasite models.
Pharmacokinetics and drug efficacy
To relate treatment outcomes to drug exposure, we assessed plasma concentrations of nitroheterocyclic drugs following single oral doses. For all compounds, the measured blood to plasma ratio was ~1, the fraction unbound in the in vitro assay media was >0.9, and the fraction unbound in plasma was 0.7 for benznidazole, 0.5 for nifurtimox, and 0.4 for fexinidazole and fexinidazole sulfone. Given the low and comparable extent of binding, data comparisons were made using the total plasma concentration throughout.
Although there are no reports of differences in pharmacokinetics between acute and chronic infections, we investigated if the disparity in efficacy between the two stages could be linked to drug exposure. Benznidazole concentrations in mouse plasma were measured in uninfected mice, and during acute and chronic infections, after a single oral dose of 100 mg kg−1. Plasma concentrations were comparable under all conditions (Fig. 5a), suggesting that the reduced ability to cure acute stage infections was not attributable to factors that influence drug exposure (Fig. 5b).
Pharmacokinetic and cure rate data for benznidazole.
(a) Plasma concentration versus time profiles of benznidazole following a single 100 mg kg−1 dose in uninfected (blue), acute (red) and chronic (green) stage infected BALB/c mice. The in vitro IC50 is shown by the dashed line. (b) Plasma concentration versus time profiles following single doses of 10 (green), 30 (red), and 100 (blue) mg kg−1 benznidazole in uninfected BALB/c mice. (c) Cure rates for benznidazole following 10 days (blue bar) and 20 days (red bar) treatment in chronic stage infected mice. (d) Cure rates for benznidazole following 10 days (blue bar) and 20 days (red bar) treatment in acute stage infected mice. Because 100 mg kg−1 was ineffective with 10 days treatment in the acute stage, 30 and 10 mg kg−1 treatments were not tested (blue asterisks).
Plasma concentrations of benznidazole reached a Cmax within 1 hour (Fig. 5b, Table 2), and then declined in a dose-dependent manner, dropping below the in vitro IC50 values after 6–12 hours (see also26). There was a clear association between benznidazole dose/exposure and effectiveness against both acute and chronic stage infections (Fig. 5c,d), although as noted above, the acute stage infections required a longer treatment duration.
When we investigated the PK properties of nifurtimox, we found that the Cmax and AUC were lower than for benznidazole, but plasma concentrations remained above the in vitro IC50 for longer (Fig. 6a). This may contribute to the greater effectiveness of nifurtimox in reducing parasite burden when acute infections are treated with 50 mg kg−1 twice daily (Fig. 2), even though curative outcomes were limited. When fexinidazole plasma concentrations were assessed, the parent drug was detected at low concentrations (<1 μM), and only for a short period (Fig. 6b), however the two active metabolites, fexinidazole sulfoxide and sulfone30 were rapidly formed, with Cmax values of 1–2 hours and 4–5 hours, respectively. Both metabolites remained above their in vitro IC50 for prolonged periods (12–14 hours for the sulfoxide, 24 hours for the sulfone). In mice where fexinidazole sulfone was administered directly, plasma concentrations reached a higher Cmax than achieved in fexinidazole-treated mice (217 vs 103 μM), with a corresponding increase in the AUC (Table 2 and ref. 27). The prolonged exposure of the sulfone could in part explain why fexinidazole and fexinidazole sulfone have greater efficacy than benznidazole at the same dose (Table 1). The steep drop-off in efficacy against acute infections when the daily dose of fexinidazole sulfone was reduced from 100 to 50 mg kg−1, or when one daily dose at 50 mg kg−1 was administered instead of two, highlights the narrow window of drug exposure that determines treatment outcome in the 5 day regimen.
Pharmacokinetic data for (a) nifurtimox, (b) fexinidazole, and (c) fexinidazole sulfone following a single 100 mg kg−1 dose (Methods). For fexinidazole (b), profiles for the metabolites fexinidazole sulfone (green) and fexinidazole sulfoxide (red) are shown together with the parent compound (blue). Data for fexinidazole sulfone reproduced from ref. 27. The in vitro IC50 values are shown by the dashed lines.
Discussion
Drug tests against chronic Chagas disease (both experimental and clinical) have been problematic, mainly because it is difficult to unambiguously confirm parasitological cure, even with PCR-based methodologies25. The dynamic, focal nature of the infection, and the extremely low parasite burden have been confounding factors23,29. Here, we used highly sensitive bioluminescence imaging coupled with cyclophosphamide-mediated immunosuppression to provide a reliable and flexible approach for systematic and comparative studies of drug efficacy. Our major finding is that nitroheterocyclic drugs cure T. cruzi infections in murine models more readily in the chronic stage than in the acute stage. This result is contrary to the widely held view that chronic infections in humans are intrinsically more difficult to treat12,13,14,15,16,17,18,19,20,21. We were able to exclude factors that influence plasma concentration for this differential drug efficacy, at least in the case of benznidazole (Fig. 5a). An alternative explanation could be that the pan-tropic nature of acute stage infections23 requires drugs to access every organ and tissue at levels sufficient to produce a sterile cure. In chronic stage infections this might be easier to achieve, as the parasite burden is considerably lower and restricted to far fewer locations than in the acute phase. In mouse models, the colon and/or stomach are the major sites of parasite persistence, with other organs and tissues sporadically infected29.
Most in vivo drug testing has focused on acute stage infections, partly because it is more straightforward to monitor parasite burden31,32,33,34. From a clinical perspective however, the ability to cure chronic infections is the primary requirement35. Therefore, the differential stage-specific responses to nitroheterocyclic drugs highlight that chronic infections should be the major focus of studies in predictive animal models. In line with this, there is a need to systematically assess the extent to which drug-induced cure of chronic infections alleviates or prevents the development of cardiac pathology. There is strong evidence that successful treatment of acute stage infections is associated with improved prognosis36,37,38,39. However, with chronic infections, the evidence is less certain40. For example, in the BENEFIT trial, improvements in clinical outcomes related to cardiac cardiomyopathy were not observed 5 years after benznidazole therapy11, although aspects of this study are the subject of debate41,42,43,44. The availability of improved predictive models of chronic Chagas disease now provides an experimental platform to dissect the link between curative therapy and disease pathology. Data from such studies will be invaluable for informing and guiding the design of future clinical trials.
The current front-line drugs are used against acute stage infections, with cure rates of 80–90%19. Because of the complex and long-term nature of Chagas disease, and difficulties in establishing parasitological cure, there have been few rigorous studies to optimise treatment of chronic infections40. Therapeutic schedules have been based on regimens that proved relatively successful against the acute stage. However, a combination of severe side-effects and treatment length (60–90 days), in a context where the infection is often asymptomatic, can lead to compliance issues that impact on curative outcome. The data from this study, which demonstrate that chronic infections in mouse models are more easily cured by nitroheterocyclic drugs than acute stage infections (Table 1), suggest the possibility of reducing treatment duration and drug dose.
Although fexinidazole has been advanced into Phase II clinical trials against T. cruzi infection, the study was not completed because of tolerability issues45. Here, we demonstrate that fexinidazole and fexinidazole sulfone are more efficacious than both of the front-line drugs in murine models at equivalent doses, particularly in the acute stage. For example, 5 days treatment with 50 mg kg−1 bid, is almost always curative with fexinidazole and fexinidazole sulfone, but rarely so with benznidazole or nifurtimox (Table 1). Similarly, the effectiveness of the fexinidazole compounds is evident when administered in single daily doses of 100 mg kg−1. The PK studies suggest that this enhanced activity could be linked to higher and more prolonged plasma exposure than is achievable with either benznidazole or nifurtimox (Figs 5 and 6; Table 2), thereby allowing more effective targeting of parasites in a wider range of organs and tissues. However, these PK-based inferences are complicated by the fact that the trypanocidal properties of nitroheterocyclic drugs result from reactive metabolites generated within the parasite following nitroreductase-mediated activity8,46,47. We also cannot exclude the possibility of exposure changes with repeat administration, given that the PK studies were all single dose experiments. Whatever the determinant of efficacy, our results highlight the potential for reducing the treatment duration and/or therapeutic dose of fexinidazole and fexinidazole sulfone as a means of circumventing toxicity and compliance issues.
In summary, the application of highly sensitive imaging technology to predictive models of Chagas disease provides new insights into drug efficacy. Of particular importance is the finding that T. cruzi infections are more readily cured in the chronic stage. It will be important to establish if these observations are transferrable to a clinical setting.
Methods
Mice
Infection experiments were approved by the LSHTM Ethics Committee and performed under UK Home Office licence PPL70/8207. All methods and manipulations were performed in accordance with the requirements of this licence. Female BALB/c and C3H/HeN mice were obtained from Charles River (UK) and CB17 SCID mice were bred in-house. Mice were maintained as described previously23 and were aged 8–12 weeks when infected with bioluminescent CL Brener23 or JRcl4 strains29. Typically, 1 × 104 bloodstream trypomastigotes (BTs) in 0.2 ml PBS were used to infect SCID mice via intraperitoneal (i.p.) inoculation. Parasitaemic blood from these mice was obtained 2–3 weeks later and adjusted to 5 × 103 BTs ml−1 with PBS. Mice were then infected i.p. with 1 × 103 BTs23. On occasions (~10%), infection with the JR strain led to a fatal outcome, prior to the initiation of treatment. These mice were not included in the data set (Table 1).
Parasites
T. cruzi strains CL Brener and JRcl4 were grown as epimastigotes at 28 °C26. Metacyclic trypomastigotes were obtained by transfer to Graces-IH medium48 and harvested after 4–7 days. Tissue culture trypomastigotes were obtained from infected L6 rat myoblasts29. Bioluminescent parasites expressing the firefly luciferase PpyRE9h gene22 were generated as described23,24.
Drug Treatment
Benznidazole, nifurtimox, fexinidazole and fexinidazole sulfone were synthesized by Epichem Pty Ltd., Australia, and prepared at 5 mg ml−1 in an aqueous suspension vehicle containing 5% (v/v) DMSO, 0.5% (w/v) hydroxypropyl methylcellulose, 0.5% (v/v) benzyl alcohol and 0.4% (v/v) Tween 80. Drugs were administered by oral gavage (~200 μl), and vehicle only was administered to control mice. To detect residual infection, mice were immunosuppressed with cyclophosphamide (200 mg kg−1) by i.p. injection every 4 days for a maximum of 3 doses.
In vivo bioluminescence imaging
Mice were injected i.p. with 150 mg kg−1 d-luciferin in Dulbecco’s Ca2+/Mg2+ free PBS, then anaesthetized using 2.5% (v/v) isofluorane in oxygen. Images were obtained using an IVIS Lumina II system (Caliper Life Science) 10–20 minutes after d-luciferin administration. Exposure times varied from 30 seconds to 5 minutes, depending on signal intensity. To estimate parasite burden, whole body regions of interest were drawn using LivingImage v4.3 to quantify bioluminescence expressed as total flux (photons/second; p/s). The detection threshold was established from uninfected mice23. Animals with bioluminescence intensity below 5 × 103 p/s/sr/cm2 in both dorsal and ventral images following immunosuppression were designated cured, subject to confirmation by ex vivo assessment.
Assessing treatment efficacy by ex vivo imaging
Organs/tissue samples were assessed by ex vivo imaging23,25. Briefly, mice were injected i.p. with 150 mg kg−1 d-luciferin, then sacrificed by ex-sanguination under terminal anaesthesia 7 minutes later. They were perfused with 10 ml 0.3 mg ml−1 d-luciferin in PBS via the heart. Organs/tissues were excised, transferred to a Petri dish, soaked in 0.3 mg ml−1 d-luciferin, then imaged. Routinely, the carcass was assessed for bioluminescence associated with skin, skeletal muscle or remaining adipose tissue.
Pharmacokinetic analysis
Pharmacokinetic (PK) studies in non-infected mice conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee. PK studies were conducted using infected and non-infected female BALB/c (benznidazole) or non-infected female (benznidazole, fexinidazole and fexinidazole sulfone) and male (nifurtimox) Swiss outbred mice. Drugs were administered by oral gavage. For the efficacy studies, blood samples (10 μl) were collected from the tail vein into tubes containing MilliQ water (20 μl) at 0.25, 0.5, 1, 2, 4, 6, 9, and 24 hours post-dose. Samples were subjected to 3x freeze/thaw cycles in liquid nitrogen before bioanalysis. The level of benznidazole in mouse blood was determined using UPLC-MS/MS (Waters Xevo TQs) following protein precipitation with acetonitrile. Blood concentrations were converted to plasma concentrations using the measured blood to plasma ratio of ~1.
For the parallel PK studies, a maximum of two blood samples were collected from each mouse (with three mice per time-point) by submandibular bleed and terminal cardiac puncture into heparinised tubes. For benznidazole, nifurtimox and fexinidazole, samples were collected for up to 24 hours post-dose, whereas for fexinidazole sulfone, samples were collected over 48 hours. Plasma was separated by centrifugation and stored at −80 °C (maximum two weeks). For analysis, samples were thawed, proteins precipitated, and the supernatant analysed by LC/MS with quantitation by comparison to a calibration curve prepared in blank plasma. Blood:plasma partitioning ratios in mouse plasma, and the fraction unbound in plasma and in vitro assay media binding, were assessed as described29.
For both efficacy and PK studies, plasma concentration versus time data were analysed by non-compartmental PK methods to obtain the maximum concentration (Cmax), the time to reach Cmax (Tmax), and the area under the plasma concentration time curve to infinity (AUC∞).
Additional Information
How to cite this article: Francisco, A. F. et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 6, 35351; doi: 10.1038/srep35351 (2016).
References
Kirchhoff, L. V. Epidemiology of American trypanosomiasis (Chagas disease). Adv. Parasitol. 75, 1–15 (2011).
Bern, C. & Montgomery, S. P. An estimate of the burden of Chagas disease in the UnitedStates. Clin. Infect. Dis. 49, e52–e54 (2009).
www.who.int/neglected_diseases/integrated_media_chagas_statement/en/index.
Carod-Artal, F. J. Trypanosomiasis, cardiomyopathy and the risk of ischemic stroke. Expert Rev. Cardiovasc. Ther. 8, 717–728 (2010).
Jabari, S., de Oliveira, E. C., Brehmer, A. & da Silveira, A. B. Chagasic megacolon: entericneurons and related structures. Histochem. Cell. Biol. 42, 235–244 (2014).
Salomon, C. J. First century of Chagas’ disease: an overview on novel approaches to nifurtimox and benzonidazole delivery systems. J. Pharm. Sci. 101, 888–894 (2012).
Gaspar, L. et al. Current and future chemotherapy for Chagas disease. Curr. Med. Chem. 22, 4293–4312 (2015).
Wilkinson, S. R., Taylor, M. C., Horn, D., Kelly, J. M. & Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 105, 5022–5027 (2008).
Mejia, A. M. et al. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Inf. Dis. 206, 220–228 (2012).
Molina, I. et al. Randomized trial of posaconazole and benznidazole for chronic Chagas disease. N. Engl. J. Med. 370, 1899–1908 (2014).
Morillo, C. A. et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 373, 1295–1306 (2015).
Rodriques Coura, J. & de Castro, S. L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97, 3–24 (2002).
Croft, S. L., Barrett, M. P. & Urbina, J. A. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 21, 508–512 (2005).
Wilkinson, S. R. & Kelly, J. M. Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev. Molec. Med. 11, e31 pp1–24 (2009).
Urbina, J. A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 115, 55–68 (2010).
Rassi, A. Jr., Rassi, A. & Marin-Neto, J. A. Chagas disease. Lancet Infect. Dis. 10, 556–570 (2010).
Jackson, Y. et al. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. 51, e69–e75 (2010).
Wilkinson, S. R., Bot, C., Kelly, J. M. & Hall, B. S. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr. Topics Med. Chem. 11, 2072–2084 (2011).
Guedes, P. M., Silva, G. K., Gutierrez, F. R. & Silva, J. S. Current status of Chagas disease chemotherapy. Expert Rev. Anti-infect. Ther. 9, 609–620 (2011).
Bern, C. Antitrypanosomal therapy for chronic Chagas’ disease. N. Engl. J. Med. 364, 2527–2534 (2011)
Bermudez, J., Davies, C., Simonazzi, A., Pablo Real, J. & Palma S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 30, 1–16 (2015).
Branchini, B. R. et al. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal. Biochem. 396, 290–297 (2010).
Lewis, M. D. et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell. Microbiol. 16, 1285–1300 (2014).
Lewis, M. D., Fortes Francisco, A., Taylor, M. C. & Kelly, J. M. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J. Biomolec. Screening 20, 36–43 (2015).
Fortes Francisco, A. et al. The limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob. Agents Chemother. 59, 4653–4661 (2015).
Bahia, M. T. et al. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Negl. Trop. Dis. 6, e1870 (2012).
Bahia, M. T. et al. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob. Agents Chemother. 58, 4362–4370 (2014).
Lewis, M. D. et al. Recent, independent and anthropogenic origins of Trypanosoma cruzi hybrids. PLoS Negl. Trop. Dis. 5, e1363 (2011).
Lewis, M. D., Fortes Francisco, A., Taylor, M. C., Jayawardhana, S. & Kelly, J. M. Host and parasite genetics shape a link betweensTrypanosoma cruzi infection dynamics and chronic cardiomyopashs. Cell. Microbiol. 18, 1429–1443 (2016).
Torreele, E. et al. Fexinidazole – a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 4, e923 (2010).
Chatelain, E. & Konar, N. Translational challenges of animal models in Chagas disease drug development: a review. J. Drug Des. Devel. Ther. 9, 4807–4823 (2015).
Buckner, F. Experimental chemotherapy and approaches to drug discovery for Trypanosoma cruzi infection. Adv. Parasitol. 75, 89–119 (2011).
Canavaci, A. M. et al. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Negl. Trop. Dis. 4, e740 (2010).
Romanha, A. J. et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 105, 233–238 (2010).
http://www.dndi.org/diseases-projects/chagas/chagas-target-product-profile/.
Assíria Fontes Martins, T. et al. Benznidazole/itraconazole combination treatment enhances anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS One 10, e0128707 (2015).
Gruendling, A. P. et al. Impact of benznidazole on infection course in mice experimentally infected with Trypanosoma cruzi I, II, and IV. Am. J. Trop. Med. Hyg. 92, 1178–1189 (2015).
Molina-Berríos, A. et al. Benznidazole prevents endothelial damage in an experimental model of Chagas disease. Acta Trop. 127, 6–13 (2013).
Davies, C. et al. Basombrío MA. Hydroxymethylnitrofurazone is active in a murine model of Chagas’ disease. Antimicrob. Agents Chemother. 54, 3584–3589 (2010).
Villar, J. C. et al. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst. Rev. 5, CD003463 (2014).
Hamers, R. L., van Gool, T. & Goorhuis, A. Benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 374, 188 (2016).
Cordeiro, M. A. Benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 374, 188–189 (2016).
Urbina, J. A., Gascon, J. & Ribeiro, I. Benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 374, 189 (2016).
Morillo, C. A., Marin-Neto, J. A. & Avezum, A. Benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 374, 189–190 (2016).
http://www.dndi.org/diseases-projects/portfolio/fexinidazole-chagas/.
Hall, B. S. & Wilkinson, S. R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 56, 115–123 (2012).
Hall, B. S., Bot, C. & Wilkinson, S. R. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 286, 13088–13095 (2011).
Isola, E. L., Lammel, E. M. & Gonzalez Cappa, S. M. Trypanosoma cruzi: differentiation after interaction of epimastigotes and Triatoma infestans intestinal homogenate. Exp. Parasitol. 62, 329–335 (1986).
Coteron, J. M. et al. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J. Med. Chem. 54, 5540–6551 (2011).
Acknowledgements
This work was supported by the Drugs for Neglected Diseases initiative (DNDi), an Australian Research Council Linkage Project grant (LP140100560) and Wellcome Trust awards (092340MF and 105021). For this project, DNDi received financial support from: Department for International Development (DFID), UK; Federal Ministry of Education and Research (BMBF) through KfW, Germany; and Médecins sans Frontières (MSF) International.
Author information
Authors and Affiliations
Contributions
J.M.K., A.F.F., K.D.R., S.A.C. and E.C. designed the experiments and analysed the data. A.F.F., S.J., M.D.L., K.L.W., D.M.S., G.C., J.S. and M.O.-C. performed the experiments. J.M.K. wrote the paper, with input from A.F.F., K.D.R., S.A.C. and E.C.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Francisco, A., Jayawardhana, S., Lewis, M. et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci Rep 6, 35351 (2016). https://doi.org/10.1038/srep35351
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35351
This article is cited by
-
Anti-Trypanosoma cruzi activity of Coptis rhizome extract and its constituents
Tropical Medicine and Health (2023)
-
Fexinidazole interferes with the growth and structural organization of Trypanosoma cruzi
Scientific Reports (2022)
-
Short-course Benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): a non-inferiority randomized controlled trial study protocol
Reproductive Health (2020)
-
Fexinidazole: First Global Approval
Drugs (2019)
-
Nitroheterocyclic derivatives: privileged scaffold for drug development against Chagas disease
Medicinal Chemistry Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.