Abstract
Anthracnose caused by Colletotrichum is one of the most severe diseases that can afflict Camellia sinensis. However, research on the diversity and geographical distribution of Colletotrichum in China remain limited. In this study, 106 Colletotrichum isolates were collected from diseased leaves of Ca. sinensis cultivated in the 15 main tea production provinces in China. Multi-locus phylogenetic analysis coupled with morphological identification showed that the collected isolates belonged to 11 species, including 6 known species (C. camelliae, C. cliviae, C. fioriniae, C. fructicola, C. karstii, and C. siamense), 3 new record species (C. aenigma, C. endophytica, and C. truncatum), 1 novel species (C. wuxiense), and 1 indistinguishable strain, herein described as Colletotrichum sp. Of these species, C. camelliae and C. fructicola were the dominant species causing anthracnose in Ca. sinensis. In addition, our study provided further evidence that phylogenetic analysis using a combination of ApMat and GS sequences can be used to effectively resolve the taxonomic relationships within the C. gloeosporioides species complex. Finally, pathogenicity tests suggested that C. camelliae, C. aenigma, and C. endophytica are more invasive than other species after the inoculation of the leaves of Ca. sinensis.
Similar content being viewed by others
Introduction
The tea plant (Camellia sinensis (L.) O. Kuntze) is an important non-alcoholic beverage crop that is widely cultivated in tropical and subtropical areas. China, one of the earliest countries to begin cultivating tea plants, cultivates the widest variety of tea plants over the largest area in the world, spanning 20 southern provinces1. According to records from the Food and Agriculture Organization (FAO) of the United Nations, China produced 1,939,457 tons of tea in 2013 (http://faostat3.fao.org/download/Q/QC/E). The raw materials for tea products, the buds and leaves of the tea plant, are affected by a number of diseases. Of these diseases, anthracnose caused by Colletotrichum spp. is one of the most serious diseases2. Leaves infected by Colletotrichum generally have water-soaked lesions at the initial stage of the disease. As the disease progresses, the lesions get larger and become necrotic, ultimately leading to serious losses in yield3.
Colletotrichum Corda is one of the most important fungal genera containing plant pathogens in the world, causing disease in a wide range of hosts4,5. Colletotrichum can inhabit plants as a pathogen, endophyte, epiphyte, or saprobe5,6,7,8. Studies of the taxonomy of Colletotrichum were once limited to the identification of strains using only inconsistent morphological characteristics and host association8,9. More recently, the use of morphology coupled with multi-gene molecular phylogeny has developed as an effective strategy for identification and has improved the understanding of Colletotrichum taxonomy4,5,8,9,10. Using this identification strategy, the Colletotrichum species were re-classified into 11 important species complexes and some independent species5,11,12,13,14,15,16. In addition, several recent studies have used the ApMat DNA locus to accurately and rapidly identify the Colletotrichum species2,17,18,19.
A single host plant can be infected by multiple Colletotrichum species20,21,22,23,24. Several studies identifying Colletotrichum as the causal agent of anthracnose in Ca. sinensis have shown that there is remarkable species diversity in Colletotrichum present as pathogens or endophytes3,25,26,27,28. However, these studies only applied either morphology or internal transcribed spacer (ITS) method to identify Colletotrichum species, and this approach has been reported inaccurate for interspecific relationship identifications4,8,9,29. Furthermore, these studies were also limited by the small area of the investigated regions and lacked information regarding the infected tea plant cultivar. For example, Guo et al.3 investigated Colletotrichum only in yellow mountain tea plants in the Anhui province of China. Liu et al.2 recently isolated Colletotrichum from healthy and diseased tissues of Camellia spp. from 7 provinces in China and from 3 other countries. However, their investigations included only partial areas of tea plant cultivation in China. Therefore, further study is necessary to identify Colletotrichum on Ca. sinensis in China to a species level by their morphological characteristics and multigene phylogenies.
To understand the diversity of Colletotrichum species on Ca. sinensis, we collected diseased leaves of tea plants from several of the major tea growing regions of China. After isolating and identifying Colletotrichum species, we summarized the Colletotrichum species associated with Ca. sinensis and their geographical distributions in China. We believe these results can provide phytopathologists and plant breeders with a reference for the prevention and control of anthracnose disease.
Results
Multilocus-based phylogenetic analysis
We collected 106 isolates of Colletotrichum spp. from diseased leaves of Ca. sinensis from the main tea growing regions in China and identified them based on phylogeny and morphological characteristics.
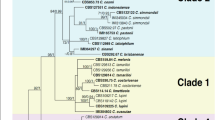
The phylogram in Fig. 1 shows the identified isolates in the C. gloeosporioides species complex. The combined aligned data matrix (ITS, ACT, GAPDH, CAL, CHS-1, TUB2, and GS) contained 152 sequences including the outgroup (C. boninense CBS 123755) and 3,879 characters including gaps. These species were determined from 81 isolates from Ca. sinensis plants in our study (the partly identical C. camelliae and C. fructicola isolates were removed from Fig. 1; the complete alignment and tree, shown in Fig. 1c, was available from TreeBASE). Of the 81 isolates, 2 clustered with C. aenigma ex-type culture, 33 clustered with C. camelliae, 3 clustered with C. endophytica, 34 clustered with C. fructicola, 8 clustered with C. siamense, 1 (SC3A3) was indistinguishable and named Colletotrichum sp., and 2 (JS1A32, JS1A44) did not cluster with any known species and formed a distinct clade with a high bootstrap support/posterior probability value (61/0.98).
Phylogenetic tree generated by maximum likelihood analysis based on combined ITS, ACT, GAPDH, CAL, CHS-1, TUB2, and GS gene sequences.
The tree shows the phylogenetic relationships between Colletotrichum species in the C. gloeosporioides species complex isolated from Ca. sinensis. Bootstrap support values above 50% and Bayesian posterior values above 0.95 are shown at each node (ML/PP). C. boninense CBS 123755 is used as outgroup. Branches crossed by diagonal lines are shortened by 50%. Ex-type strains are emphasized in bold.
The phylogram in Fig. 2 shows the isolates of Colletotrichum that were not in the C. gloeosporioides species complex. The combined aligned data matrix (ITS, ACT, GAPDH, TUB2, and CHS-1) contained 59 sequences including the outgroup (C. lindemuthianum CBS 144.31) and 1,928 characters including gaps. These data were determined from 25 isolates from Ca. sinensis in our study. The maximum likelihood tree (tree topology) had bootstrap support values greater than 50% and Bayesian posterior probability values of ≥0.95. The 25 isolates were grouped into 4 subclades: C. cliviae, C. fioriniae, C. karstii, and C. truncatum. Thirteen isolates clustered with the ex-type strain of C. karstii CORCG6, 1 isolate clustered with C. truncatum, 6 isolates clustered with C. fioriniae, and 5 isolates clustered with C. cliviae.
Phylogenetic tree generated by maximum likelihood analysis based on combined ITS, ACT, GAPDH, TUB2, and CHS-1 gene sequences.
The tree shows the phylogenetic relationships between Colletotrichum species outside of the C. gloeosporioides species complex isolated from Ca. sinensis. Bootstrap support values above 50% and Bayesian posterior values above 0.95 are shown at each node (ML/PP). C. lindemuthianum CBS 144.31 is used as outgroup. Ex-type strains are emphasized in bold.
ApMat & GS-based phylogenetic analysis of the C. gloeosporioides species complex
Forty-seven strains of the C. gloeosporioides species complex isolated from tea plants and 35 reported strains were used for phylogenetic tree construction (rooted with C. xanthorrhoeae) based on ApMat and GS sequences. The dataset comprised 1,829 characters with alignment gaps (Fig. 3). All species could be separated with high support values, followed by multi-locus phylogenetic tree analysis, with the exception of isolates GX2A1 and GX2A3. These 2 isolates belonged to C. siamense rather than C. aeschynomenes (Fig. 1). Sequence alignment of ApMat and GS showed that the isolates GX2A1 and GX2A3 differed from C. aeschynomenes ICMP 17673 by only 5 bp in ApMat but by 19 bp in GS. The strain SC3A3 clustered with C. kahawae sensu lato.
Pairwise homoplasy index (PHI) test
Application of the PHI test to the concatenated 6-locus sequences (ITS, ACT, GAPDH, CAL, TUB2, and GS) revealed the recombination level within phylogenetically related species (Φw < 0.05). Significant recombination events were detected between C. camelliae and three closely related strains (JS1A35, SH1B4, and YN2A1) (Φw = <0.01) (Fig. 4a). No significant recombination events were observed between C. wuxiense and phylogenetically related species such as C. camelliae, C. jiangxiense, and C. kahawae s. l. (Φw = 0.3) (Fig. 4b).
Pathogenicity tests
Pathogenicity tests showed that C. aenigma CGMCC 3.17883, C. camelliae CGMCC 3.17884, and C. endophytica CGMCC 3.17887 have the typical brown lesions of anthracnose disease around wounded areas. Conidia from wounds were re-isolated and cultured on potato dextrose agar (PDA), which showed pathogenesis analogous to that of infected strains. However, wounded leaves of Ca. sinensis cv. Longjing 43 did not have obvious disease spots after inoculation with Colletotrichum sp. CGMCC 3.17890, C. siamense CGMCC 3.17892, or C. wuxiense CGMCC 3.17894 (see Supplementary Figs S4l, S9m and S10m). A possible explanation for the few disease spots caused by these strains is that the strains have weak virulence or lack necessary pathogenesis genes for Longjing 43 (the gene-for-gene hypothesis)30,31.
Taxonomy
Based on their multi-locus phylogeny and morphological characteristics, the 106 isolates from Ca. sinensis were identified as 11 species of Colletotrichum (Figs 1, 2, 3), including 1 new species (named C. wuxiense), 3 new record species (C. aenigma, C. endophytica, and C. truncatum), 6 previously described species (C. camelliae, C. cliviae, C. fioriniae, C. fructicola, C. karstii, and C. siamense), and 1 indistinguishable strain (described as Colletotrichum sp.).
Colletotrichum aenigma B.S. Weir & P.R. Johnston, Studies in Mycology 73: 135. 2012. See Supplementary Fig. S1.
Description: Colonies on PDA were flat with entire edges, aerial mycelium sparse, cottony, pale white, scattered acervuli with yellow conidial mass near the center, pale white on reverse, had a growth rate of 11.4–12.3 mm per day at 25 °C after 5 days. Sexual morph was observed on PDA agar after 7 days. Ascomata globose, brown to dark brown, covered by sparse and white aerial mycelium, outer walls composed of dark brown verruculose angular cells. Asci clavate, falciform, 64.0–111.0 × 12.0–17.0 μm, n = 8, 8-spored. Ascospores arranged biseriately, hyaline, aseptate, smooth, ellipsoidal or ovoid, 13.4–16.6 × 5.9–8.3 μm, av ± SE = 14.8 ± 1.1–6.7 ± 0.8 μm, L/W ratio = 2.2, n = 30. Asexual morph was observed, Chlamydospores were not observed. Conidiomata acervular. Setae observed, dark brown, smooth, 2-septate, 112.0–123.0 μm long, the base rounded, 1.6–4.8 μm diameter, tip somewhat acute. Conidiophores and conidiogenous cells were not observed. Conidia hyaline, aseptate, smooth, cylindric with broadly rounded ends, 13.2–20.0 × 5.2–7.3 μm, av ± SE = 17.2 ± 1.5–6.1 ± 0.5 μm, L/W ratio = 2.8, n = 30. Appressoria subglobose or with a few broad lobes, brown, branched, 8.6–16.6 × 5.2–10.6 μm, av ± SE = 11.8 ± 2.0–7.9 ± 1.2 μm, L/W ratio = 1.5, n = 30.
Materials examined: CHINA, Jiangsu Province, Wuxi City, from diseased leaves of Ca. sinensis, 20 Aug 2014. Y.C. Wang, culture JS1A9; ibid., culture CGMCC 3.17883 = JS1A29.
Notes: Colletotrichum aenigma was first reported on Persea americana from Israel and has been subsequently reported on Pyrus pyrifolia from Japan13, Pyrus communis, Citrus sinensis, and Olea europaea from Italy32, Hylocereus undatus from Thailand33, Poplar sp. from China22,34, and Vitis vinifera from China24. However, none of these studies described the sexual morph and setae of this species. This is the first report of C. aenigma associated with anthracnose of Ca. sinensis in China and the first description of the morphological characteristics of its sexual morph and seta.
Colletotrichum camelliae Massee, Bull. Misc. Inform. Kew. 1899: 91. 1899. See Supplementary Fig. S2.
Description: Colonies on PDA raised centers, aerial mycelium dense, cottony, iron-grey. Chlamydospores not observed, reverse buff, a growth rate of 11.9–12.9 mm per day at 25 °C after 5 days. Sexual morph was not observed. Chlamydospores black, hidden in medium. Only one acervular was observed, conidiophores and setae either directly formed from hyphae or on a cushion of roundish hyaline cells. Setae dark brown, smooth-walled, 1–2 septate, 56.3 μm long, base inflated or cylindrical, 2.0–5.4 μm diameter, tip more or less acute. Conidiophores abundant, hyaline, smooth, septate, branched. Conidiogenous cells hyaline, cylindrical, 8.1–16.2 × 4.9–6.2 μm. Conidia hyaline, aseptate, smooth, cylindrical with obtuse ends or narrowed towards the base, 14.4–18.2 × 5.6–7.4 μm, av ± SE = 16.5 ± 1.0 × 6.5 ± 0.4 μm, L/W ratio = 2.5, n = 30. Appressoria irregularly shaped, fusiform, crenate, lobed, brown to dark brown, branched, 8.0–20.3 × 5.2–12.7 μm, av ± SE = 12.3 ± 2.5 × 8.5 ± 1.7 μm, L/W ratio = 1.4, n = 30.
Materials examined: CHINA, Chongqing City, Yongchuan County, from diseased leaves of Ca. sinensis, 20 Jul. 2014, Y.C. Wang, culture CQ1A10. Jiangsu Province, Wuxi City, from diseased leaves of Ca. sinensis, 20 Jul. 2014, Y.C. Wang; ibid., culture CGMCC 3.17884 = JS1A35. Shaanxi Province, Hanzhong City, Xixiang County, from diseased leaves of Ca. sinensis cv. Fuding Dabaicha, 30 Jun. 2014, Y.C. Wang, culture SH1B4. Yunan Province, Puer City, Menghai County, from diseased leaves of Ca. sinensis, 10 Sept. 2014, Y.C. Wang, culture YN2A1.
Notes: The morphological characteristics of Colletotrichum camelliae were systematically described in detail based on methodology used in previous studies2. Although C. camelliae had been described previously as the dominant Colletotrichum species on Camellia plants in China2,13,18, the characteristics of its seta had not been investigated. In the present study, the morphological characteristics of setae were described. Interestingly, 3 strains clustered with C. camelliae LF789 and formed a distinct subclade as shown in Fig. 1c. However, ApMat and GS-based phylogenetic analysis showed that these 3 strains were distinctly clustered with C. camelliae (Fig. 3). The PHI test also revealed significant genetic recombination levels between these 3 strains and C. camelliae, suggesting that they are conspecific. In addition, conidia and appressorium dimensions of the 2 strains from Jiangsu and Yunnan Province (JS1A35, conida: 14.4–18.2 × 5.6–7.4 μm, av = 16.5 × 6.5 μm, appressoria: 8.0–20.3 × 5.2–12.7, av = 12.3 × 8.5 μm; YN2A1, conida: 15.7–20.1 × 4.9–6.8 μm, av = 18.1 × 5.8 μm, appressoria: 6.1–18.0 × 6.3–11.8, av = 11.3 × 8.6 μm) were in accordance with C. camelliae ex-type culture, while the mean values were larger than those of ex-type culture (GMCC 3.14925, conida: 9.0–25 × 3.5–7.5 μm, av = 15.5 × 5.0 μm, appressoria: 6.5–13.5 × 5.0–10.5 μm, av = 10.0 × 7.5 μm).
Colletotrichum cliviae Y.L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai, Fungal Diversity 39: 133. 2009.
Description and illustrations: see Yang et al.35 and Liu et al.2.
Materials examined: CHINA, Anhui Province, Huangshan City, Qimen County, from diseased leaves of Ca. sinensis cv. Keemenzhong, 27 Aug. 2014, Y.C. Wang, culture AH1A2; ibid., culture CGMCC 3.17885 = AH1B5; ibid., culture AH1B6. Zhejiang Province, Hangzhou city, from diseased leaves of Ca. sinensis cv. Longjing 43, 8 Jun. 2015, Y.C. Wang, culture ZJ3A4; ibid., culture ZJ3A9.
Notes: Colletotrichum cliviae was identified previously on a healthy tea leaf from Guilin, Guangxi Province, China2. In the present study, 5 strains of pathogenic C. cliviae were isolated from Ca. sinensis cv. Keemenzhong from Anhui and cv. Longjing 43 from Zhejiang Province, China.
Colletotrichum endophytica Manamgoda, Udayanga, L. Cai & K.D. Hyde, Fungal Diversity 61: 107–115. 2013. See Supplementary Fig. S3.
Description: Colonies on PDA flat with entire edge, aerial mycelium sparse, pale white, scattered numerous acervuli with orange conidial mass, reverse scattered orange colored pigmentation around the center and white aerial mycelium near the margin, a growth rate of 12.6–13.6 mm per day at 25 °C after 5 days. Sexual morph was not observed. Conidiomata acervular. Chlamydospores not observed. Setae brown, 2–3 septate, 73.6–105.5 μm long, base more or less inflated, 1.8–3.3 μm diameter, tip usually acute. Conidiophores abundant, hyaline, smooth, septate, branched. Conidiogenous cells hyaline, cylindrical, ampulliform, 11.1–15.1 × 3.6–4.9 μm. Conidia hyaline, aseptate, smooth, cylindrical, 16.2–19.9 × 4.2–6.0 μm, av ± SE = 18.4 ± 0.9 × 5.0 ± 0.5 μm, L/W ratio = 3.7, n = 30. Appressoria irregularly shaped, unlobed or slightly lobed, brown to dark brown, unbranched, 7.9–18.0 × 4.6–11.6 μm, av ± SE = 12.6 ± 2.8 × 7.8 ± 1.5 μm, L/W ratio = 1.6, n = 30.
Materials examined: CHINA, Yunnan Province, Lincang City, from diseased leaves of Ca. sinensis cv. Menku, 17 Jul. 2014, Y.C. Wang, culture CGMCC 3.17886 = YN1A3; ibid., culture CGMCC 3.17887 = YN1A4; ibid., culture YN1A5.
Notes: Colletotrichum endophytica had been found only as an endophyte on Pennisetum purpureum6 and on unknown wild fruit from Thailand36. In the present study, 3 pathogenic strains were isolated from diseased leaves of Ca. sinensis. This is the first report of C. endophytica causing anthracnose in Ca. sinensis and the first report of C. endophytica in China. Setae of C. endophytica are observed and described in this study for the first time.
Colletotrichum fioriniae (Marcelino & Gouli) R.G. Shivas & Y.P. Tan, Fungal Diversity 39: 117. 2009.
Description and illustrations: see Damm et al.11.
Materials examined: CHINA, Sichuan Province, Meishan City, Hongya County, from diseased leaves of Ca. sinensis cv. Mingshanzao 132, 10 Sept. 2014, Y.C. Wang, culture CGMCC 3.17888 = SC3A2. Zhejiang Province, Hangzhou City, on Ca. sinensis cv. Longjing 43, 20 Jul. 2014, Y.C. Wang, culture ZJ1A1.
Notes: Colletotrichum fioriniae has been reported on various host plants, including Camellia plants in Kunming, Yunnan Province, and Fuzhou, Fujian Province, China11,28. In the present study, the species were isolated from Ca. sinensis cv. Fuding Dahaocha and cv. Huangdan from Fuzhou, Fujian Province, from cv. Mingshanzao 132 from Meishan, Sichuan Province, and from cv. Longjing 43 from Hangzhou, Zhejiang Province, China.
Colletotrichum fructicola Prihastuti, L. Cai & K.D. Hyde, Fungal Diversity 39: 158. 2009.
Description and illustrations: see Prihastuti et al.23 and Liu et al.2.
Materials examined: CHINA, Chongqing City, Yongchuan County, from diseased leaves of Ca. sinensis, 20 Jul. 2014, Y.C. Wang, culture CGMCC 3.17889 = CQ1A5. Hubei Province, Enshi City, from diseased leaves of Ca. sinensis, 18 Jul. 2014, Y.C. Wang, culture HB1A5. Sichuan Province, Yibin City, Jiangan County, from diseased leaves of Ca. sinensis cv. Zhongcha 302, 16 Jul. 2014, Y.C. Wang, culture SC1A1. Zhjiang Province, Hangzhou City, from diseased leaves of Ca. sinensis cv. Longjing 43, 20 Jul. 2014, Y.C. Wang, culture ZJ3A6.
Notes: Colletotrichum fructicola was first found in coffee berries and has since been found to cause disease in several plants23. It was recently reported to cause anthracnose in Ca. sinensis in 5 Provinces in China2,28. In the present study, the species was found on multiple Ca. sinensis cultivars from almost all of the main tea growing areas in China.
Colletotrichum karstii Y.L. Yang, Z.Y.Liu, K.D. Hyde & L.Cai, Cryptogamie Mycologie 32: 241. 2011.
Description and illustrations: see Yang et al.37 and Damm et al.12.
Materials examined: CHINA, Fujian Province, Quanzhou City, Anxi County, from diseased leaves of Ca. sinensis cv. Huangjingui, 21 Jul. 2014, Y.C. Wang, culture FJ2A1; ibid., from diseased leaves of Ca. sinensis cv. Maoxie, 21 Jul. 2014, Y.C. Wang, culture FJ2C6. Hunan Province, Changsha City, from diseased leaves of Ca. sinensis, 15 Sept. 2014, Y.C. Wang, culture HUN2A7. Jiangsu Province, Wuxi City, from diseased leaves of Ca. sinensis, 20 Aug. 2014, Y.C. Wang, culture JS1A8. Yunnan Province, Lincang City, from diseased leaves of Ca. sinensis cv. Menku, 17 Jul. 2014, Y.C. Wang, culture YN1A6.
Notes: Colletotrichum karstii is the most common Colletotrichum and is present in a wide range of hosts. It was previously reported to be pathogenic to Ca. sinensis from Fujian28 and Zhejiang provinces2. In this study, species were isolated from the Fujian, Zhejiang, Yunnan, Jiangsu, and Hunan Provinces of China.
Colletotrichum sp. See Supplementary Fig. S4.
Description: Colonies on PDA raised centers, aerial mycelium dense, cottony, grey, reverse olivaceous to grey colored pigmentation, a growth rate of 7.3–12.4 mm per day at 25 °C after 5 days. Sexual morph was not observed. Chlamydospores black, among the aerial mycelium. Conidiomata, seta and Conidiophores not observed. Conidia hyaline, aseptate, smooth, cylindrical, clavate, straight, 14.5–20.7 × 5.2–7.5 μm, av ± SE = 17.3 ± 1.7 × 6.1 ± 0.5 μm, L/W ratio = 2.8, n = 30. Appressoria irregularly shaped, unlobed or slightly lobed, brown to dark brown, unbranched, 8.9–14.7 × 6.6–12.2 μm, av ± SE = 11.6 ± 1.6 × 8.9 ± 1.5, L/W ratio = 1.3, n = 30.
Materials examined: CHINA, Sichuan Province, Meishan City, Hongya County, from diseased leaves of Ca. sinensis cv. Mingshanzao 132, 10 Sept. 2014, Y.C. Wang, culture CGMCC 3.17890 = SC3A3.
Notes: In the phylogenetic tree, strain SC3A3 appears as a sister clade between C. kahawae s. l. and C. jiangxiense (Fig. 1). Its sequences (ITS, ACT, GAPDH, CAL, CHS-1, and TUB2) were identical to those of ICMP 12952, but its GS sequence differed by 8 bp. The GS sequence of the SC3A3 strain also differed from that of the ex-type culture of C. jiangxiense (CGMCC 3.17363) by 25 bp and 1 bp indel. Conidia of strain SC3A3 (av = 17.3 × 6.1 μm) were similar to the extype culture of C. kahawae subsp. ciggaro (ICMP 18539, av = 17.8 × 5.1) but larger than those of the ex-type culture of C. jiangxiense (CGMCC 3.17363, av = 15.2 × 5.2 μm).
Colletotrichum siamense Prihastuti, L. Cai & K.D. Hyde, Fungal Diversity 39: 158. 2009. See Supplementary Fig. S5.
Description: Colonies on PDA raised centers, aerial mycelium dense, pale white, scattered little acervuli with orange conidial mass around the center, reverse viridescent to pale white, a growth rate of 12.4–13.5 mm per day at 25 °C after 5 days. Sexual morph was not observed. Conidiomata acervular. Chlamydospores black, hidden in medium. Setae brown, 4 septate, 69.0 μm long, base more or less inflated, 1.5–3.3 μm diameter, tip usually acute. Conidiophores formed on a cushion of roundish with medium brown cells, hyaline, smooth, aseptate, branched. Conidiogenous cells hyaline, cylindrical, ampulliform, 10.7–16.8 × 5.1–5.9 μm. Conidia hyaline, aseptate, smooth, cylindrical, straight or slight curved, 16.4–19.4 × 4.4–6.1 μm, av ± SE = 16.8 ± 1.2 × 5.3 ± 0.4 μm, L/W ratio = 3.1, n = 30. Appressoria circular or ellipsoidal, brown, branched, 6.9–15.6 × 6.1–10.1 μm, av ± SE = 10.1 ± 2.0 × 8.0 ± 1.2 μm, L/W ratio = 1.3, n = 30.
Materials examined: CHINA, Fujian Province, Fuzhou City, from diseased leaves of Ca. sinensis cv. Fujian Shuixian, 20 Jul. 2014, Y.C. Wang, culture FJ1A3; ibid., from diseased leaves of Ca. sinensis cv. Fujian Shuixian, 20 Jul. 2014, Y.C. Wang, culture FJ1A4; ibid., Quanzhou City, Anxi County, from diseased leaves of Ca. sinensis cv. Tie guanyin, 21 Jul. 2014, Y.C. Wang, culture FJ2D4. Guangxi Province, Guilin City, from diseased leaves of Ca. sinensis cv. Jiukang, 29 Aug. 2014, Y.C. Wang, culture CGMCC 3.17891 = GX2A1; ibid., CGMCC 3.17892 = GX2A3. Jiangxi Province, Nanchang City, from diseased leaves of Ca. sinensis cv. Fuding Dabaicha, 2 Sept. 2014, Y.C. Wang, culture JX1A1; ibid., JX1A3. Yunan Province, Puer City, Menghai County, from diseased leaves of Ca. sinensis, 10 Sept. 2014, Y.C. Wang, culture YN2A9.
Notes: Colletotrichum siamense was originally found on coffee berries from Thailand, has been found on various hosts, and now is considered a biologically and geographically diverse species13,23,35,38. A previous study reported that C. siamense caused anthracnose of several varieties of Ca. sinensis from many regions of China28. The species can be distinguished from other species in the C. gloeosporioides species complex through analysis of concatenated ApMat and GS sequences2,17,18. In the present study, multi-locus phylogenetic trees and morphological characterization were used to identify strains GX2A1 and GX2A3 as C. siamense (Fig. 1). However, in a phylogenetic tree constructed from ApMat and GS sequences, these 2 strains clustered with the ex-type strain of C. aeschynomenes ICMP 17673 rather than with C. siamense s. l. The ApMat sequences of the 2 strains (GX2A1 and GX2A3) were identical to those of ICMP 17673 with the exception of just 5 bp. Therefore, analysis of a single gene sequence or analysis of ApMat and GS gene sequences together were not sufficient to accurately separate the C. siamense.
Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore, Phytopathology 25: 122. 1935.
Description and illustrations: see Andrus & Moore39 and Damm et al.16.
Materials examined: CHINA, Zhejiang Province, Hangzhou City, from diseased leaves of Ca. sinensis cv. Longjing 43. 10 Aug. 2015, Y.C. Wang, culture CGMCC 3.17893 = ZJ3A3.
Notes: Colletotrichum truncatum is present in a wide range of hosts16. This is the first report showing that this species can infect Ca. sinensis in China.
Colletotrichum wuxiense Y.C. Wang, X.C. Wang & Y.J. Yang. sp. nov. Fig. 5.
MycoBank: MB 816242.
Etymology: This fungus was first collected from Wuxi city, Jiangsu province in China.
Description: Colonies on PDA were flat with entire edge, aerial mycelium dense, cottony, white, reverse olivaceous colored pigmentation around the center to white near the margin, a growth rate of 13.4–14.1 mm per day at 25 °C after 5 days. Sexual morph was not observed. Chlamydospores black, among the aerial mycelium, conidiomata and seta not observed. Conidiophores rare, formed directly on aerial mycelium, hyaline, aseptate, unbranched. Conidiogenous cells hyaline, cylindrical. Conidia hyaline, aseptate, smooth, cylindrical with obtuse ends or narrowed towards the base, straight or slightly curved, 16.5–23.1 × 4.6–6.7 μm, av ± SE = 19.0 ± 1.4 × 5.6 ± 0.5 μm, L/W ratio = 3.8, n = 30. Appressoria elliptic to subfusoid, deeply lobed, brown, unbranched, 5.2–14.6 × 5.8–10.5 μm, av ± SE = 10.2 ± 2.1 × 7.7 ± 1.1 μm, L/W ratio = 1.3, n = 30.
Materials examined: CHINA, Jiangsu Province, Wuxi City, from diseased leaves of Ca. sinensis, 20 Aug. 2014, Y.C. Wang, Holotype HMAS 246948, culture ex-type CGMCC 3.17894 = JS1A32; ibid., culture JS1A44.
Notes: Multi-locus (ITS, ACT, GAPDH, CAL, CHS-1, TUB2, and GS) phylogenetic tree analysis showed that Colletotrichum wuxiense is a sister clade of the closely related C. jiangxiense and C. kahawae s. l., and it clustered with C. camelliae (Fig. 1). C. wuxiense can be distinguished from these other species by the morphological features of its conidia, which are larger than those of these similar species2,13 and are slightly curved. In addition, C. wuxiense can be directly separated from other species of the C. gloeosporioides species complex using its concatenated ApMat and GS gene tree (Fig. 3). A PHI test also showed that no significant recombination events between C. wuxiense and closely phylogenetically related species occurred.
Prevalence of Colletotrichum species
Of the 106 isolates of Colletotrichum, 33 isolates of C. camelliae were isolated from 14 provinces/cities, and 34 isolates of C. fructicola were isolated from 11 provinces/cities (Table 1). Approximately 61.3% of the total isolates were harvested from tea-producing areas of China (Table 1, Fig. 6). These results suggest that C. camelliae and C. fructicola are the dominant species causing anthracnose of Ca. sinensis. Moreover, 7 of 11 species belonged to the C. gloeosporioides species complex (Figs 1 and 2, Table 1, Supplementary Table S1).
Isolation Rate (IR%) of Colletotrichum species that were isolated from tea plants from China.
The pie chart is created by Microsoft Excel 2010 (https://products.office.com/en-us/buy/using-your-office-2010-product-key-card). Only data from our study were used.
Discussion
In this study, a total of 106 Colletotrichum isolates obtained from the diseased leaves of tea cultivars in China were identified as 11 species, of which 9 had been described previously, 1 was identified as a new species, and 1 was unidentifiable.
C. gloeosporioides was previously considered the dominant Colletotrichum species on tea plants in China3. In our study, C. camelliae and C. fructicola were the most prevalent species in China. These results were similar to those of Liu, et al.2. C. camelliae were collected from 14 out of 15 provinces of China (Table 1, Fig. 6, Supplementary Table S1). Nevertheless, 3 strains of C. camelliae (YN2A1, JS1A35, and SH1B4) formed a separate clade and were close to C. camelliae (CGMCC 3.14925) in the phylogenetic tree (Fig. 1). Moreover, conidiophores and setae of strain JS1A35 were directly produced from hyphae or on a cushion of roundish hyaline cells, rather than on aerial mycelium (CGMCC 3.14925). In addition, we also observed intraspecific differences in the colonial morphology and growth rate of C. camelliae isolates. Therefore, the genetic differentiation among the above isolates with different geographic distribution and morphology should be further clarified. C. fructicola, which was obtained from 11 provinces across China, was the second most prevalent species in our study (Table 1, Fig. 6, Supplementary Table S1). Liu28 reported that this species could infect several varieties of Ca. sinensis in the Fujian province of China. Liu, et al.2 further corroborated this finding using 32 isolates. Hence, we believe that the C. fructicola was a latent dominant species.
Research on new record species of microorganisms in hosts can provide helpful information for understanding the interactions between hosts and microorganisms as well as their geographical distribution. Colletotrichum species could switch their lifestyle from endophytic to pathogenic, for which both internal and external environmental factors play important roles5,40. In our study, 3 new record species from Ca. sinensis were reported for the first time, including C. aenigma, C. endophytica and C. truncatum. C. endophytica was described as an endophyte or saprobe in Pennisetum purpureum and an unknown wild fruit6,36. Interestingly, our study showed that C. endophytica also can be a pathogen that infects Ca. sinensis (see Supplementary Fig. S3j). We speculated that there is a specific interaction between Ca. sinesis and C. endophytica31,41.
We collected a strain (SC3A3) that was not well distinguished and we classified it as Colletotrichum sp. using the multi-locus phylogenetic tree (Fig. 1). Its morphological characteristics were more similar to C. kahawae subsp. ciggaro than to C. jiangxiense (see Supplementary Fig. S4). Therefore, further studies are required to clarify the phylogenetic relationships among these species.
Previous studies indicated that Ca. sinensis can harbor various Colletotrichum species. C. acutatum25 and C. gloeosporioides42 were generally considered as dominantly endophytic. A few other species were considered as pathogenic or potentially pathogenic on Camellia, such as C. lupini, C. acutatum, C. carveri, C. coccodes and C. queenslandicum11,13. In this study, we collected the common species, such as C. cliviae, C. fioriniae, C. karstii, and C. siamense, as well as a novel species that was named C. wuxiense. However, C. acutatum and C. gloeosporioides were not the dominant species. Similar results were also reported by Liu, et al.2 in the Colletotrichum classification study. These differences in dominant species identification may be caused by the variation of sampling range between studies.
In conclusion, we investigated the diversity of Colletotrichum species in tea plants in China and identified 11 species including 1 novel species. Moreover, we found that C. camelliae and C. fructicola are the dominant species in Ca. sinensis. Unfortunately, our study failed to characterize endogenetic Colletotrichum species due to the lack of healthy tissue collected from tea plants. In future studies, we will isolate endophytic Colletotrichum species from healthy tissues of tea plants and elucidate their geographical distribution, the evolutionary relationship between Colletotrichum and Ca. sinensis, and the differences in intraspecific virulence and morphology of C. camelliae.
Materials and Methods
Collection and isolation
Diseased leaves with visible anthracnose symptoms were collected from the following 15 provinces or cities of China: the provinces of Anhui, Fujian, Guangdong, Guangxi, Guizhou, Henan, Hunan, Hubei, Jiangsu, Jiangxi, Shaanxi, Sichuan, Yunnan, and Zhejiang and the city of Chongqing. Five randomly selected diseased leaves were sampled from each cultivar and region. Colletotrichum species were isolated by a single spore isolation technique as described by Cai, et al.4. Spore masses were picked off with a sterilized wire loop and suspended in sterilized water. The spore suspension was diluted to a reasonable concentration and spread onto the surface of PDA, followed by an incubation overnight at 25 °C. Single germinating spores were picked up with a sterilized needle and transferred to a new PDA plate.
DNA extraction, PCR amplification, and sequencing
Fungal isolates were grown for 5–7 days on PDA. Mycelia were collected in a sterile centrifuge tube and stored at −80 °C for DNA extraction. Total genomic DNA of the isolate was extracted using a Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech Shanghai Company Limited, Shanghai, China) and stored at −20 °C. The ribosomal internal transcribed spacer (ITS), actin (ACT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-tubulin (TUB2), partial sequences of the chitin synthase 1 (CHS-1), calmodulin (CAL), glutamine synthetase (GS), mating type protein, and the Apn2-Mat1-2 intergenic spacer (ApMat) were amplified. The protocols for amplification and the PCR primers used in this study are listed in Supplementary Table S2. Each 50 μL PCR mixture included 25 μL of Premix TaqTM (Takara Biomedical Technology Company Limited, Beijing, China), 22 μL of ddH2O, 1 μL of each primer, and 1 μL of genomic DNA. PCR purification and sequencing were performed by ShangHai Huagene Biotech Company Limited, Shanghai, China.
Phylogenetic analysis
The accession numbers of all sequences in this study were obtained from NCBI-GenBank and are listed in Supplementary Table S3. A phylogenetic tree was constructed using Multi-locus sequences. The dataset was assembled using MAFFT v. 743 and manually adjusted using MEGA v. 6.044. All gaps were treated as missing data. Nucleotide substitution models were generated using MrModeltest v. 2.345, and the GTR + I + G model with gamma-distributed rate was selected for constructing all phylogenic trees. A maximum likelihood phylogenetic analysis of the dataset was performed with RAxML46. Markov Chain Monte Carlo (MCMC) sampling was used to reconstruct phylogenies in Mrbayes v. 3.247. Analyses of 6 MCMC chains based on the full dataset were run for 1 × 107 generations and sampled every 100 generations. The first 25% of the generations were discarded as burn-in. Figures of trees were created in FigTree v 1.3.148.
Morphological characterization
Mycelial discs (9 mm diameter) were taken from 5-day-old cultures, plated on PDA, and incubated at 25 °C in the dark. Daily growth rate was calculated after 5 days of growth and was based on values from three replicates. Colony characteristics were also recorded. Conidial, conidiophores, and appressoria characteristics were determined using methods described by Cai, et al.4. Additionally, appressoria were produced and measured using a slide culture technique and induced on synthetic nutrient-poor agar (SNA) medium. After 7 days, the shapes and sizes of 30 conidia, conidiophores, and appressoria were recorded (Eclipse 80i, Nikon, Japan).
Pathogenicity tests
Inoculations were based on the method described by Liu, et al.2. Six strains were selected for pathogenicity testing: C. aenigma CGMCC 3.17883, C. camelliae CGMCC 3.17884, C. endophytica CGMCC 3.17887, Colletotrichum sp. CGMCC 3.17890, C. siamense CGMCC 3.17892, and C. wuxiense CGMCC 3.17894. Healthy and non-wounded mature tea leaves, collected from 5-year-old Ca. sinensis cv. Longjing 43 grown in a tea garden in Hangzhou, Zhejiang province, China, were washed with tap water and then disinfected in 1% sodium hypochlorite for 3 min. Disinfected mature leaves were washed three times with sterilized water and then dried on the benchtop. Using sterile needles, 20 μL of conidial suspension (106 spores/mL) was added to three wounded leaves for each strain. Leaves inoculated with sterile water were used as controls. The inoculated samples were laid on plastic petri dishes 12 cm in diameter and cultured in a growth cabinet at 25 °C with a light cycle of 12 h fluorescent light and 12 h darkness for 14 d. Finally, conidia of each strain were collected from diseased leaves and cultured on a new PDA plate. They were then checked for morphological characteristics to confirm Koch’s postulates4.
Genealogical concordance phylogenetic species recognition analysis
We used the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) model, as described by Liu, et al.2, by performing a pairwise homoplasy index (Φw, PHI) test to analyze related but ambiguous species in the phylogenetic tree. The PHI test was performed in Splits Tree 449,50 using 6-locus concatenated datasets (ITS, ACT, GAPDH, CAL, TUB2 and GS), and both the LogDet transformation and splits decomposition options were selected51. PHI results below a 0.05 threshold (Φw < 0.05) were considered indicative of significant recombination in the dataset.
Prevalence of Colletotrichum species
The Isolation Rate (IR), calculated as IR% = (Cx/Ct) × 100, where Cx is the number of isolates of the same species and Ct was the total number of isolates52, was determined as a measure of the prevalence of Colletotrichum species in Ca. sinensis in China.
Statistical Analysis
SPSS (SPSS Inc., USA) was used to conduct statistical analyses. The average value of all measurements including sizes of conidia, appressoria and daily growth rate were used for statistical analyses, and values were expressed as average ± standard error (av ± SE).
Additional Information
How to cite this article: Wang, Y.-C. et al. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 6, 35287; doi: 10.1038/srep35287 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Chen, Z. M. & Yang, Y. J. The classic of tea in china. 390 (Shanghai culture press, 2011).
Liu, F. et al. Unravelling Colletotrichum species associated with Camellia: employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 35, 63–86 (2015).
Guo, M., Pan, Y., Dai, Y. & Gao, Z. First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui province, China. Plant Dis. 98, 284–284 (2014).
Cai, L. et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 39, 183–204 (2009).
Cannon, P. F., Damm, U., Johnston, P. R. & Weir, B. S. Colletotrichum-current status and future directions. Stud. Mycol. 73, 181–213 (2012).
Manamgoda, D. S., Udayanga, D., Cai, L., Chukeatirote, E. & Hyde, K. D. Endophytic Colletotrichum from tropical grasses with a new species C. endophytica. Fungal Divers. 61, 107–115 (2013).
Tao, G., Liu, Z. Y., Liu, F., Gao, Y. H. & Cai, L. Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new speices. Fungal Divers. 61, 139–164 (2013).
Hyde, K. D. et al. Colletotrichum: a catalogue of confusion. Fungal Divers. 39, 1–17 (2009).
Hyde, K. D. et al. Colletotrichum-names in current use. Fungal Divers. 39, 147–182 (2009).
Damm, U. et al. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 61, 29–59 (2013).
Damm, U., Cannon, P. F., Woudenberg, J. H. & Crous, P. W. The Colletotrichum acutatum species complex. Stud. Mycol. 73, 37–113 (2012).
Damm, U. et al. The Colletotrichum boninense species complex. Stud. Mycol. 73, 1–36 (2012).
Weir, B. S., Johnston, P. R. & Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 73, 115–180 (2012).
Damm, U., O’Connell, R. J., Groenewald, J. Z. & Crous, P. W. The Colletotrichum destructivum species complex-hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 79, 49–84 (2014).
Liu, F., Cai, L., Crous, P. W. & Damm, U. The Colletotrichum gigasporum species complex. Persoonia 33, 83–97 (2014).
Damm, U., Woudenberg, J. H. C., Cannon, P. F. & Crous, P. W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 39, 45–87 (2009).
Sharma, G., Kumar, N., Weir, B. S., Hyde, K. D. & Shenoy, B. D. The ApMat marker can resolve Colletotrichum species: a case study with Mangifera indica. Fungal Divers. 61, 117–138 (2013).
Sharma, G., Pinnaka, A. K. & Shenoy, B. D. Resolving the Colletotrichum siamense species complex using ApMat marker. Fungal Divers. 71, 247–264 (2014).
Silva, D. N. et al. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycol. 104, 396–409 (2012).
Huang, F. et al. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 61, 61–74 (2013).
Lima, N. B. et al. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 61, 75–88 (2013).
Liu, F., Damm, U., Cai, L. & Crous, P. W. Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Divers. 61, 89–105 (2013).
Prihastuti, H., Cai, L., Chen, H., McKenzie, E. & Hyde, K. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 39, 89 (2009).
Yan, J. Y. et al. Diverse species of Colletotrichum associated with grapevine anthracnose in China. Fungal Divers. 71, 233–246 (2014).
Fang, W., Yang, L., Zhu, X., Zeng, L. & Li, X. Seasonal and habitat dependent variations in culturable endophytes of Camellia sinensis. J. Plant Pathol. Microb. 4, 169 (2013).
Lu, D., Wang, J., Wu, X. & Ye, J. The species and distribution of endophytic fungi in tea trees. J. Henan Agric. Sci. 10, 54–56 (2007).
Dai, Q., Xu, Y., Lin, Q., Wang, G. & Yang, M. Distribution and establishment of Colletotrichum sp. as an endophyte in tea plants (Camellia sinensis). Sci. Silv. Sin. 44, 84–89 (2008).
Liu, W. Anthracnose pathogens identification and the genetic diversity of tea plant. PhD thesis, Fujian Agriculture and Forestry University, China (2013).
Crouch, J. A., Clarke, B. B. & Hillman, B. I. What is the value of ITS sequence data in Colletotrichum systematics and species diagnosis? A case study using the falcate-spored graminicolous Colletotrichum group. Mycol. 101, 648–656 (2009).
Brown, J. K. Durable resistance of crops to disease: a darwinian perspective. Annu. Rev. Phytopath. 53, 513–539 (2015).
O’Connell, R. J. et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 44, 1060–1065 (2012).
Schena, L. et al. Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 63, 437–446 (2014).
Meetum, P., Leksomboon, C. & Kanjanamaneesathian, M. First report of Colletotrichum aenigma and C. siamense, the causal agents of anthracnose disease of dragon fruit in Thailand. J. Plant Pathol. 97 (2015).
Li, Z., Liang, Y. M. & Tian, C. M. Characterization of the causal agent of poplar anthracnose occurring in the Beijing region. Mycota. 120, 277–286 (2012).
Yang, Y. et al. Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers. 39, 123–146 (2009).
Udayanga, D., Manamgoda, D. S., Liu, X., Chukeatirote, E. & Hyde, K. D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 61, 165–179 (2013).
Yang, Y., Cai, L., Yu, Z., Liu, Z. & Hyde, K. D. Colletotrichum species on Orchidaceae in southwest China. Cryptogamie, Mycol. 32, 229–253 (2011).
Wikee, S. et al. Colletotrichum species from Jasmine (Jasminum sambac). Fungal Divers. 46, 171–182 (2011).
Andrus, C. & Moore, W. Colletotrichum truncatum (Schw.), n. comb., on garden and lima beans. Phytopathology 25, 121–125 (1935).
Newton, A. C., Fitt, B. D., Atkins, S. D., Walters, D. R. & Daniell, T. J. Pathogenesis, parasitism and mutualism in the trophic space of microbe-plant interactions. Trends Microbiol. 18, 365–373 (2010).
Guidarelli, M., Zoli, L., Orlandini, A., Bertolini, P. & Baraldi, E. The mannose-binding lectin gene FaMBL1 is involved in the resistance of unripe strawberry fruits to Colletotrichum acutatum. Mol. Plant Pathol. 15, 832–840 (2014).
Osono, T. Endophytic and epiphytic phyllosphere fungi of Camellia japonica: seasonal and leaf age-dependent variations. Mycol. 100, 387–391 (2008).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Nylander, J. A., Wilgenbusch, J. C., Warren, D. L. & Swofford, D. L. AWTY: A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 581–583 (2008).
Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Rambaut, A. & Drummond, A. FigTree: Tree figure drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh (2008).
Huson, D. H. SplitsTree: Analyzing and visualizing evolutionary data. Bioinformatics 14, 68–73 (1998).
Huson, D. H. & Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (2006).
Quaedvlieg, W. et al. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 33, 1–40 (2014).
Vieira, W. A., Michereff, S. J., de Morais Jr, M. A., Hyde, K. D. & Câmara, M. P. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 67, 181–202 (2014).
Chen, Y. J., Tong, H. R., Wei, X. & Yuan, L. Y. First report of brown blight disease on Camellia sinensis caused by Colletotrichum acutatum in China. Plant Dis. 100, 227 (2015).
Acknowledgements
This work was supported by the Earmarked Fund for China Agriculture Research System (CARS-23), the Chinese Academy of Agricultural Sciences through an Innovation Project for Agricultural Sciences and Technology (CAAS-ASTIP-2014-TRICAAS), and the Major Project for New Agricultural Varieties Breeding of Zhejiang Province (2012C2905-3). The authors thank the Tea Experimental Stations of China Agriculture Research System in Huangshan, Chongqing, Qingyuan, Tongren, Enshi, Xinyang, Chansha, Wuxi, Yibing, Leshan, Hanzhong, Quanzhou, Guilin, Pu’er, Lishui, and Nanchang for pathogen collection. The authors would like to thank Professor L. Cai for inspiration and suggestions for the manuscript and Dr. F. Huang for help with the phylogenetic analysis.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y.-C.W., X.-C.W. and Y.-J.Y. Performed the experiments: Y.-C.W. and X.-Y.H. Analyzed the data: Y.-C.W., X.-Y.H. and X.-C.W. Contributed reagents/materials/analysis tools: Y.-C.W., X.-Y.H., L.W., B.X., X.-C.W. and Y.-J.Y. Wrote the paper: Y.-C.W. and X.-C.W. This is the first submission of this manuscript and no parts of it are being considered for publication elsewhere. All authors have approved the submission of this manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, YC., Hao, XY., Wang, L. et al. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci Rep 6, 35287 (2016). https://doi.org/10.1038/srep35287
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35287
This article is cited by
-
What’s in my Pot? Six Colletotrichum Species Causing Anthracnose in Brazilian Pecan Orchards
Current Microbiology (2024)
-
Genomic analysis of Colletotrichum camelliae responsible for tea brown blight disease
BMC Genomics (2023)
-
Molecular and biological investigating of tea plant necrotic ring blotch virus as a worldwide threat
Scientific Reports (2023)
-
24-Epibrassinolide Enhances Resistance Against Colletotrichum fructicola by Promoting Lignin Biosynthesis in Camellia sinensis L.
Journal of Plant Growth Regulation (2023)
-
First report of leaf spot caused by Colletotrichum siamense on Sophora tonkinensis
Australasian Plant Disease Notes (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.