Abstract
As one of the three major human pathogens that cause schistosomiasis, Schistosoma japonicum is the only one that is endemic in China. Despite great progress on schistosomiasis control over the past 50 years in China, S. japonicum transmission still occurs in certain endemic regions, which causes significant public health problems and enormous economic losses. During different life stages, parasites are able to survive dramatic osmolality changes between its vector, fresh water, and mammal host. However, the molecular mechanism of parasite osmoregulation remains unknown. To address this challenging question, we report the first cloning of an S. japonicum aquaglyceroporin (SjAQP) from an isolate from Jiangsu province, China. Expressing SjAQP in Xenopus oocytes facilitated the permeation of water, glycerol, and urea. The water permeability of SjAQP was inhibited by 1 mM HgCl2, 3 mM tetraethylammonium, 1 mM ZnCl2, and 1 mM CuSO4. SjAQP was constitutively expressed throughout the S. japonicum life cycle, including in the egg, miracidia, cercaria, and adult stages. The highest expression was detected during the infective cercaria stage. Our results suggest that SjAQP plays a role in osmoregulation throughout the S. japonicum life cycle, especially during cercariae transformation, which enables parasites to survive osmotic challenges.
Similar content being viewed by others
Introduction
Schistosomiasis is a neglected tropical disease that is caused by Platyhelminthes of the Schistosoma genus. It remains one of the most serious parasitic diseases in clinics and public health, especially in Asia, south America and Africa1,2. In 2014, at least 258 million people required preventive treatment and 61.6 million people were treated for schistosomiasis3. As one of the three major causative agents of human schistosomiasis, Schistosoma japonicum is the most malignant and the only human blood fluke that is endemic in regions of China, the Philippines, and parts of Indonesia4,5. It has more than 40 kinds of potential hosts that serve as reservoirs for human infections, and this unique feature complicates the transmission patterns of S. japonicum. S. japonicum infection leads to Katayama fever, as well as liver fibrosis, cirrhosis, portal hypertension, and splenomegaly. Repeated infections also cause chronic impairment of the liver3,6. Despite the remarkable success of schistosomiasis control over the past 50 years in China, this disease still remains endemic in certain lake and marshland regions, and it causes significant public health problems and enormous economic losses7,8; Hu et al. 2015). Compared with intensive epidemiological surveys, functional characterizations of proteins are important for understanding parasite physiology and transmission, especially the channel-forming aquaporins (AQPs), which have not been studied extensively and whose functions need to be determined to improve our knowledge of this important pathogen.
AQPs are a family of channel proteins that facilitate the movement of water and small neutral solutes across cell membranes. A deficiency of human AQPs causes disequilibria of water and solutes in the body, leading to clinical complications such as nephrogenic diabetes insipidus9,10. Widely distributed in nature, AQPs have been found in almost every known organism9,11. Based on their permeation specificities, aquaporins are further divided into two sub-families, water-selective AQPs or aquaglyceroporins. The former subgroup is permeable to water, while the latter exhibit permeability to small neutral solutes such as glycerol and urea, as well as water9,12. Aquaglyceroporins are the only known glycerol channels in mammals, and they play essential roles in osmoregulation by facilitating the import or export of glycerol, a major intracellular osmolyte13. The expression of certain aquaglyceroporins is up-regulated up to 20-fold under stresses such as low osmolality13, heat14, cold15, or starvation16. Glycerol is the major osmolyte, and its content in yeast cells increases under restrict regulation of the mitogen-activated protein kinase pathway, which increases intracellular osmolality and helps yeast survive osmotic stresses17. Moreover, glycerol is also a well-known stabilizer that protects proteins against denaturation through preferential hydration18,19.
S. japonicum has a complex life cycle that requires transformations among the free-living stage in fresh water, and intracellular stages in intermediate vectors or hosts20. It must have developed a mechanism to adapt to different environments, which may provide a novel and specific means of schistosomiasis control. However, thus far, there have been no reports of S. japonicum osmoregulation at the molecular level. The objectives of the present study were to clone and characterize the functions of an AQP in S. japonicum (hereafter SjAQP), as well as to determine its expression pattern throughout the parasite life cycle. Our results reveal the contributions of SjAQP to parasite survival during dramatic osmolality changes.
Materials and Methods
Ethics statement
All experiments using the S. japonicum parasite, Oncomelania hupensis snails, and mice were performed under protocols approved by the Jiangsu Parasitic Disease Institute (Wuxi, Jiangsu Province, China) with China guidelines (permit no. [2006]398). Parasite and snail was approved by the Biological Studies Animal Care and Use Committee, Peoples Republic of China. All the methods were performed in accordance with the relevant guidelines and regulations of China.
Parasites, animals, protocols, sequences, and phylogenesis
Recombinant DNA procedures were performed by protocols approved by the Johns Hopkins University with National Institutes of Health (NIH) guidance. Cercariae were removed from O. hupensis snails that were artificially infected with S. japonicum. Each BALB/c mouse was percutaneously infected with 30 S. japonicum cercariae through shaved abdominal skin. Adult S. japonicum worms were later harvested by portal perfusion of infected mice at 35 d post-infection. Eggs were collected from dissected livers of infected mice, and then hatched into miracidia. O. hupensis snails were cultivated under standard protocols in the Jiangsu Parasitic Disease Institute, exposed to S. japonicum miracidia for infection, and then harvested for RNA isolation and other molecular biology experiments.
The mRNA sequence of SjAQP determined in this study was submitted to the National Center for Biotechnology Information (NCBI) (GenBank accession no. KR709301.1). Detailed methods of phylogenetic analysis, RNA isolation, reverse transcription (RT), as well as quantitative polymerase chain reaction (qPCR) with the delta-delta Ct analysis were described in a previous paper21. Briefly, SYBR Green qPCR master mix (Applied Biosystems, Foster City, CA, USA) was used. Each 25-μl reaction was repeated in triplicate. The optimized qPCR program was one cycle at 50 °C for 2 min, followed by 1 cycle of 95 °C for 2 min, followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by a final 10-min elongation step at 72 °C. Primer concentrations were 200 nM. Specific primers designed for SjAQP cloning, qPCR, and quality controls are listed in Table 1. The NADH gene is one of the most stably expressed housekeeping genes under different developmental stages22; thus, it was chosen as the control for qPCR normalizations.
The sequence of the primers for cloning AQP in our research was designed based on the published mRNA sequence (GenBank: AY813118.1)23. AQP sequences and GenBank accession numbers used in our phylogenetic analysis are: from Homo sapiens and Bos taurus hosts (HsAQP1, NP_001171989.1; BtAQP1, NP_001073262.1); the liver fluke Opisthorchis viverrini (OvAQP1, KF697690; OvAQP2 KF697691); Schistosoma mansoni (SmAQP ACI31185.1); the Chinese liver fluke Clonorchis sinensis (CsAQP3, GAA31414; CsAQP9, GAA55320); the rodent malaria-causing agent Plasmodium berghei (PbAQP #XM_671432); the malignant human malaria pathogen Plasmodium falciparum (PfAQP #AJ413249); and Trypanosome brucei (TbAQP1, 2, and 3, AJ697889, AJ697890, and AJ697891, respectively). A multiple sequence alignment was performed with ClustalW. The phylogenetic tree is presented using pairwise scores, which are simply the number of identities between two sequences, divided by the length of the alignment, and they are represented as percentages. A neighbor-joining tree was created by ClustalW, downloaded, and presented by TreeView 0.5.0 software. According to the software provider, the unit of the phylogenetic tree represents 0.1 amino acid substitutions.
In vitro complimentary RNA (cRNA) transcription, Xenopus oocyte injection, and osmotic swelling assays
Plasmid was constructed by ligating the SjAQP fragment between the BglII sites of the pXβG-ev1 vector. cRNA transcription, oocyte preparation, microinjection, an osmotic swelling assay for water permeability measurement, and inhibition assays were described previously24. Glycerol and urea permeabilities were measured with previously described methods16. Briefly, cRNA of SjAQP, without or with a carboxyl-terminal myc-tag, was in vitro transcribed using the linearized pXβG-SjAQP plasmid as the template. The size and quality of the cRNA product were confirmed by denaturing gel electrophoresis. For protein expression in Xenopus laevis oocytes, 5 ng (in 69 nL) of cRNA were injected into each oocyte. Control oocytes were injected with the same volume of nuclease-free water. After growing in modified Barth’s solution for 3 d, oocytes were tested in osmotic swelling assays, and the coefficient of osmotic water permeability (Pf) and solute permeability (Ps) were determined as previously described16,24. Briefly, the relative volume (V/V0) was calculated from the area at the initial time (A0) and after a time interval (At) as follows: V/V0 = (At/A0)3/2. Pf was determined from the initial slope of the time course [d(V/V0)/dt], the initial oocyte volume (V0 = 9 × 10−4 cm3), the initial oocyte surface area (S = 0.045 cm2), and the molar volume of water (Vw = 18 cm3/mol) as follows: Pf = [Osmtotal × Vo × d(V/Vo)/dt]/[S × Vw × (Osm_in − Osm_out)]. Non-isotopic solute permeabilities (Ps) were measured by placing oocytes in 200 mOsm modified Barth’s solution containing 100 mOsm of solute, which caused water influx and oocyte swelling. Ps was calculated from the oocyte surface area (S = 0.045 cm2), the initial oocyte volume (Vo = 9 × 10−4 cm3), the initial slope of the relative volume increase d(V/Vo)/dt, the total osmolality of the system (Osmtotal = 200 mOsm), and the osmotic solute gradient (Osm_out − Osm_in) as follows: Ps = [Osmtotal × Vo × d(V/Vo)/dt]/[S × (Osm_out − Osm_in)]. At least six individual oocytes were measured in each treatment, and statistical significance was determined by a Student’s t-test.
In the inhibition assays, oocytes were pre-incubated with inhibitors for 5 min at room temperature at the indicated final concentrations, and Pf and Ps were determined. The proper concentration of inhibitor ions tested in this study was determined by previous research on other AQPs by other groups21,25,26,27,28.
Results
SjAQP cDNA and its deduced protein sequence
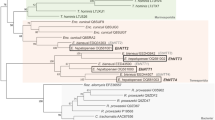
As shown in Fig. 1A, we determined the full-length SjAQP cDNA sequence of a Jiangsu S. japonicum isolate. This mRNA sequence has been submitted to the NCBI under GenBank accession no. KR709301.1. The deduced protein sequence shares 59.5% identity with an SmAQP (Fig. 1A). Typical AQPs contain two canonical Asn-Pro-Ala (NPA) motifs that line the pore region to restrict proton conductance. Interestingly, in SjAQP, the first motif is Asn-Pro-Gly (NPG) rather than NPA. Similarly, the aquaglyceroporin SmAQP also carries an amino acid exchange in the first loop, which replaces the conventional NPA motif with Asn-Pro-Ser (Fig. 1A, underlined). SjAQP shares the highest sequence homology with homologs from O. viverrini (OvAQP2), C. sinensis (CsAQP9), and S. mansoni (SmAQP). The amino acid sequence identities are 62.4, 62.1, and 59.5%, respectively, as shown in the phylogenetic tree in Fig. 1B. SjAQP also shares 20.7 and 38.0% amino acid sequence identities with AQPs from humans (HsAQP1) and bovines (BtAQP1), respectively.
Deduced primary protein sequence and phylogenetic analysis of SjAQP.
(A) Deduced amino acid sequence of SjAQP and alignment with its homolog SmAQP from Schistosoma mansoni. Asterisks indicate fully conserved residues; colons indicate strongly conserved similar residues with scores >0.5 in the Gonnet PAM 250 matrix; and periods indicate weakly similar residues with scores ≤0.5 in the matrix. The two highly conserved loops of the AQP family are underlined. (B) Phylogenetic analysis of SjAQP and characterized homologs from Homo sapiens (HsAQP1), Bos taurus (BtAQP1), Plasmodium berghei (PbAQP), Plasmodium falciparum (PfAQP), Opisthorchis viverrini (OvAQP), Clonorchis sinensis (CsAQP), Toxoplasma gondii (TgAQP), and Trypanosome brucei (TbAQP). Unit, 0.1 amino acid substitutions per site.
Functional characterization of SjAQP in Xenopus oocytes
cRNA of myc-tagged SjAQP was transcribed in vitro. The size and quality of the cRNA were confirmed, and then this cRNA was injected into X. laevis oocytes to express the SjAQP protein. Osmotic swelling assays showed that the Pf in SjAQP-expressing oocytes was 21.9-fold greater than that of the controls. Water permeation through SjAQP was significantly reduced to only 31% of its natural level by incubation with 1.0 mM HgCl2, a typical AQP inhibitor, while control oocytes were not significantly affected by HgCl2 (Fig. 2A). We also found that certain non-mercurial blockers significantly inhibited the water-permeating activity of SjAQP. After the addition of 3.0 mM tetraethylammonium, 1.0 mM copper, or 1.0 mM zinc ions, SjAQP-expressing oocytes only exhibited 42, 37, or 39%, respectively, of their full activity (Fig. 2B). More interestingly, oocytes expressing SjAQP exhibited glycerol and urea Ps values that were 29.8- and 21.2-fold, respectively, greater than those in control oocytes (Fig. 3A,B); thus, SjAQP is a functional aquaglyceroporin subfamily member.
Water-permeating activity of SjAQP.
(A) SjAQP-expressing oocytes significantly facilitate water movement across cell membranes, and the permeation is significantly inhibited by 1.0 mM HgCl2. Black and white bars represent permeation coefficients of control or SjAQP-expressing oocytes, respectively. (B) Water permeation by SjAQP-expressing oocytes is significantly inhibited by 3.0 mM tetraethylammonium, 1.0 mM CuSO4, and 1.0 mM ZnCl2. The x-axis is the coefficient of osmotic water permeability, Pf, with the unit 10−4 cm/s. Data are represented as means ± SDs. *p ≤ 0.05, **p ≤ 0.01 by a Student’s t-test.
Solute-permeating activities of SjAQP.
(A) SjAQP-expressing oocytes significantly facilitate glycerol movement across oocyte membranes compared with control oocytes (B) SjAQP-expressing oocytes significantly facilitate urea movement across cell membranes compared with control oocytes. The x-axis is the coefficient of solute permeability, Ps, with the unit 10−6 cm/s. Data are represented as means ± SDs. **p ≤ 0.01 by a Student’s t-test.
Expression of SjAQP during the S. japonicum life cycle
Using quantitative RT-PCR with total RNA extracted from S. japonicum at different developmental stages, we found that SjAQP mRNA was constitutively present in the egg, miracidia, infected snail, cercaria, and adult stages. Notably, the SjAQP expression level was highest (approximately four times higher than that of the other stages) in the cercaria stage (Fig. 4).
Expression profiles of SjAQP in different stages of the parasite life cycle.
Reverse transcription-quantitative polymerase chain reaction revealed the expression of SjAQP in S. japonicum developmental-specific stages – eggs, miracidia, cercariae, and adults. cDNA levels were normalized using the NADH housekeeping gene as an internal control. neg_snail, naive snails as the negative control; i_snail, S. japonicum infected snails.
Discussion
Schistosoma spp. undergo tremendous osmoregulatory stresses as they develop from one life stage to another in different environments29. The environment of fluke parasites alternates from eggs in the feces of humans and livestock to miracidia and cercariae in fresh water and snails and, finally, to warm-blooded mammalian hosts. It is critical for the parasite to survive dramatic osmolality changes from feces to freshwater snails, and later from snails to hosts. Additionally, blood-sucking parasites take in a huge amount of blood relative to their bodyweights. For example, S. mansoni female adults ingested 330,000 erythrocytes per hour, and take in a total fluid equivalent of 4.4 body volumes per day30. To survive the extreme osmolality changes between life stages, or to concentrate nutrients and exclude excess water, parasites must develop an effective system to permeate osmolytes and water.
AQP channels are widely expressed in organisms, and they confer unique trans-cellular movements of water and certain small solutes9. AQPs in S. mansoni and two liver flukes, O. viverrini and C. sinensis, play important roles in parasite physiology and water equilibrium31,32,33,34. Recently, using transcriptomic and proteomic analyses, we found that there is another annotated SjAQP, “MIP” (GenBank accession no. AAW24850.1)23. It has 63.0% amino acid sequence identity to our SjAQP; however, there was no functional study of an SjAQP until we reported the results of SjAQP-expressing oocytes in this study. Further investigations are needed to confirm that MIP is a functional AQP.
SjAQP shares slightly higher sequence similarity with orthologs in liver flukes from different genera, O. viverrini and C. sinensis, compared with S. mansoni from the same genus (Fig. 1B). S. mansoni mainly resides in South America, the Caribbean, Africa, and the Middle East35,36, while O. viverrini and C. sinensis are both common fish-borne liver flukes in Asia, including China37; thus, there may be a geographical influence on the molecular evolution of parasite AQPs.
In the oocyte-swelling assay, we observed significantly faster water or solute movements in SjAQP-expressing oocytes, which implied that SjAQP was well expressed and performed its functions. Generation of a specific polyclonal antibody against SjAQP is ongoing, and additional investigations will be performed in the future.
We identified several inhibitors that specifically reduce water permeation through SjAQP (Fig. 2). They have much lower cell toxicities compared with mercurial chloride and, thus, have great potential as new SjAQP antagonists.
Distinct from water-selective orthologs from O. viverrini33, SjAQP shows permeability to glycerol and urea in addition to water (Fig. 3). During S. japonicum development stages, water, glycerol, and urea are all fundamental for parasite physiology. Water accounts for up to 80% of the living organism bodyweight38. Glycerol is a building block of membrane synthesis, and it also enters metabolic pathways, such as glycolysis or gluconeogenesis pathways, to provide energy for growth39,40. The elevated glycerol level in mice following P. berghei infection proves the high demand for glycerol during parasite proliferation41. Urea is a metabolic waste product that needs to be excreted to maintain normal physiology21,42. Therefore, as an aquaglyceroporin, SjAQP may be essential for parasite physiology, and it may serve as a novel target to block parasite invasion.
We found an interesting expression pattern of SjAQP, which is constitutive throughout the S. japonicum life cycle, with a peak during the cercaria stage (Fig. 4). This differs from SmAQP, which shows peak expression in adults32 S. japonicum infection occurs when cercariae are released by snails into fresh water, and then cercariae penetrate human skin to achieve invasion43. Our finding of peak SjAQP expression in cercariae suggests that SjAQP probably protects parasites from osmolality changes when moving from fresh water (zero osmolality) to hosts and vectors (physiological osmolalities) and vice versa. We would expect that if SjAQP expression was reduced, or if the SjAQP gene was deleted or deactivated, the S. japonicum parasite would become significantly sensitive to osmolality changes. A similar trend has been observed in O. viverrini, in which suppression of two AQPs disabled water influx into parasites under hypoosmotic conditions33.
S. japonicum is currently endemic in the low reaches of the Yangtze River in China, and it causes major health problem8. Current research focuses on immune genes. However, channel-, transporter-, or metabolism-related genes also contribute to parasite proliferation. For instance, deletion of PbAQP reduces the virulence of a malaria parasite40,44. A reduction in the expression of a trehalose transporter leads to decreased P. falciparum parasite intensity in the vector midgut45. The disruption of P. falciparum glycerol kinase results in deficient parasite growth46. HsAQP8 facilitates hydrogen peroxide transport and mitigates oxidative stress during Plasmodium infection in red blood cells47. Therefore, SjAQP may be important for parasite invasion, and our study will fill knowledge gaps regarding pathogen-vector interactions during the S. japonicum transmission cycle.
Conclusions
Taken together, we have identified SjAQP as the first aquaglyceroporin from S. japonicum that functions in vitro and the results suggest its important roles in parasite water and osmolyte equilibria.
Additional Information
How to cite this article: Huang, Y. et al. Cloning and in vitro characterization of a Schistosoma japonicum aquaglyceroporin that functions in osmoregulation. Sci. Rep. 6, 35030; doi: 10.1038/srep35030 (2016).
References
Mwai, J., Njenga, S. & Barasa, M. Knowledge, attitude and practices in relation to prevention and control of schistosomiasis infection in Mwea Kirinyaga county, Kenya. BMC public health 16, 819 (2016).
Wangchuk, P., Giacomin, P. R., Pearson, M. S., Smout, M. J. & Loukas, A. Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms. Scientific reports 6, 32101 (2016).
Yin, M. et al. Co-dispersal of the blood fluke Schistosoma japonicum and Homo sapiens in the Neolithic Age. Scientific reports 5, 18058 (2015).
Rudge, J. W. et al. Population genetics of Schistosoma japonicum within the Philippines suggest high levels of transmission between humans and dogs. PLoS Negl Trop Dis 2, e340 (2008).
Huang, Y. et al. Proteomic patterns as biomarkers for the early detection of schistosomiasis japonica in a rabbit model. International Journal of Mass Spectrometry 299, 191–195 (2011).
Huang, Y. et al. New detection method in experimental mice for schistosomiasis: ClinProTool and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Parasitology research (2016).
Ross, A. G. et al. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev 14, 270–295 (2001).
Li, Y. S. et al. Large water management projects and schistosomiasis control, Dongting Lake region, China. Emerg Infect Dis 13, 973–979 (2007).
King, L. S., Kozono, D. & Agre, P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5, 687–698 (2004).
Agre, P. & Kozono, D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS letters 555, 72–78 (2003).
Liu, K., Tsujimoto, H., Huang, Y., Rasgon, J. L. & Agre, P. Aquaglyceroporin function in the malaria mosquito Anopheles gambiae. Biology of the cell/under the auspices of the European Cell Biology Organization (2016).
Carbrey, J. M. & Agre, P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol 3–28 (2009).
Kayingo, G., Sirotkin, V., Hohmann, S. & Prior, B. A. Accumulation and release of the osmolyte glycerol is independent of the putative MIP channel Spac977.17p in Schizosaccharomyces pombe. Antonie Van Leeuwenhoek 85, 85–92 (2004).
Winkler, A. et al. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot Cell 1, 163–173 (2002).
North, M. J. Cold-induced increase of glycerol kinase activity in Neurospora crassa: rapid inactivation of the enzyme in vivo. J Bacteriol 120, 741–747 (1974).
Carbrey, J. M. et al. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proceedings of the National Academy of Sciences of the United States of America 100, 2945–2950 (2003).
Hohmann, S. Osmotic adaptation in yeast--control of the yeast osmolyte system. Int Rev Cytol 215, 149–187 (2002).
Diamant, S., Eliahu, N., Rosenthal, D. & Goloubinoff, P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem 276, 39586–39591 (2001).
Meng, F. G. et al. Osmophobic effect of glycerol on irreversible thermal denaturation of rabbit creatine kinase. Biophys J 87, 2247–2254 (2004).
Gryseels, B., Polman, K., Clerinx, J. & Kestens, L. Human schistosomiasis. Lancet 368, 1106–1118 (2006).
Liu, K., Tsujimoto, H., Cha, S. J., Agre, P. & Rasgon, J. L. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proceedings of the National Academy of Sciences of the United States of America 108, 6062–6066 (2011).
Yang, C., Pan, H., Liu, Y. & Zhou, X. Selection of reference genes for expression analysis using quantitative real-time PCR in the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera, Aphidiae). PLoS One 9, e110454 (2014).
Liu, F. et al. New perspectives on host-parasite interplay by comparative transcriptomic and proteomic analyses of Schistosoma japonicum. PLoS Pathog 2, e29 (2006).
Liu, K. et al. Conversion of aquaporin 6 from an anion channel to a water-selective channel by a single amino acid substitution. Proceedings of the National Academy of Sciences of the United States of America 102, 2192–2197 (2005).
MacIver, B. et al. Expression and functional characterization of four aquaporin water channels from the European eel (Anguilla anguilla). J Exp Biol 212, 2856–2863 (2009).
Lazowski, K. W., Li, J., Delporte, C. & Baum, B. J. Evidence for the presence of a Hg-inhibitable water-permeability pathway and aquaporin 1 in A5 salivary epithelial cells. J Cell Physiol 164, 613–619 (1995).
Mulders, S. M. et al. Importance of the mercury-sensitive cysteine on function and routing of AQP1 and AQP2 in oocytes. Am J Physiol 273, F451–F456 (1997).
Nemeth-Cahalan, K. L., Kalman, K., Froger, A. & Hall, J. E. Zinc modulation of water permeability reveals that aquaporin 0 functions as a cooperative tetramer. J Gen Physiol 130, 457–464 (2007).
Maroulis, S. L., Schofield, P. J. & Edwards, M. R. Osmoregulation in the parasitic protozoan Tritrichomonas foetus. Appl Environ Microbiol 69, 4527–4533 (2003).
Skelly, P. J., Da’dara, A. A., Li, X. H., Castro-Borges, W. & Wilson, R. A. Schistosome feeding and regurgitation. PLoS Pathog 10, e1004246 (2014).
Da’dara, A., Krautz-Peterson, G., Faghiri, Z. & Skelly, P. J. Metabolite movement across the schistosome surface. J Helminthol 86, 141–147 (2012).
Faghiri, Z. & Skelly, P. J. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J 23, 2780–2789 (2009).
Thanasuwan, S. et al. Suppression of aquaporin, a mediator of water channel control in the carcinogenic liver fluke, Opisthorchis viverrini. Parasit Vectors 7, 224 (2014).
Geadkaew, A. et al. Bi-functionality of Opisthorchis viverrini aquaporins. Biochimie 108, 149–159 (2015).
Wangchuk, P. et al. Compounds Derived from the Bhutanese Daisy, Ajania nubigena, Demonstrate Dual Anthelmintic Activity against Schistosoma mansoni and Trichuris muris. PLoS Negl Trop Dis 10, e0004908 (2016).
Lu, Z. et al. Schistosome sex matters: a deep view into gonad-specific and pairing-dependent transcriptomes reveals a complex gender interplay. Scientific reports 6, 31150 (2016).
Petney, T. N., Andrews, R. H., Saijuntha, W., Wenz-Mucke, A. & Sithithaworn, P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int J Parasitol 43, 1031–1046 (2013).
Agre, P. et al. Aquaporin water channels–from atomic structure to clinical medicine. J Physiol 542, 3–16 (2002).
da Silva, G. P., Mack, M. & Contiero, J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27, 30–39 (2009).
Promeneur, D. et al. Aquaglyceroporin PbAQP during intraerythrocytic development of the malaria parasite Plasmodium berghei. Proceedings of the National Academy of Sciences of the United States of America 104, 2211–2216 (2007).
Basant, A., Rege, M., Sharma, S. & Sonawat, H. M. Alterations in urine, serum and brain metabolomic profiles exhibit sexual dimorphism during malaria disease progression. Malar J 9, 110 (2010).
McDonald, M. D., Gilmour, K. M. & Walsh, P. J. New insights into the mechanisms controlling urea excretion in fish gills. Respir Physiol Neurobiol 184, 241–248 (2012).
Aguiar, P. H. et al. Functional complementation of a yeast knockout strain by Schistosoma mansoni Rho1 GTPase in the presence of caffeine, an agent that affects mutants defective in the protein kinase C signal transduction pathway. Mem Inst Oswaldo Cruz 101 Suppl 1, 323–326 (2006).
Liu, Y. et al. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proceedings of the National Academy of Sciences of the United States of America 104, 12560–12564 (2007).
Liu, K., Dong, Y., Huang, Y., Rasgon, J. L. & Agre, P. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proceedings of the National Academy of Sciences of the United States of America 110, 17504–17509 (2013).
Naidoo, K. & Coetzer, T. L. Reduced glycerol incorporation into phospholipids contributes to impaired intra-erythrocytic growth of glycerol kinase knockout Plasmodium falciparum parasites. Biochim Biophys Acta 1830, 5326–5334 (2013).
Almasalmeh, A., Krenc, D., Wu, B. & Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J 281, 647–656 (2014).
Acknowledgements
We are grateful to the Gene Array Core Facilities at the Johns Hopkins Malaria Research Institute (JHMRI) for help with the qPCRs. We also thank the animal facility at the Jiangsu Parasitic Diseases Institute for aiding in the mouse experiment. This study was supported by grants R01 HL48268 and U19 AI089680 from the NIH, two pilot grants from the JHMRI and the Bloomberg Philanthropies to P Agre and K Liu, and grants to Y Huang, including those from the Natural Science Foundation of China (no. 31201893, no. 81673673), the Natural Science Foundation of Jiangsu Province (no. BK2011164), Jiangsu Province’s Key Medical Center (no. 201108), Open project of Jiangsu branch of CACMS (H1604), the Jiangsu Health Science Project (nos. X201110, X201416 and X201505), Project of Preventive Medicine Association of Jiangsu Province(grant No. Y2015071), the Jiangsu Health International Exchange Program, the Open Project of Key Laboratory of Jiangsu Preventive Veterinary Medicine (no. K13045), the Open Project of Jiangsu Provincial Key Laboratory on Biological Pharmacy of Veterinary Medicine (no. GCZXKF1404), and Jiangsu Provincial Science and Technology Project of Traditional Chinese Medicine (Z2011189). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.H. and K.C.L. conceived and designed the experiments; Y.H., W.L., K.C.L., C.X., Y.Y., H.Y. and P.C. performed the experiments; K.C.L. and Y.H. analyzed the data; Y.H. and K.C.L. wrote the paper. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, Y., Li, W., Lu, W. et al. Cloning and in vitro characterization of a Schistosoma japonicum aquaglyceroporin that functions in osmoregulation. Sci Rep 6, 35030 (2016). https://doi.org/10.1038/srep35030
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35030
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.