Abstract
The development of new drugs to disrupt malaria transmission requires the establishment of an in vivo model to address the biology of Plasmodium falciparum sexual stages (gametocytes). Herein we show that chemically immune-modulated NSG mice grafted with human erythrocytes support complete sexual development of P. falciparum parasites and generate high gametocytemia. Immunohistochemistry and RT-qPCR analyses indicate an enrichment of immature gametocytes in the bone marrow and the spleen, suggesting a sequestration mechanism reminiscent to that observed in humans. Upon primaquine treatment, elimination of gametocytes from peripheral blood and from sequestration sites was observed, providing a proof of concept that these mice can be used for testing drugs. Therefore, this model allows the investigation of P. falciparum sexual commitment, gametocyte interactions with the bone marrow and spleen and provides the missing link between current in vitro assays and Phase I trials in humans for testing new malaria gametocytidal drugs.
Similar content being viewed by others
Introduction
The eradication of malaria requires the development of new transmission-blocking drugs that have to be tested in animal models1. Plasmodium falciparum transmission from humans to mosquitoes is ensured by the parasite sexual stages called gametocytes. Immature gametocytes, from stage I to IV, develop in erythrocytes that sequester approximately 10 days in internal organs. Only the mature stages (stage V) are found in the peripheral blood where they are available for ingestion by mosquitoes. Recent molecular and histological studies of post-mortem specimens and clinical studies from infected individuals revealed that immature gametocytes are present in the extravascular compartment of the human bone marrow2,3,4. In addition, early post-mortem observations and a recent clinical case report on a splenectomized patient suggested that immature gametocytes might also sequester in the spleen5,6,7. However, it is still unclear whether the spleen is a site for maturation or clearance of immature gametocytes. The molecular mechanisms underlying the sequestration of gametocytes, followed by their release upon maturation into the circulation, remains one of the unanswered questions in the biology of malaria parasites that needs to be addressed by in vivo studies8.
One of the major challenges in studying P. falciparum gametocytes in laboratory animals is the parasite’s specificity for its human host and the important biological differences that exist between P. falciparum and rodent malaria gametocytes. Moreover, the use of non-human primates is limited due to economic and ethical considerations. In addition, P. falciparum infection with a gametocyte-producing parasite strain can only be achieved in splenectomized animals9,10, precluding the use of monkeys to address gametocytes’ interactions with the spleen. Consequently, mechanisms underlying P. falciparum gametocytogenesis have never been addressed in vivo. In addition, the lack of appropriate experimental models has restricted the evaluation of new transmission-blocking drugs to in vitro assays, which do not account for factors such as drug metabolism or gametocyte sequestration that might complicate intervention approaches. The generation of mouse strains with severe immunodeficiency and grafted with human red blood cells (hRBC) has allowed the establishment of humanized mouse models for P. falciparum erythrocyte infection that are currently being used to test anti-malarial drugs that target asexual parasites11,12. Further development of transgenic immune-deficient mice has made it possible to study the parasite pre-erythrocytic cycle and the complete life cycle was obtained in mice co-grafted with human hepatocytes and hRBC13,14. However, parasite sexual development in these humanized mouse models is still challenging due to the high turnover rate of infected hRBC, which is not optimal for the complete maturation of gametocytes. A few authors have reported presence of gametocytes in peripheral blood of P. falciparum-infected humanized mice, however gametocytes were only detected at very low levels (below 0.001%)13,15,16. High gametocytemia have only been reported when mouse splenic function was reduced by splenectomy17 or by using an immunomodulation protocol that led to gametocyte formation and maturation in the peritoneum11,18. Consequently, these models have not been developed further and to date there are no reports describing their usefulness to test the ability of compounds to eliminate P. falciparum gametocytes, nor their relevance as a model to address gametocyte interactions with the bone marrow and the spleen.
To overcome the limitations of these humanized models, we optimized an immunosuppression protocol to decrease the macrophage load in the spleen and liver18, thereby increasing the half-life of grafted hRBC and allowing gametocyte sequestration in internal organs. We applied this protocol to the severe immune-deficient mouse strain NOD SCID gamma c (NSG) and followed gametocyte development and distribution in different mice organs after P. falciparum infection.
Results and Discussion
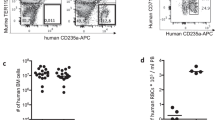
In previous reports we have shown that an immunomodulation protocol in immune-deficient mice allows the engraftment of hRBC and subsequent P. falciparum infection by intraperitoneal route18,19. In this protocol, the depletion of neutrophils and macrophages in the peritoneum, the spleen and the liver was induced by NIMP-R14 mAb and by clodronate encapsulated in liposomes (lip-clod), respectively. However, the high concentrations of NIMP-R14 mAb and lip-clod used in this protocol resulted in hRBC retention in the mouse peritoneum and consequently a high proportion of hRBC did not reach the peripheral blood, leading to gametocyte formation and maturation in the peritoneum11,18. This artificial model did not reflect P. falciparum gametocyte development in humans and precluded its use to address gametocyte interactions with host organs. To improve hRBC engraftment in peripheral blood, we optimized a new immunosuppression protocol in the severe immune-deficient mouse strain NOD SCID gamma c (NSG) (Fig. 1a). This mouse strain lacks mature T cells, B cells and Natural Killer cells, and is an excellent recipient mouse model for engraftment of human cells15,20. To overcome hRBC retention in mouse peritoneum, we tested three different combinations of lip-clod and mAb NIMP-R14 (Fig. 1b). Our results showed that the combination of 6.25 mg/kg of lip-clod, 1 mg/kg of NIMPR-14 and 0.75 mL of hRBC allowed the graft of human cells in peripheral blood without residual retention in peritoneum. This combination also reduced mouse mortality without inducing any major changes in other hematological parameters (Fig. 1b). Eighteen NSG mice were humanized with this immunomodulation protocol and infected with P. falciparum asexual parasites in four independent experiments (Fig. 1a). The parasitemia and gametocytemia were monitored daily in thin tail-blood smears from day 3 post infection (dpi) until sacrifice at different time points. Asexual parasites were detected in the peripheral circulation of the 18 mice and gametocytes at different stages of maturity (stage I to V) were observed in 15 mice (Fig. 2a), with peaks of gametocytemia ranging from 0.042% to 1% (Fig. 2b,e). During the course of gametocyte development, engraftment of hRBC in peripheral blood increased from 50% to 85% whereas other hematological parameters remained stable (Fig. 2c,d). Appearance of stage II gametocytes in the peripheral blood occurred on average 8 days after injection of asexual parasites and stage I gametocytes were still observed up to 35 days post-infection (Fig. 2e). These results indicate that parasite sexual commitment occurred in mice rather than in vitro prior to injection, thus supporting the use of this humanized mouse model to address in vivo the mechanisms underlying gametocytogenesis.
Establishment of a new protocol for Plasmodium falciparum sexual development in humanized mouse.
(a) Experimental procedure for engraftment of NSG mice with hRBC before infection with P. falciparum parasites. (b) Hematological parameters and mortality in 4 groups of mice (n = 4 mice per group) treated with different immunomodulation protocols. Protocol A: 33.3 mg/kg of lip-clod +10 mg/kg of mAb NIMP-R14, protocol B: 13.3 mg/kg of lip-clod +2 mg/kg of mAb NIMP-R14 and protocol C: 6.25 mg/kg of lip-clod +1 mg/kg of mAb NIMP-R14. Control mice received RPMI 1460 medium. Measurement of total RBC (mouse and human RBC, 106/mm3), platelets (PLT, 103/mm3), leucocytes (LCT, 103/mm3), hematocrit (HTC, %) and percentage of hRBC (hRBCpb) in mice peripheral blood samples were performed by using an automatic hematology analyzer at day 9 of the protocol. Mortality was recorded during 9 days and surviving mice (4 in Control group, 3 in group A, 2 in group B and 4 in group C) were sacrificed at day 9. Presence of hRBC in peritoneum (hRBCp) was determined after sacrifice. Complete coverage of this cavity with hRBC was noted as 2 points, partial coverage was noted as 1 point and absence of hRBC was noted as 0 point.
P. falciparum gametocytes develop in humanized NSG mice.
(a) Representative pictures of stage II, III, IV and V gametocytes in peripheral blood as observed in Giemsa-stained thin blood smears. Bars represent 2 μm. (b) Kinetics of gametocytemia for stage II, III, IV and V gametocytes in peripheral blood during 35 days of infection. Results represent the average gametocytemia of 15 mice from 4 independent infection experiments. Mice were sacrificed at different time points (3 mice at day 10, 5 mice at day 14, 5 mice at day 19 and 2 mice at day 35). (c,d) Follow up of hematological parameters in 8 mice during gametocyte development. Measurement of platelets, leucocytes, hematocrit and % of hRBC in mice peripheral blood samples were performed with an automatic hematology analyzer. (e) Kinetics of asexual stages and gametocytes in peripheral blood during the course of infection. Parasitemia, gametocytemia (% of parasites in total RBCs) and prepatent period (time between inoculation and detection of parasites on thin blood smears) for each gametocyte stage were determined by counting parasites on Giemsa-stained thin blood smears. Data represent the average of 15 mice from four independent infection experiments. dpi: days post-infection.
To evaluate whether this new immunosuppression protocol in NSG mice allows the parasite to sequester and mature in the spleen and bone marrow, we investigated the parasite distribution in different mouse organs. Immunohistochemical labeling was performed on samples of bone marrow, spleen, liver and lung of 5 mice with antibodies against the constitutively expressed heat shock protein 70 (HSP70), that detects all parasites, or the gametocyte specific antigen Pfg27 that detects stage I to stage V gametocytes21 (Fig. 3a). The proportion of gametocytes in the parasite population was 2 to 3-fold higher in the bone marrow and spleen than in the lung and liver (Fig. 3b). To further confirm this observation, we performed a real-time RT-qPCR analysis to measure gene expression of the gametocyte specific gene Pfs48/45 related to the asexual stage specific genes hypoxanthine phosphorybosyltransferase (hprt) and skeleton binding protein 1 (sbp1) in peripheral blood, bone marrow, spleen, liver and lung of 8 mice (Fig. 3c). The mean expression of Pf48/45 was about 3-fold higher in the bone marrow and spleen compared to peripheral blood, lung and liver. This indicates that gametocytes were more abundant in the bone marrow and spleen than in peripheral blood and other organs, in agreement with the immunohistochemical analysis. To determine the spatial distribution of parasites within the bone marrow, sternum bone marrow sections were stained with anti-laminin antibody to label the basement membrane of the host vasculature22 and hemozoin malaria pigments were localized relative to the laminin-positive microvasculature (Fig. 3d). In three mice with 54–65% gametocytes in their bone marrow, as determined by the ratio Pfg27/HSP70, we observed an average of 47% of parasites associated with laminin and 53% in the extravascular space (Fig. 3e). Although presence of hemozoin pigments is not specific for gametocytes, these observations suggest that a proportion of gametocytes were enriched in the extravascular compartment of bone marrow, as observed in humans4.
Gametocytes are enriched in the bone marrow and the spleen.
(a) Histological sections of sternum bone marrow, spleen, liver and lung were stained with antibodies directed against Pfg27 and HSP70 to detect gametocytes and all parasites, respectively. n = total number of HSP70- and Pfg27-positive parasites observed in 30 fields at 630X magnification for each tissue sample for 5 mice (a total of 1, 200 fields was analyzed). Bars represent 10 μm. (b) Proportion of Pfg27-positive gametocytes in the HSP70-positive parasite population in bone marrow (BM), spleen (SP), lung and liver. Results represent the average ratio of Pfg27-positive cells in Pfg27-stained sections to HSP70-positive cells in HSP70-stained sections observed in 30 fields. Stars represent significant differences in proportion (**P < 0.01, *P < 0.05). ns: not significant. (c) Quantitative analysis by real time RT-qPCR of gametocytes distribution in peripheral blood (PB), femur bone marrow (BM), spleen (SP), lung and liver. Relative copy numbers (RCN) of the gametocyte marker Pfs48/45 were calculated as the ratio between copy numbers of Pfs48/45 transcripts and the geometric mean of the ring upregulated gene sbp1 and the trophozoite upregulated gene hprt transcripts. Results represent the average quantification for 8 mice from two independent infection experiments. (d) Histological sections of bone marrow (sternum) were stained with antibodies directed against laminin to detect basement membrane of microvasculature (V). Hemozoin malaria pigment allows detection of parasites in intra- (green arrow) or extra- (yellow arrow) vascular space. Bars represent 10 μm. (e) Proportion of intra- and extra-vascular parasites observed in a population of 200 parasites on laminin-stained histological sections from three mice.
To address the distribution of each gametocyte stage in the bone marrow and spleen compared to peripheral blood, we performed an immunostaining analysis using an antibody against the gametocyte marker Pfg27 on smears of peripheral blood, bone marrow aspirate, and spleen extract (Fig. 4a). Results showed that only immature gametocytes from stage I to III were significantly enriched in the bone marrow and spleen, whereas mature gametocytes were more abundant in the peripheral blood (Fig. 4b–d). These results suggest that immature gametocytes sequester in the bone marrow and spleen of these mice and are released into the circulation upon maturation, although enrichment of gametocytes in the spleen may also reflect their retention in splenic slits before clearance by macrophages. In either case, our results are in accordance with observations made in humans2,4,7. However, the observation that one third of the immature gametocyte population is present in the peripheral circulation suggests that the sequestration mechanism occurring in this mouse model is incomplete. A possible explanation is that gametocyte sequestration in humans may be due to a combination of cytoadhesion and mechanical retention mechanisms, and the lack of human specific receptor(s) in NSG mice might only allow rigidity-mediated sequestration due to the important stiffness of immature gametocyte-infected erythrocytes23. The development of a long-term humanized mouse model with human bone marrow transplantation would allow to decipher the putative respective roles of cytoadhesion and mechanical retention in sequestration mechanisms. Altogether these exciting observations indicate that this humanized mouse model provides a unique opportunity to address in vivo the mechanisms underlying sequestration of immature gametocytes in human bone marrow and potentially in the spleen.
Immature gametocytes are enriched in the bone marrow and the spleen.
(a) The proportion of gametocytes in peripheral blood, femur bone marrow aspirate or spleen extract was analyzed by immunostaining and determined by calculating the percentage of Pfg27-positive parasites in the total parasite population detected by Hoechst staining. Different developmental stages were determined by analysis of gametocyte morphology on differential interference contrast images. Bars represent 2 μm. (b) Average proportion of gametocytes in the parasite population observed in peripheral blood (PB), bone marrow (BM) and spleen (SP). PB: 311 gametocytes observed in 12 mice, BM: 213 gametocytes observed in 12 mice, SP: 123 gametocytes observed in 6 mice. (c) Average proportion of asexual stages, immature gametocytes and mature gametocytes in the parasite population observed in PB, BM and SP. (d) Average proportion of each gametocyte stage in the parasite population observed in PB, BM and SP. (b–d) Results represent the average proportion for 12 mice (PB and BM) or 6 mice (SP) from four independent infection experiments. Stars represent significant differences in proportion (***P < 0.001, **P < 0.01, *P < 0.05). ns: not significant. Graphs were generated using GraphPad Prism software. Error bars represents Standard Error of the Mean (SEM).
We next explored the potential of the NSG mice model as a platform for testing drugs against P. falciparum transmission stages. Indeed, humanized mice have a crucial role for drug discovery in malaria, as they offer the opportunity to assess in vivo the activity of drugs. As a proof of concept, we studied the effect of primaquine, which is currently the only licensed drug that has demonstrated efficacy to eliminate P. falciparum gametocytes in humans24. A group of 4 mice were treated with primaquine (2 mg/kg; intraperitonealy) for 4 days, starting 1 to 4 days after appearance of P. falciparum gametocytes in peripheral blood (Fig. 5a). In the 4 mice, with gametocytemia ranging from 0.1% to 0.66% at day 0, gametocytes of all stages were totally eliminated from peripheral blood after 3 to 6 days of treatment. In contrast, in 10 untreated mice from 3 independent infection experiments, the average gametocytemia increased from 0.045% to 0.325% during the 9 days following appearance of gametocytes in peripheral blood. As expected, asexual stages were also eliminated from peripheral blood after 3 to 6 days, since primaquine treatment at 2 mg/kg represents a dose 1000-fold upper the IC50 of primaquine on asexual stages25,26 (Supplemental Figure S1). In addition, the efficacy of primaquine to eliminate gametocytes from sequestration sites was evaluated in Giemsa-stained smears of bone marrow aspirates and spleen extracts and compared to the average of gametocytemia in 10 untreated mice (Fig. 5b,c). Gametocytes were totally eliminated from both organs in 3 treated mice, whereas one mouse still harbored a significant gametocytemia, corresponding to 10% (in bone marrow) and 16% (in spleen) of the initial gametocytemia in peripheral blood at day 0 of primaquine treatment. These data show that the immunosuppression protocol used to engraft mice does not interfere with the assessment of gametocytidal treatments and provides the proof of concept that this model is suitable for testing new drugs against P. falciparum transmission stages. Importantly, this protocol can be used to address the effect of a drug on the persistence of gametocytes in their sequestration sites, which is crucial information that could not be provided by clinical trials in humans.
Humanized NSG mice provide a suitable model for testing malaria gametocytidal drugs.
(a) Kinetics of gametocytemia for stage II, III, IV and V gametocytes in peripheral blood in 10 untreated mice (Control mice) and in 4 primaquine-treated mice during 8 to 11 days following appearance of gametocytes in peripheral blood (blue star). Gametocytemia (% of gametocytes in total RBCs) for each gametocyte stage were determined by counting parasites on Giemsa-stained thin blood smears. Primaquine treatment (2 mg/kg) was started 1 to 4 days after appearance of gametocytes and was daily administered for 4 days (red arrows). Mice were sacrificed seven days after beginning of treatment (black arrow). (b) Gametocytemia in bone marrow after sacrifice as observed in Giemsa-stained smears of bone marrow aspirate. Control represents an average of gametocytemia for 10 mice from three independent infection experiments. (c) Gametocytemia in spleen after sacrifice as observed in Giemsa-stained smears of crushed spleen. Control represents the average gametocytemia of 6 mice from two independent infection experiments.
In summary, these humanized mice are a suitable model to investigate P. falciparum sexual commitment in vivo, gametocyte interactions with the bone marrow and the spleen, and they may facilitate the discovery of new gametocytidal drugs, which is urgently needed to achieve the goal of malaria eradication.
Methods
Ethics statements
This study was carried out in strict accordance with the guide for the care and use of laboratory animals from the Centre d’Expérimentation Fonctionnelle (CEF, La Pitié-Salpêtrière, Paris) and with the French and European regulations (2010/63/EU). The experimental protocols were approved by the Ministère de l′Education Nationale, de l’Enseignement Supérieur et de la Recherche (Authorization Number 01737.03).
Immunomodulation treatment and mouse infection
NSG mice aged 9–11 weeks (Charles River, US) were bred at the CEF under strict pathogen-free conditions. They were provided with UV light-exposed commercial food and autoclaved water ad libitum. Human red blood cells (hRBC) were obtained from donors without history of malaria (Etablissement Français du Sang Ile-de-France, Rungis). Before peritoneal injection into mice, hRBC were washed twice with RPMI-1640 medium (Gibco/BRL) at 900 g, 10 min at 25 °C. The depletion of tissue macrophages was induced by clodronate (Roche Diagnostics) encapsulated in liposomes (lip-clod) as described27. Neutrophils were depleted using the monoclonal antibody (mAb) NIMP-R14 produced by a hybridoma kindly provided by Dr M. Strath (National Institute for Medical Research, London, U.K.)28. To obtain the graft of hRBC and subsequent P. falciparum NF54 infection, each mouse received by intraperitoneal injection a dose of 1 mg/kg of mAb NIMP-R14 at day 0 and 6.25 mg/kg of lip-clod at day 1. At day 5 and day 7 each mouse received 1.5 mL of hRBC at 50% hematocrit in RPMI mixed with 1 mg/kg of mAb NIMP-R14 and 6.25 mg/kg of lip-clod. At day 9 each mouse received the same doses of immunomodulators in 1.5 mL of hRBC at 50% hematocrit containing 5.10e5 P. falciparum NF54 asexual parasites. Parasites used for infection were obtained from an in vitro culture maintained below 1.5% parasitemia to avoid in vitro induction of gametocytogenesis. After P. falciparum infection, mice were grafted with 1 to 1.5 mL of hRBC at 50% hematocrit containing 1 mg/kg of mAb NIMP-R14 and 6.25 mg/kg of lip-clod every 2–3 days. Hematological parameters (hematocrit, leucocytes, platelets, percentage of hRBC in peripheral blood) were followed up during the assay in blood samples taken from mouse tails and analyzed with an automatic hematology analyzer (Scil Vet abc Plus, Scil Animal Care). Mice exhibiting hematocrit up to 60% and percentage of hRBC higher than 70% only received the graft of hRBC once the hematocrit decreased to 50%. Presence of residual hRBC in the peritoneum was assessed after sacrifice of the mice.
RT-qPCR analysis
Peripheral blood samples and femur bone marrow aspirate samples were added to Trizol (Life technologies) and vortexed. Spleen, liver and lung sections were processed through grinding in Trizol. RNA was prepared using the PureLink RNA Mini kit (Life technologies) and treated using on-column DNase-Treatment with Pure Link DNase (Life technologies). Quantity and purity of RNA were assessed with Epoch spectrophotometer. Contamination with genomic parasite DNA was assessed by qPCR with primers for P. falciparum seryl-tRNA synthetase (PF07_0073) and positive samples were retreated with DNAse and retested for lack of genomic DNA. RNA (100–200 ng for peripheral blood and total RNA for other organs) was used for complementary DNA (cDNA) synthesis with the SuperScript III First-Strand Synthesis System (Life technologies). Positive cDNA synthesis was confirmed by qPCR targeting P. falciparum seryl-tRNA synhtetase. Primers for the gametocyte marker Pfs48/45 (PF13_0247)2, the trophozoite upregulated gene hprt (PF10_0121)29 and the ring upregulated gene sbp1 (PFE0065w)29 were used to detect transcripts from gametocytes, trophozoites and rings, respectively (Supplemental Table S1). Final reaction volumes of 20 uL included 4 uL of cDNA (3–30 ng/uL) and 10 uL of Power SYBRGreen Master Mix (Applied Biosystems). qPCRs were performed in a ABi Prism 7500 (Applied Biosystems) with 2 minutes at 50 °C, 10 minutes at 95 °C (1 cycle), 30 seconds at 95 °C, and 1 minute at 60 °C (40 cycles). Each sample was analyzed in triplicate together with a serial dilution of 3D7 gDNA for absolute quantification of transcript copy numbers. To normalize for the level of parasitemia and amount of cDNA loaded in each reaction, relative copy numbers (RCN) were calculated as the ratio between copy numbers of Pfs48/45 transcripts and the geometric mean of sbp1 and hprt transcripts.
Immunofluorescence assays
Thin smears of peripheral blood, femur bone marrow aspirate or spleen extract were air-dried and fixed for 10 minutes in methanol chilled at −20 °C. Samples were incubated in 2% bovine serum albumin in PBS for 1 h at room temperature to block non-specific binding. The preparations were then incubated overnight at 4 °C with a rabbit polyclonal anti-Pfg27 antibody30 diluted 1/1000 and with AlexaFluor 594-conjugated goat anti-rabbit antibody (Molecular Probes) for 1 hour. Nuclei were stained with Hoechst 33342. Samples were observed at 1000X magnification using a Leica DM 5000 B. At least 50 fields at 1000X magnification were analyzed for each sample.
Histology and immunohistochemistry
Samples were fixed in 10% neutral buffered formalin, embedded in paraffin and 4 μm-thick sections were cut and stained with standard Hematoxylin-Eosin staining. In order to detect parasites, immunohistochemistry analyses were carried out using a mouse monoclonal anti-Plasmodium HSP70 antibody31 at dilution 1/1000 and a rabbit polyclonal anti-Pfg27 antibody30 at 1/2000. Gametocyte enrichment in different organs was calculated as the ratio of Pfg27-positive cells in Pfg27-stained sections to HSP70-positive cells in HSP70-stained sections observed in 30 fields at 630X magnification for each tissue sample. To determine the location of parasites, indirect labeling of blood vessels was carried out using a rabbit anti-laminin polyclonal antibody (Sigma Aldrich, dilution 1:100) to detect basement membrane of microvasculature. Positive signal was revealed using Histofine Simple Stain MAX PO (mouse or rabbit depending on the primary antibody; Nichirei Biosciences Inc.) according to the manufacturer’s protocol. Color was developed with 3-Amino-9-EthylCarbazole (AEC chromogen; BD Pharmingen). Samples were observed at 630X magnification using a LEICA DM 400B.
Drug treatment
Primaquine (Sigma Aldrich) at 2 mg/kg in RPMI-1640 (Gibco, BRL) was administered daily by intraperitoneal injection during 4 days. Primaquine treatment was started 1 to 4 days after appearance of gametocytes in peripheral blood. For each mouse, gametocytemia was monitored in thin tail-blood smears collected daily until sacrifice at day 7 post drug treatment.
Statistical analysis
Statistical significance for differences in gametocyte proportions in different organs was established using Student’s t-test and Wilcoxon Mann-Whitney rank sum test.
Additional Information
How to cite this article: Duffier, Y. et al. A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci. Rep. 6, 35025; doi: 10.1038/srep35025 (2016).
References
Wells, T. N., Alonso, P. L. & Gutteridge, W. E. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov 8, 879–891, doi: 10.1038/nrd2972 (2009).
Aguilar, R. et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123, 959–966, doi: 10.1182/blood-2013-08-520767 (2014).
Farfour, E., Charlotte, F., Settegrana, C., Miyara, M. & Buffet, P. The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malaria journal 11, 285, doi: 10.1186/1475-2875-11-285 (2012).
Joice, R. et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med 6, 244re245, doi: 10.1126/scitranslmed.3008882 (2014).
Bachmann, A. et al. Absence of erythrocyte sequestration and lack of multicopy gene family expression in Plasmodium falciparum from a splenectomized malaria patient. PLoS One 4, e7459, doi: 10.1371/journal.pone.0007459 (2009).
Bastianelli, G. & Bignami, A. Studi Sulla Infezione Malarica. Bullettino R Accademia Med 20, 151–220 (1893).
Thomson, J. G. & Robertson, A. The structure and development of Plasmodium falciparum gametocytes in the internal organs and peripheral circulation. Trans R Soc Trop Med Hyg 29, 31–40 (1935).
Tiburcio, M., Sauerwein, R., Lavazec, C. & Alano, P. Erythrocyte remodeling by Plasmodium falciparum gametocytes in the human host interplay. Trends Parasitol 31, 270–278, doi: 10.1016/j.pt.2015.02.006 (2015).
Herrera, S., Perlaza, B. L., Bonelo, A. & Arevalo-Herrera, M. Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int J Parasitol 32, 1625–1635 (2002).
Collins, W. E. et al. The Santa Lucia strain of Plasmodium falciparum in Aotus monkeys. Am J Trop Med Hyg 80, 536–540 (2009).
Moreno, A., Perignon, J. L., Morosan, S., Mazier, D. & Benito, A. Plasmodium falciparum-infected mice: more than a tour de force. Trends Parasitol 23, 254–259, doi: 10.1016/j.pt.2007.04.004 (2007).
Vaughan, A. M., Kappe, S. H., Ploss, A. & Mikolajczak, S. A. Development of humanized mouse models to study human malaria parasite infection. Future Microbiol 7, 657–665, doi: 10.2217/fmb.12.27 (2012).
Soulard, V. et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun 6, 7690, doi: 10.1038/ncomms8690 (2015).
Vaughan, A. M. et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest 122, 3618–3628, doi: 10.1172/JCI62684 (2012).
Arnold, L. et al. Further improvements of the P. falciparum humanized mouse model. PLoS One 6, e18045, doi: 10.1371/journal.pone.0018045 (2011).
Wijayalath, W. et al. Humanized HLA-DR4.RagKO.IL2RgammacKO.NOD (DRAG) mice sustain the complex vertebrate life cycle of Plasmodium falciparum malaria. Malar J 13, 386, doi: 10.1186/1475-2875-13-386 (2014).
Moore, J. M., Kumar, N., Shultz, L. D. & Rajan, T. V. Maintenance of the human malarial parasite, Plasmodium falciparum, in scid mice and transmission of gametocytes to mosquitoes. J Exp Med 181, 2265–2270 (1995).
Moreno, A. et al. The course of infections and pathology in immunomodulated NOD/LtSz-SCID mice inoculated with Plasmodium falciparum laboratory lines and clinical isolates. Int J Parasitol 36, 361–369, doi: 10.1016/j.ijpara.2005.10.012 (2006).
Moreno Sabater, A. et al. Experimental infection of immunomodulated NOD/LtSz-SCID mice as a new model for Plasmodium falciparum erythrocytic stages. Parasitol Res 95, 97–105, doi: 10.1007/s00436-004-1249-7 (2005).
Ito, M. et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182, doi: 10.1182/blood-2001-12-0207 (2002).
Alano, P., Premawansa, S., Bruce, M. C. & Carter, R. A stage specific gene expressed at the onset of gametocytogenesis in Plasmodium falciparum. Mol Biochem Parasitol 46, 81–88 (1991).
Yousif, L. F., Di Russo, J. & Sorokin, L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr 7, 101–110, doi: 10.4161/cam.22680 (2013).
Tiburcio, M. et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood 119, e172–180, doi: 10.1182/blood-2012-03-414557 (2012).
White, N. J. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 13, 175–181, doi: 10.1016/S1473-3099(12)70198-6 (2013).
Adjalley, S. H. et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proceedings of the National Academy of Sciences of the United States of America 108, E1214–1223, doi: 10.1073/pnas.1112037108 (2011).
Cabrera, M. & Cui, L. In Vitro Activities of Primaquine-Schizonticide Combinations on Asexual Blood Stages and Gametocytes of Plasmodium falciparum. Antimicrobial agents and chemotherapy 59, 7650–7656, doi: 10.1128/AAC.01948-15 (2015).
Van Rooijen, N. & Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174, 83–93 (1994).
Tacchini-Cottier, F. et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 165, 2628–2636 (2000).
Rovira-Vallbona, E. et al. Transcription of var genes other than var2csa in Plasmodium falciparum parasites infecting Mozambican pregnant women. J Infect Dis 204, 27–35, doi: 10.1093/infdis/jir217 (2011).
Olivieri, A. et al. The Plasmodium falciparum protein Pfg27 is dispensable for gametocyte and gamete production, but contributes to cell integrity during gametocytogenesis. Mol Microbiol 73, 180–193, doi: 10.1111/j.1365-2958.2009.06762.x (2009).
Renia, L. et al. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur J Immunol 20, 1445–1449, doi: 10.1002/eji.1830200706 (1990).
Acknowledgements
CL, YD, FD, and AL acknowledge the financial support from the Fonds Inkermann and the Cnrs (ATIP-Avenir grant). We thank Pietro Alano for providing the anti-Pfg27 antibody. We thank Eliane Jibouayo for technical support. We are grateful to Geneviève Milon for critical reading of the manuscript. We thank Patricia Baldacci for English editing.
Author information
Authors and Affiliations
Contributions
Y.D., A.L., P.C., F.D., C.L. and A.M.S. performed experiments; Y.D., A.L., P.C., A.M., C.L. and A.M.S. analysed data; F.D. performed statistical analysis; G.J., L.F., D.M. and A.M. contributed new reagents or analytical tools; C.L. and A.M.S. designed the research; C.L. and A.M.S. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Duffier, Y., Lorthiois, A., Cisteró, P. et al. A humanized mouse model for sequestration of Plasmodium falciparum sexual stages and in vivo evaluation of gametocytidal drugs. Sci Rep 6, 35025 (2016). https://doi.org/10.1038/srep35025
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35025
This article is cited by
-
Plasmodium asexual growth and sexual development in the haematopoietic niche of the host
Nature Reviews Microbiology (2020)
-
A cryptic cycle in haematopoietic niches promotes initiation of malaria transmission and evasion of chemotherapy
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.