Abstract
Melanocortin-3 and 4 receptors (MC3R and MC4R) can regulate energy homeostasis, but their respective roles especially the functions of MC3R need more exploration. Here Mc3r and Mc4r single and double knockout (DKO) rats were generated using CRISPR-Cas9 system. Metabolic phenotypes were examined and data were compared systematically. Mc3r KO rats displayed hypophagia and decreased body weight, while Mc4r KO and DKO exhibited hyperphagia and increased body weight. All three mutants showed increased white adipose tissue mass and adipocyte size. Interestingly, although Mc3r KO did not show a significant elevation in lipids as seen in Mc4r KO, DKO displayed even higher lipid levels than Mc4r KO. DKO also showed more severe glucose intolerance and hyperglycaemia than Mc4r KO. These data demonstrated MC3R deficiency caused a reduction of food intake and body weight, whereas at the same time exhibited additive effects on top of MC4R deficiency on lipid and glucose metabolism. This is the first phenotypic analysis and systematic comparison of Mc3r KO, Mc4r KO and DKO rats on a homogenous genetic background. These mutant rats will be important in defining the complicated signalling pathways of MC3R and MC4R. Both Mc4r KO and DKO are good models for obesity and diabetes research.
Similar content being viewed by others
Introduction
The melanocortin system plays an important physiological role in energy homeostasis1. Melanocortin-3 and 4 receptors (MC3R and MC4R) are two neural melanocortin receptors that belong to the rhodopsin-like G protein-coupled receptor family. MC4R is expressed throughout the central nervous system (CNS)2, and mutations in the MC4R gene are the most common cause of monogenic obesity in human3,4,5,6. Many researchers reported that MC4R participates in regulating feeding behavior and energy expenditure7,8. In addition, MC4R is expressed in peripheral such as adipose tissue9. Recently it was recognized as the second most highly expressed G protein-coupled receptor in enteroendocrine L cells, which suggests that peripheral MC4R is important in specific physiological functions10. MC3R is also expressed in CNS especially in the arcuate nucleus, as well as some peripheral tissues such as heart and peritoneal macrophages11,12,13,14. Studies in human and rodents have confirmed MC3R expression in the kidney, where it participates in modulation of natriuresis13,15. Importantly, MC3R has been found to play a subtle role in regulating energy homeostasis12,16. There are also some reports about the influence of variant Mc3r alleles on human obesity17,18,19, especially the recent paper about replacing mouse locus with human obesity related MC3R double-mutant suggesting an important role of MC3R in human metabolism20.
Mc4r and Mc3r knockout (KO) mouse models have provided some evidence for their respective role in energy homeostasis. Mc4r KO mice exhibited obesity, hyperphagia, hyperglycaemia and hyperinsulinemia8,21, while Mc3r KO mice displayed almost the same body weight as WT control up to 26 weeks of age, but they did exhibit a mild obesity phenotype characterized by increased fat mass, reduced lean mass and reduced body length16. Although not much data is available about Mc4r/Mc3r double KO (DKO) mice, they were reported to be significantly more obese than Mc4r KO mice16. As genetic background can significantly influence the phenotype of obesity22,23, a true phenotypic comparison among Mc3r KO, Mc4r KO and DKO in the same congenic background would give more valuable information.
The laboratory rat is a valuable mammalian model organism for many human diseases and has advantages over the mouse such as in metabolic and pharmacological studies. The first functional knockout rat (Wistar/Crl background) for Mc4r (Mc4rK314X) was reported in 2011 as the result of an N-ethyl-N-nitrosourea-induced point mutation24, and exhibited increased body weight, food intake and white adipose mass, and altered substrate preference25. In 2013 we published the generation of Mc4r KO, and Mc4r/Mc3r DKO rat by CRISPR-Cas9 and observed a similar phenotype of the Mc4r KO rat in body weight, food intake, insulin and leptin level as compared to the N-ethyl-N-nitrosourea-induced Mc4r KO rat, although these observations were only based on founders with biallelic mutants26. Meanwhile, no report on phenotypic analysis of the Mc3r KO rat or Mc3r/Mc4r DKO rat was found. In this study, an investigation on Mc3r KO, Mc4r KO, and DKO rats was carried out and data were compared systematically regarding their metabolic phenotypes in a single genetic background, in order to provide an overall understanding of these two receptors in energy metabolism, and to evaluate the possibility of using these KO rats as suitable animal models for obesity or type 2 diabetes. Results showed that MC4R deficiency led to a dramatic alteration in energy homeostasis including obesity and hyperglycaemia, whereas MC3R deficiency showed hypophagia and reduced body weight. However, MC3R deficiency appeared to exert an additive effect on MC4R deficiency in several aspects including lipid profile and glucose metabolism which implies that different pathways mediated MC3R’s effect on different functions. Data from our extensive phenotypic comparison suggested that Mc4r KO and DKO might be good rat models for obesity or type 2 diabetes (T2D).
Results
Effect of MC3R and MC4R deficiency on growth curve, size and Lee’s index
Heritable total Mc3r and Mc4r knockout (KO) rats were generated using CRISPR-Cas9 system in Sprague Dawley (SD) rats26. DNA sequences of Mc3r or Mc4r genomic loci in mutant rats were verified as correct. The expression of Mc3r and Mc4r in liver, brain and visceral total white adipose tissue (WAT) of mutants reduced significantly as compared with WT (Supplementary Fig. S1). This indicates that premature stop codons decreased mutant mRNA stability, as it is well established that frameshift or nonsense mutations induce mRNA decay27. All Mc3r, Mc4r single KO and double KO rats appeared normal phenotypically except for changed body size. We monitored the body weight of male and female Mc3r KO and Mc4r KO rats from 4–18 weeks old, and DKO rats from postnatal day (PND) 45 to 85. It was found that Mc3r KO displayed a significantly reduced body weight as compared to WT littermates, beginning at PND 58 in males and PND 28 in females (Fig. 1a,b, n = 8, male p = 0.0006, female p < 0.0001, Supplementary Fig. S2). Meanwhile, Mc4r KO and DKO showed approximate same degree of increased body weight, with the only exception at time points near PND 50 (Fig. 1a,b).
Effect of MC4R and MC3R deficiency on body weight, body length and Lee’s index.
(a) Body weight and (b) Relative body weight were measured from postnatal day (PND) 45 to 85 for Mc3r KO, Mc4r KO, DKO and WT littermates (n = 8 except 6 for DKO, repeated measures analysis followed by Bonferroni post-tests). Nose-anus length (c,d) and Lee’s index (e,f) of Mc3r KO, Mc4r KO, and DKO rats vs. WT littermates (n = 8 except 4 for DKO) at 14 weeks of age (one-way ANOVA test). Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT control.
The body length of different groups of rats was measured at 14 weeks of age. As shown in Fig. 1c,d, Mc4r KO and DKO rats were significantly longer than WT littermates (n = 4–8, male and female p = 0.02 for Mc4r KO, male p = 0.02, female p = 0.04 for DKO). However, the average body length of Mc3r KO rats was significantly shorter (Fig. 1c,d, n = 8, male p = 0.002, female p = 0.0008), which was consistent with previous reports on mice. As an indicator of obesity, Lee’s index was calculated based on body weight and body length. No change was observed in Mc3r KO, while in Mc4r KO and DKO the same significant increase was revealed (Fig. 1e,f, n = 4–8, male p = 0.001, female p = 0.006 for Mc4r KO, male p = 0.01, female p = 0.005 for DKO). Thus, MC3R deficiency resulted in a shorter, lighter rat, while in Mc4r KO and DKO, increase in body weight was consistent with the increase in body length and Lee’s index.
Food and water intake in Mc3r, Mc4r KO and DKO rats
Next we proceeded to monitor food and water consumption in different groups. Consistent with their body weight changes, Mc3r KO rats displayed a decreased food intake especially in females, as well as a reduction in water intake that did not reach significant levels as compared to WT littermates. Conversely, Mc4r KO and DKO had an increased food intake as well as water intake as compared to WT rats (Fig. 2). Thus the Mc3r KO rat was hypophagic while Mc4r KO and DKO rats were both hyperphagic.
Food and water intake in different mutant rat models.
(a) Food intake in Mc3r KO, Mc4r KO and DKO vs. WT littermates. (b) Water intake in Mc3r KO, Mc4r KO and DKO vs. WT littermates. Data are shown as mean ± SEM (n = 6). *Difference between mutant and WT littermates. #Difference between Mc3r KO vs. Mc4r KO and DKO. &Difference between Mc4r KO and DKO. #,& and *p < 0.05; ##,&& and **p < 0.01; ###,&&& and ***p < 0.001 vs. WT control (one-way ANOVA test).
Characterization of serum lipid profile and adipose accumulation
Serum obtained from Mc4r KO and DKO rats appeared thick and milky white, obviously abnormal as compared to those of WT and Mc3r KO which were clear and yellowish (Fig. 3a). As expected, the lipid profile of Mc4r KO and DKO rats showed significantly increased total cholesterol (p = 0.01 for Mc4r KO, p < 0.0001 for DKO), triglyceride (p = 0.008 for Mc4r KO, p < 0.0001 for DKO) and free fatty acid (p = 0.007 for Mc4r KO, p = 0.0005 for DKO) (Fig. 3b–d, n = 10–16). Not much change was found in low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c), except an increase in LDL-c was manifested in DKO rats (Fig. 3e,f). Interestingly, although Mc3r KO did not induce any significant alteration in lipid profile, it did show an additive effect to Mc4r KO on elevation of serum lipids (Fig. 3b–d). In addition, all three mutant rats displayed significantly increased WAT weight as well as percentage of body weight (Fig. 4a,b, n = 4–8, p ≤ 0.0001; Fig. 4c,d, n = 6–8, p < 0.0001). In males, the mean visceral WAT rose from 3.3% of body weight in WT to 5.4% in Mc3r KO, 7.7% in Mc4r KO, and 6.8% in DKO respectively (Fig. 4b, n = 4–8, p ≤ 0.0001). In females, it rose from 2.2% of body weight in WT to 8.9% in Mc3r KO, 9.4% in Mc4r KO, and 8.1% in DKO (Fig. 4d, n = 6–8, p < 0.0001). These differences in body fat were also reflected in adipocytes. All three mutants had an enlarged average size for adipocytes, with DKO displaying the greatest enlargement (Fig. 4e, n = 6, p = 0.05 for Mc3r KO, p = 0.02 for Mc4r KO, p = 0.0002 for DKO). Altogether, the above data showed that Mc4r KO and DKO had a higher serum lipid content, while Mc3r KO only showed an additive effect on top of Mc4r KO. Furthermore, all mutant rats displayed increased visceral WAT as well as enlarged adipocyte size.
Serum lipid profile.
(a) Typical appearance of serum from different groups of rats. (b) Total cholesterol (c) Triglyceride, (d) Free fatty acid, (e) Low-density lipoprotein cholesterol (LDL-c) and (f) High density lipoprotein cholesterol (HDL-c) of Mc3r KO, Mc4r KO, DKO rats and WT littermates at 8 weeks of age (n = 10–16). Data from male and female rats were merged as they showed a similar pattern and are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT littermates (one-way ANOVA test).
Visceral total WAT and adipocytes.
Visceral total white adipose tissue (WAT) weight (a,c) and percentage of body weight (b,d) of Mc3r KO, Mc4r KO, DKO and WT controls of 14-week old male and female rats (n = 8 except 4–6 for DKO). Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT control (one-way ANOVA test). (e) Representative images of adipocytes of Mc3r KO, Mc4r KO, DKO rats and WT littermates, together with quantitation (magnification ×100 and ×400), from at least 3 animals of each genotype.
Effect of MC3R and MC4R deficiency on liver and kidney
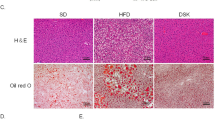
Next we examined whether there was any change in the liver of mutant rats. Results showed that Mc3r KO rats had a relatively smaller liver, whereas Mc4r KO and DKO rats had significantly larger livers as compared to WT littermates (Fig. 5a, n = 8, p = 0.0005 for Mc4r KO, p < 0.0001 for DKO). Mc3r KO and DKO rats showed elevated alanine aminotransferase and aspartate aminotransferase (Fig. 5b,c). However, when we checked up hematoxylin-eosin (HE) staining of liver sections, it was obvious that the Mc3r KO liver was indistinguishable from WT liver, while livers of Mc4r KO and DKO showed accumulation of lipid in the intracellular vesicles, with the phenotype more severe in DKO livers (Fig. 5d,e). To confirm the result, we subjected liver slides to oil Red-O staining. There were significantly more red stained areas in Mc4r KO and DKO liver sections indicating the presence of higher level of lipids, whereas no significant change was observed in the liver of Mc3r KO (Fig. 5f,g, n = 6, p < 0.0001).
Alteration of liver in MC3R and MC4R deficient rats.
(a) Relative liver weight (as percentage of body weight) of Mc3r KO, Mc4r KO, DKO and WT littermates at 14 weeks (n = 8). (b) Alanine aminotransferase and (c) Aspartate aminotransferase of Mc3r KO, Mc4r KO, DKO and WT littermates (n = 8). Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. WT littermates (one-way ANOVA test). (d,e) Representative image of HE staining and (f,g) Oil Red-O staining of livers from at least 3 animals per group (magnification ×100). Relative areas of vacuolation or Oil Red O staining were quantified using Image-Pro® Plus version 6.0 software.
Effects of MC3R and MC4R deficiency on the kidney were also examined. A smaller relative kidney size was found in Mc3r KO and DKO rats. Elevation of uric acid was only observed in DKO. Creatinine was reduced in Mc4r KO. Importantly, HE staining of kidneys from KO rats did not show any significant alteration (see Supplementary Fig. S3). Therefore, MC4R deficiency led to liver steatosis but did not cause significant change in kidney histology. MC3R deficiency alone was not enough to produce a significant alteration in the liver, but it did aggravate the pathological effect of MC4R deficiency.
Characterization of glucose metabolism
When blood glucose homeostasis was evaluated in different groups of rats, we found that glucose tolerance states were unchanged in all Mc3r KO rats. At 8 weeks of age only DKO rats showed a reduced glucose tolerance, while both Mc4r KO and DKO rats demonstrated a reduced tolerance by the end of 14 weeks (Fig. 6a,b). We then evaluated whether there was any change in the postprandial blood glucose (PBG) level. Only DKO rats showed elevated PBG levels at 7 weeks (n = 8, p = 0.0008), but both Mc4r KO and DKO displayed hyperglycaemia by 13 weeks of age (Fig. 6c, n = 6, p = 0.02 for Mc4r KO, p = 0.002 for DKO). These results were partly in line with the previous report in which serum glucose was unchanged at 4–8 week old, but started to show hyperglycemia at 10–14 weeks of age in male Mc4r KO mice8, although in our study, both male and female Mc4r KO or DKO rats presented the same glucose metabolism characteristics. In addition, a significant elevation of glycosylated hemoglobin (HbA1C) was observed in both Mc4r KO and DKO rats at 14 weeks of age, whereas no change was manifested in Mc3r KO (Fig. 6d,e, n = 6–8, p = 0.0005 for Mc4r KO and p < 0.0001 for DKO). Similarly, insulin was elevated in both Mc4r KO and DKO rats at 14 weeks of age (Fig. 6f, n = 6–8, p = 0.01 for Mc4r KO, p = 0.04 for DKO). Leptin levels were also elevated in Mc4r KO as well as DKO rats, although the latter did not reach the significant level (Fig. 6g, n = 6–8, p = 0.007 in Mc4r KO, p = 0.07 for DKO). At the same time, Mc3r KO did not display any significant alteration in insulin or leptin levels (Fig. 6f,g). The above results suggested that although Mc3r KO did not show a significant change in glucose metabolism, it showed an additive effect on top of Mc4r KO and led to an earlier and more severe hyperglycaemia in DKO.
Effect of MC3R and MC4R deficiency on glucose metabolism.
OGTT of different groups of rats at (a) 8 weeks and (b) 14 weeks of age. (c) Postprandial blood glucose (PBG) of mice at indicated ages. (d,e) Hb1Ac concentration, (f) Insulin and (g) Leptin levels of Mc3r KO, Mc4r KO, DKO and WT littermates at 14 week of age. Only data (mean ± SEM, n = 6–8) from males are shown. Females showed a similar trend. *p < 0.05, ***p < 0.001 vs. WT littermates (one-way ANOVA test).
Discussion
In this study, we report rat models of Mc3r total KO, Mc4r total KO and Mc3r/Mc4r DKO in a homogenous background, and provide a systematic comparison of MC3R and MC4R functions in energy metabolism. Our data reinforce previous reports about MC4R’s role in energy metabolism. However, for the first time MC3R deficiency was found to display a reduced body weight, whereas at the same time exhibited additive effects on top of MC4R deficiency in lipid and glucose metabolism. This report will be useful not only in understanding the effects of MC3R and MC4R deficiency in obesity and obesity-related diseases, but also provides better options of rat models that can be used in obesity and type 2 diabetes studies. Compared with other obesity rat models, our Mc4r KO and DKO manifested a relatively earlier hyperglycaemia on a normal chow diet (Table 1). Zucker rats have normal blood glucose28, while Mc4rK314x rats were reported have to be maintained on a moderately high-fat diet, with a limited commercial availability29.
The effect of MC4R deficiency on obesity and metabolism homeostasis is fairly consistent. Compared with Mc4rK314x rats, our Mc4r KO founders had a similar phenotype in body weight, food intake, insulin and leptin level26, which was further confirmed in this study. We speculate that our Mc4r KO rats may have a greater food intake increase if we use a chow diet with a higher fat content, as Mc4r KO mice were reported to show a fat-induced hyperphagia30,31. Additionally, our Mc4r KO rats have similar body size and hyperglycaemia phenotypic characteristics as Mc4r KO mice (Table 2). However, the function of MC3R is more complicated. Initial studies of the phenotypes of Mc3r KO mice indicated a very small body weight increase under chow diet, despite the increased fat mass16,32. In addition, there was no hyperphagia or glycaemia, indicating that Mc3r KO mice are more protected from metabolic syndrome compared to other obesity models with similar levels of adiposity33. More recent studies have demonstrated an exaggerated diet-induced obese phenotype in Mc3r KO12,34. However, while Mc3r KO fed on a high fat diet achieved a level of adiposity comparable to that observed in Mc4r KO mice, the insulin resistant phenotype remained modest and less severe35. While most of our data related to Mc3r KO were consistent with previous mouse data including increased adiposity, it is worth noting that there were some significant difference (Table 2). First, our Mc3r KO rats displayed a significant reduction in body weight and food intake as compared to WT littermates. Furthermore, from PND 45 to 85 (which is the usual age for rats used in biological research), DKO rats displayed almost the same body weight and weight gain as Mc4r KO rats. Previous reports stated that Mc3r/Mc4r DKO mice were significantly heavier than Mc4r single KO mice at the age of 26 weeks, although no data demonstrated whether any difference existed before this age16,36. Although it seems different from results in mice, the reduction or lack of additive effect in body weight of Mc3r deficient rats is consistent with the reported notion that MC3R is an inhibitory autoreceptor on proopiomelanocortin (POMC) neurons. This inhibitory role of MC3R was suggested when the MC3R agonist, D-trp8-γ-MSH (melanocyte stimulating hormone) was found to cause an increase in food intake which was absent in MC3R KO mice37. However, it is also possible that the reduced body size of our Mc3r KO rats is due to other reasons, such as reduced hunger sensations owing to an attenuated response of AgRP/Npy neurons38. There were no MC3R null humans. Although MC3R mutations had been reported to associate with obesity in human17,18,19,39,40, whether MC3R variants play a causative role still needs more investigation. Therefore it is urgent that the physiological function and signalling of MC3R be clarified. Up till now, the signal pathway of MC3R is not well defined. Results generated from various models were quite different. Some groups reported that MC3R coupled to adenylyl cyclases through Gs, leading to stimulation of cAMP production12,41. Others demonstrated calcium as well as MAP kinase activation in ex vivo or in vivo models41,42,43,44.
Despite the fact that MC3R and MC4R deficiency showed an opposite effect on food consumption and body weight in our mutant rats, we observed additive effects of Mc3r KO to Mc4r KO in phenotypes including adipocyte size, hepatic steatosis, lipid profile, OGTT and hyperglycaemia. This suggests that MC3R has a more complicated role in energy regulation. It may exert different functions on feeding behavior and lipid regulation through different signalling pathways. In fact, MC4R was recently found to exert distinct physiological functions through different G proteins. The research found that in addition to the effect of MC4R on glucose metabolism and energy expenditure which are mediated by Gαs, a pathway for appetite regulation was identified which was mediated by Gαq/1145. It is known that melanocortin receptors share some common natural agonists, but with different affinities and different signalling pathways. This maybe one of the reasons why MC3R and MC4R have additive effects in energy metabolism. In addition, both MC3R and MC4R are expressed in the CNS as well as in distinct peripheral sites such as gut, muscle, pancreas13,46,47. They had been reported to exert central and peripheral actions in energy metabolism48. It is very likely that MC3R and MC4R regulate adiposity and lipid metabolism using different mechanisms, and both of them together could more accurately regulate energy balance.
Our data confirmed the notion that MC3R has a critical role in inhibition of energy storage, and its ablation led to the increased body fat16,32. However, despite the obvious increase in body fat, we did not observe alterations in lipid contents of Mc3r KO rats, indicating that MC3R by itself does not have a dominant function in lipid metabolism. While Mc3r KO showed a subtle phenotype, DKO exhibited worse phenotypic features than single KO rats, suggesting that both receptors are important and non-redundant in energy balance and that some interactions probably exist between them. The phenotype of Mc3r KO might even be dependent upon the presence of MC4R. A number of MC3R and MC4R agonists have been developed, yet none of them has demonstrated satisfactory selectivity12,49. It is important to identify more selective agonists for MC3R and MC4R in order to elucidate their precise physiological functions in vivo. For obesity or diabetic drug development, it may be more attractive to look for pathway selective compounds of these receptors to regulate specific functions.
In conclusion, this study is the first systematic comparison of MC3R and MC4R single deficiency and double deficiency in rats with the same genetic background. It seems these two receptors possess non-redundant but somewhat overlapping functions in energy metabolism. Although MC3R deficiency led to a reduction of food consumption and body weight, it did show some additive effects on top of MC4R deficiency in both lipid and glucose metabolic disorders, which suggests different signalling pathways exist for MC3R. Data presented in this paper shed some new lights on the mechanism of MC3R and MC4R function in metabolic regulation and revealed potential interactions between MC3R and MC4R deficiency. The Mc4r KO and DKO rats generated in our lab will be beneficial for future studies to further elucidate MC3R and MC4R’s function and signalling pathways, and provide better rat models for novel anti-obesity or anti-diabetic drug development. In addition, the Mc3r KO rat might have its own value as a unique model in which high adiposity is not linked with body weight increase and insulin resistance.
Methods
Animals
Heritable total Mc3r or Mc4r gene knockout were generated using CRISPR-Cas9 system in our lab26, using Sprague Dawley (SD) rats from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The single KO homozygotes were breed from heterozygotes, and Mc3r/Mc4r DKO were generated by intercrossing double-heterozygous rats. The mutant rats appeared phenotypically normal except for changes in body weight and size. No obvious developmental and reproductive defects were noticed which was consistent with that reported in mouse models50. Animals were housed 2 per cage, maintained in a specific pathogen-free facility on 12-hour light/12-hour dark cycles at a constant room temperature (22 ± 1 °C), with free access to water and chow diet (18% protein and 6% fat, Xietong Medical and Biological Engineering Co. Ltd., Jiangsu, China). Food and water were weighed before and after each change, and the difference was calculated as estimated intake. Minimal bedding was used for easy inspection of possible abnormal spillage, which was not observed. In fact spillage was very little and similar in all four genotypes. All animal experimental procedures and techniques were approved by the Animal Ethics Committee of East China Normal University (Permit number: R20151504), and methods were carried out in accordance with the approved guidelines.
Genotype of Mc3r and Mc4r KO rats
Tail clips were subjected to a standard DNA extraction procedure. Identification of the Mc4r mutation was done by PCR, with primers 5′-GTCCGCCACAGCCAGACTAC-3′ (sense) and 5′-CGCTGCTTCTGACCCTGTTC-3′ (antisense). The Mc3r mutation was identified by PCR with primers 5′-CCCAGCAGCTTGCTCAGGAC-3′ (sense) and 5′-CTCCAGGGAGTTGGACAGGC-3′ (antisense), together with bpm1 (New England Biolabs Inc., USA) digestion analysis. Mutations in Mc3r and Mc4r were successfully transmitted to the following generations. The genotypes of all of the rats were verified as correct.
Quantitative real-time PCR analysis
Rats were sacrificed and total RNA was isolated from brain, liver and WAT with TRIzol (Invitrogen). Possible DNA contamination was eliminated using DNase I (Amplification Grade, Invitrogen). The purified RNA was then reverse-transcribed to cDNA using the Prime Script RT kit (Takara). Real-time PCR was performed in quadruplicate with a SYBR Green PCR Master Mix (Takara) according to the manufacturer’s instruction and ran on the MX3005p system (Stratagene, USA). Data were calculated through MXProv4.1. (Stratagene, USA). Primers (synthesized by Shanghai Biosune, China) were as follows: Mc3r, (forward) 5′-TGCTGCCCGTCCTCCTCTTA-3′ and (reverse) 5′-CCAGGATCACCAGGATGTTTT-3′; Mc4r, (forward) 5′-GGACCACTTCAAGGAGGATT-3′ and (reverse) 5′-CACCCAGAGTCACAAACACC-3′; the reference gene GAPDH, (forward) 5′-TCTCTGCTCCTCCCTGTTCT-3′ and (reverse) 5′-TACGGCCAAATCCGTTCACA-3′.
Bodyweight, size and Lee’s index calculation
Weight gain was monitored throughout the study. For animal size, rats were anesthetized with isoflurane (Hebei Yipin Pharmaceutical Co. Ltd, Hebei, China) and body length (from anus to nose) was measured. Because there is possible body length change in the mutant rats, we used Lee’s index as an additional measurement for the degree of obesity. Lee’s index was calculated as bodyweight (gram)1/3/body length (cm) × 100051.
Biochemical analysis
Blood was collected from the retro-orbital plexus and serum was obtained by centrifugation at 3000 rpm for 15 minutes at 4 oC, which was kept frozen at −80 oC until analysis. Serum triglyceride, total cholesterol, aspartate aminotransferase, alanine aminotransferase, uric acid, creatinine, LDL-c and HDL-c were analyzed using the AU680 Automatic Biochemistry Analyzer (Beckman Coulter, USA). Serum insulin, leptin and free fatty acid levels were measured using ELISA kits (R&D systems, USA). For HbA1c measurement, plasma samples were tested in a rat HbA1c kit (Shensuo Youfu Medical Diagnosis Products Co. Ltd, Shanghai, China) according to the manufacturer’s instruction. PBG levels of overnight fasted rats were measured 2 h after an oral glucose challenge at 2 g/kg, using a portable glucose meter (ACCU-CHEK Performa Nano, Roche).
Oral Glucose Tolerance Test (OGTT)
Animals were fasted overnight and blood glucose was measured from tail bleeds before and 15, 30, 45, 60, 90, and 120 minutes after intragastric administration of 50% glucose (2.5 g/kg), using the Roche glucose meter.
Hematoxylin-Eosin (HE) staining
Rats were sacrificed by CO2 inhalation. Visceral adipose tissue, liver and kidney samples were collected and fixed overnight in ice-cold 4% paraformaldehyde solution, then embedded in paraffin. Serial 5 μm sections were cut and stained with hematoxylin-eosin for histological analysis. Images were captured on a Leica DM4000 B LED microscope with a Leica DFC310FX Camera and software kit. Quantification of HE staining was done by Image-Pro® Plus version 6.0 software. A minimum of 5 independent fields per sample was evaluated.
Oil Red-O staining
Liver samples were fixed overnight in 4% paraformaldehyde, followed by immersion in 15% and then 30% sucrose. Serial 5 μm sections were cut and stained with the Oil Red-O staining kit (Jiancheng Scientific Inc., Nanjing, China) for 3~5 minutes in accordance with the manufacturer’s instruction, and finally counterstained with hematoxylin. Relative areas of lipid accumulation (expressed as percentage Oil Red-O staining) were quantified using Image-Pro® Plus version 6.0 software. A minimum of 5 independent fields per sample was evaluated.
Statistical analyses
Data are expressed as mean ± SEM. One way ANOVA followed by Turkey’s multiple comparisons test was applied to analyse differences among groups, except for body weight (Fig. 1a,b and Supplementary Fig. S2), where the comparisons were analysed using repeated measures analysis followed by Bonferroni post-tests. p < 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: You, P. et al. Effects of Melanocortin 3 and 4 Receptor Deficiency on Energy Homeostasis in Rats. Sci. Rep. 6, 34938; doi: 10.1038/srep34938 (2016).
References
Cone, R. D. Studies on the physiological functions of the melanocortin system. Endocr Rev 27, 736–749, doi: 10.1210/er.2006-0034 (2006).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8, 571–578, doi: 10.1038/nn1455 (2005).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348, 1085–1095, doi: 10.1056/NEJMoa022050 (2003).
Hinney, A. et al. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J Clin Endocrinol Metab 88, 4258–4267, doi: 10.1210/jc.2003-030233 (2003).
Ho, G. & MacKenzie, R. G. Functional characterization of mutations in melanocortin-4 receptor associated with human obesity. J Biol Chem 274, 35816–35822 (1999).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 20, 113–114, doi: 10.1038/2407 (1998).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505, doi: 10.1016/j.cell.2005.08.035 (2005).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141, doi: 10.1016/S0092-8674(00)81865-6 (1997).
Chagnon, Y. C. et al. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study. Mol Med 3, 663–673 (1997).
Panaro, B. L. et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab 20, 1018–1029, doi: 10.1016/j.cmet.2014.10.004 (2014).
Roselli-Rehfuss, L. et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90, 8856–8860 (1993).
Renquist, B. J., Lippert, R. N., Sebag, J. A., Ellacott, K. L. & Cone, R. D. Physiological roles of the melanocortin MC(3) receptor. Eur J Pharmacol 660, 13–20, doi: 10.1016/j.ejphar.2010.12.025 (2011).
Chhajlani, V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int 38, 73–80 (1996).
Getting, S. J. et al. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol 170, 3323–3330 (2003).
Ni, X. P., Pearce, D., Butler, A. A., Cone, R. D. & Humphreys, M. H. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest 111, 1251–1258, doi: 10.1172/JCI16993 (2003).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 26, 97–102, doi: 10.1038/79254 (2000).
Feng, N. et al. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes 54, 2663–2667 (2005).
Lee, Y. S., Poh, L. K. & Loke, K. Y. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab 87, 1423–1426, doi: 10.1210/jcem.87.3.8461 (2002).
Lee, Y. S., Poh, L. K., Kek, B. L. & Loke, K. Y. The role of melanocortin 3 receptor gene in childhood obesity. Diabetes 56, 2622–2630, doi: 10.2337/db07-0225 (2007).
Lee, B. et al. A mouse model for a partially inactive obesity-associated human MC3R variant. Nat Commun 7, 10522, doi: 10.1038/ncomms10522 (2016).
Ste Marie, L., Miura, G. I., Marsh, D. J., Yagaloff, K. & Palmiter, R. D. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97, 12339–12344, doi: 10.1073/pnas.220409497 (2000).
Qiu, J., Ogus, S., Mounzih, K., Ewart-Toland, A. & Chehab, F. F. Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology 142, 3421–3425, doi: 10.1210/endo.142.8.8323 (2001).
Stoehr, J. P. et al. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes 49, 1946–1954 (2000).
van Boxtel, R. et al. Systematic generation of in vivo G protein-coupled receptor mutants in the rat. Pharmacogenomics J 11, 326–336, doi: 10.1038/tpj.2010.44 (2011).
Mul, J. D. et al. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 20, 612–621, doi: 10.1038/oby.2011.81 (2012).
Li, D. et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol 31, 681–683, doi: 10.1038/nbt.2661 (2013).
Hentze, M. W. & Kulozik, A. E. A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96, 307–310 (1999).
Bray, G. A. The Zucker-fatty rat: a review. Fed Proc 36, 148–153 (1977).
Roth, J. D. et al. Interactions of amylinergic and melanocortinergic systems in the control of food intake and body weight in rodents. Diabetes Obes Metab 14, 608–615, doi: 10.1111/j.1463-1326.2012.01570.x (2012).
Butler, A. A. et al. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4, 605–611, doi: 10.1038/88423 (2001).
Srisai, D. et al. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology 152, 890–902, doi: 10.1210/en.2010-0716 (2011).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521, doi: 10.1210/endo.141.9.7791 (2000).
Ellacott, K. L., Murphy, J. G., Marks, D. L. & Cone, R. D. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 148, 6186–6194, doi: 10.1210/en.2007-0699 (2007).
Sutton, G. M. et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147, 2183–2196, doi: 10.1210/en.2005-1209 (2006).
Trevaskis, J. L. et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 15, 2664–2672, doi: 10.1038/oby.2007.318 (2007).
Atalayer, D., Robertson, K. L., Haskell-Luevano, C., Andreasen, A. & Rowland, N. E. Food demand and meal size in mice with single or combined disruption of melanocortin type 3 and 4 receptors. Am J Physiol Regul Integr Comp Physiol 298, R1667–R1674, doi: 10.1152/ajpregu.00562.2009 (2010).
Marks, D. L., Hruby, V., Brookhart, G. & Cone, R. D. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R). Peptides 27, 259–264, doi: 10.1016/j.peptides.2005.01.025 (2006).
Girardet, C., Mavrikaki, M., Southern, M. R., Smith, R. G. & Butler, A. A. Assessing interactions between Ghsr and Mc3r reveals a role for AgRP in the expression of food anticipatory activity in male mice. Endocrinology 155, 4843–4855, doi: 10.1210/en.2014-1497 (2014).
Lee, Y. S. The role of leptin-melanocortin system and human weight regulation: lessons from experiments of nature. Ann Acad Med Singapore 38, 34–11 (2009).
Mencarelli, M. et al. Sporadic mutations in melanocortin receptor 3 in morbid obese individuals. Eur J Hum Genet 16, 581–586, doi: 10.1038/sj.ejhg.5202005 (2008).
Kim, C. S. et al. Identification of domains directing specificity of coupling to G-proteins for the melanocortin MC3 and MC4 receptors. J Biol Chem 277, 31310–31317, doi: 10.1074/jbc.M112085200 (2002).
Mountjoy, K. G., Kong, P. L., Taylor, J. A., Willard, D. H. & Wilkison, W. O. Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiol Genomics 5, 11–19 (2001).
Chai, B., Li, J. Y., Zhang, W., Ammori, J. B. & Mulholland, M. W. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept 139, 115–121, doi: 10.1016/j.regpep.2006.11.003 (2007).
Konda, Y. et al. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. J Biol Chem 269, 13162–13166 (1994).
Li, Y. Q. et al. Gq/11alpha and Gsalpha mediate distinct physiological responses to central melanocortins. J Clin Invest 126, 40–49, doi: 10.1172/JCI76348 (2016).
Gantz, I. et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem 268, 8246–8250 (1993).
Ni, X. P., Butler, A. A., Cone, R. D. & Humphreys, M. H. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens 24, 2239–2246, doi: 10.1097/01.hjh.0000249702.49854.fa (2006).
Begriche, K. et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J Biol Chem 286, 40771–40781, doi: 10.1074/jbc.M111.278374 (2011).
Huang, H. & Tao, Y. X. A small molecule agonist THIQ as a novel pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Int J Biol Sci 10, 817–824, doi: 10.7150/ijbs.9625 (2014).
Butler, A. A. & Cone, R. D. The melanocortin receptors: lessons from knockout models. Neuropeptides 36, 77–84 (2002).
Lei, F. et al. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 31, 1023–1029, doi: 10.1038/sj.ijo.0803502 (2007).
Bao, D. et al. Preliminary Characterization of a Leptin Receptor Knockout Rat Created by CRISPR/Cas9 System. Sci Rep 5, 15942, doi: 10.1038/srep15942 (2015).
Reinwald, S., Peterson, R. G., Allen, M. R. & Burr, D. B. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am J Physiol Endocrinol Metab 296, E765–E774, doi: 10.1152/ajpendo.90937.2008 (2009).
Tamasi, J. A., Arey, B. J., Bertolini, D. R. & Feyen, J. H. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J Bone Miner Res 18, 1605–1611, doi: 10.1359/jbmr.2003.18.9.1605 (2003).
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2013ZX09507001) and National Basic Research Program of China (2012CB910401) to M.L., the National Natural Science Foundation of China (31271468) and the Science and Technology Commission of Shanghai Municipality (12ZR1408700) to H.C. The authors thank Dr. Stefan Siwko of Texas A&M University Health Science Center for scientific editing of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.C., H.C., D.L. and M.L. conceived the experiments. P.Y., Y.Z., Y.Y., T.W., Y.S. and R.X. conducted the experiments. H.C., D.L., Y.C., P.Y., W.Z. and H.H. analysed the results. H.C., Y.Z. and H.H. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
You, P., Hu, H., Chen, Y. et al. Effects of Melanocortin 3 and 4 Receptor Deficiency on Energy Homeostasis in Rats. Sci Rep 6, 34938 (2016). https://doi.org/10.1038/srep34938
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep34938
This article is cited by
-
Evaluation of the MC3R gene pertaining to body weight and height regulation and puberty development
Scientific Reports (2023)
-
Molecular cloning and functional characterization of melanocortin-3 receptor in grass carp (Ctenopharyngodon idella)
Fish Physiology and Biochemistry (2023)
-
Hypothalamic MC4R regulates glucose homeostasis through adrenaline-mediated control of glucose reabsorption via renal GLUT2 in mice
Diabetologia (2021)
-
Identification and characterization of the melanocortin 1 receptor gene (MC1R) in hypermelanistic Chinese tongue sole (Cynoglossus semilaevis)
Fish Physiology and Biochemistry (2020)
-
Mouse models of atherosclerosis and their suitability for the study of myocardial infarction
Basic Research in Cardiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.